Abstract
Objective
The aim was to estimate the cost-effectiveness of inotuzumab ozogamicin (InO) versus standard of care chemotherapy (SoC) for adults with relapsed or refractory B cell acute lymphoblastic leukaemia (R/R ALL) in Sweden and Norway, and compare this to evaluations made by the health technology assessment (HTA) authorities Tandvårds- och läkemedelsförmånsverket (TLV) and the Norwegian Medicines Agency (NoMA).
Materials and methods
A partitioned survival model was developed to determine incremental cost-effectiveness ratios (ICERs) for InO versus SoC. Parametric survival models were fit to overall survival and progression-free survival Kaplan-Meier data from the INO-VATE ALL phase III trial. Two base cases were run using (1) Swedish and (2) Norwegian inputs (costs and discount rates). Core clinical inputs and utilities did not differ between countries. Analyses were then conducted to reflect the preferred assumptions of TLV and NoMA. Univariate and multivariate sensitivity analyses were performed.
Results
The base case deterministic ICERs for InO versus SoC were €16,219/quality-adjusted life years (QALY) in Sweden (probabilistic €19,415) and €44,405/QALY in Norway (probabilistic €47,305). The ICERs using our model but applying the preferred assumptions of TLV or NoMA were €74,061/QALY (probabilistic €77,484) and €59,391/QALY (probabilistic €63,632), respectively. Differences between our base cases and the ICERs with TLV and NoMA settings were mainly explained by the exclusion of productivity costs and use of pooled post-haematopoietic stem-cell transplant (post-HSCT) survival in Sweden and use of higher HSCT costs in Norway. All ICERs remained below the approximated willingness-to-pay thresholds. The probability of InO being cost-effective ranged from 77 to 99% versus SoC.
Conclusions
InO can likely be considered cost-effective versus SoC under our and the HTA-preferred settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the INO-VATE acute lymphoblastic leukaemia (ALL) phase III trial, inotuzumab ozogamicin (InO) demonstrated improved overall survival and remission rate, favourable patient-reported outcomes, and a manageable safety profile versus standard of care chemotherapy (SoC) for relapsed or refractory acute lymphoblastic leukaemia (R/R ALL). |
Prior to public reimbursement, in Sweden and Norway, a health technology assessment (HTA) including a cost-effectiveness analysis is conducted. |
This article explores the cost-effectiveness of InO versus SoC and examines the differences between our preferred set of cost-effectiveness analyses and the evaluations made by the Swedish and Norwegian HTA authorities in order to aid future decision making on where and how to use InO for the treatment of R/R ALL. |
1 Introduction
Acute lymphoblastic leukaemia (ALL) is a malignant disorder of lymphoid progenitor cells that affects children and adults [1]. In Sweden and Norway around 50 and 44 adults are diagnosed with ALL annually, respectively [2,3,4]. Upon diagnosis, patients are stratified by a number of factors to help understand the prognosis and guide therapeutic decisions (e.g. T vs B cell leukaemia; Philadelphia-negative vs Philadelphia-positive leukaemia). For patients newly diagnosed with ALL, treatment consists of an induction phase, a consolidation phase, and a maintenance phase [2, 5, 6]. Chemotherapy is typically administered in the induction phase with the aim of achieving a complete remission (CR) or complete remission with incomplete haematological recovery (CRi), with treatment in the following consolidation and maintenance phases aiming to keep the leukaemia in remission [7]. If remission is achieved following previous conditioning pharmacological treatment, a subsequent allogeneic haematopoietic stem-cell transplant (HSCT) [8] currently offers the best opportunity for long-term disease-free survival [7, 9,10,11,12]. Only around 30–40% of adults with ALL achieve long-term disease-free survival, with the primary reason for failure being disease recurrence [13]. If a patient relapses following treatment or HSCT or if they are refractory to treatment (R/R ALL), the aim of salvage treatment is again to induce haematological remission (CR/CRi) so that patients might be able to bridge to subsequent HSCT. To induce haematological remission in the R/R setting, patients have historically been treated with a salvage regimen consisting of combination chemotherapy [2, 6].
Over recent years, new options have been added to the R/R treatment arsenal, with the targeted therapy inotuzumab ozogamicin (InO) (Besponsa®;) now indicated for adults with B cell R/R ALL, [14] based on results versus standard of care chemotherapy (SoC) in the INO-VATE 1022 ALL trial [15, 16]. The INO-VATE ALL trial was a multicentre, parallel, open-label, phase 3 trial that randomised 326 adults with R/R ALL to InO (n = 164) or SoC (n = 162). The study demonstrated that patients in the InO arm versus the SoC arm experienced a significantly higher CR/CRi rate (73.8% vs 30.9%; P < 0.0001), improved minimal residual disease rate (78.4% vs 28.1% in the initially studied population; P < 0.001), and longer duration of remission (median 5.4 months vs 4.2 months; P = 0.0071). Median overall survival (OS) was 7.7 months with InO and 6.2 months with SoC, with 2-year OS rates of 22.8% and 10.0%, respectively (overall hazard ratio [HR] 0.75; P = 0.0105). Correlated with these improvements, the percentage of patients reaching HSCT after achieving CR/CRi was higher for InO versus SoC (39.6% vs 10.5%; P < 0.0001) [15, 16].
Prior to public reimbursement in many countries covered by the European Medicines Agency, health technology assessments (HTAs) must often be conducted, including in Norway and Sweden. Such HTAs typically include a cost-effectiveness analysis to assess whether an intervention brings value for money. Based on HTAs conducted by Tandvårds- och läkemedelsförmånsverket (TLV) and the Norwegian Medicines Agency (NoMA), InO was considered to be cost-effective and recommended for use by the New Therapies (NT) council in Sweden and publicly reimbursed in Norway [4, 17, 18].
The aim of this paper was to estimate the cost-effectiveness of InO compared to SoC for the treatment of B cell R/R ALL in Sweden and Norway, and to compare the results of our two base cases to the results when applying to our model the preferred assumptions stated by TLV and NoMA in their recent appraisals of InO [4, 18]. Whilst in most part the models were similar between our base cases and the preferred base cases of the two agencies, we have sought to highlight where assumptions differed and provide justification and rationale for our preferences. By presenting a full set of assumptions, where they differed, and then the resulting impact these have on cost-effectiveness estimates via a side-by-side examination, we aim to provide future decisions makers with transparent and full information to aid their choices and decisions.
2 Methods
The cost-effectiveness for InO versus SoC was assessed using a health economic model. The following sections describe the model structure and incorporation of clinical and economic inputs. Two sets of base case analyses were performed, specific to the perspective of each country: (1) using Swedish and (2) using Norwegian cost inputs and annual discount rates. These base case analyses were then compared to analyses using the preferred assumptions of TLV and NoMA. Both TLV and NoMA appraised InO using fundamentally the same model as the one presented in this study, although some assumptions differed.
2.1 Model Structure
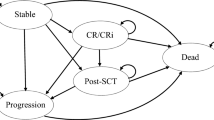
The cost-effectiveness analysis used a time-dependent, partitioned survival model with states defined based on treatment response (CR/CRi), HSCT, progression, and death (Fig. 1). A comparable model structure has been used in the National Institute of Health and Care Excellence (NICE) appraisal of InO (appraisal TA541) and was deemed appropriate for decision-making [19].
All patients entered the model in the ‘stable’ state. The average age of the patient cohort at the start of the model was 46 years, based on the INO-VATE ALL trial [16, 20]. A monthly model cycle length (30.4375 days) was used. From the ‘stable’ health state, patients could move to the ‘CR/CRi’ state after one cycle (the average time to response in INO-VATE ALL was 1 month) or to the ‘post-HSCT’ state. Almost all patients in INO-VATE ALL who underwent HSCT did so having first achieved CR/CRi; however, a small number of patients underwent HSCT without CR/CRi. Transition to ‘post-HSCT’ from either the ‘stable’ or ‘CR/CRi’ state occurred after 4 and 5 months for InO and SoC, respectively, mirroring the average time to HSCT in the INO-VATE ALL trial’s respective arms [20]. This transition at 4 and 5 months was considered appropriate as alternatively allowing patients to transition to HSCT in different cycles through tunnel states led to similar results but overcomplicated the model. From all health states, patients were at risk of disease progression and/or death. This risk was specific to each arm and each state and was determined by progression-free survival (PFS) and OS Kaplan-Meier data from INO-VATE ALL’s latest cut (April 2017) [16, 20]. To capture all relevant costs and effects, a life-time time horizon was used. Discount rates for future costs and effects of 3.0% per annum for Sweden and 4.0% for Norway were applied in line with TLV and NoMA guidelines [21, 22].
2.2 Model Estimation
Health-state specific PFS and OS were based on the intention-to-treat (ITT) population from the INO-VATE ALL trial (patient characteristics were presented in the trial publications) [16, 20]. PFS and OS for InO and SoC were estimated by fitting parametric survival distributions to Kaplan-Meier data using Flexsurv [23] (Gompertz and generalised gamma) and Survival [24] (exponential, Weibull, lognormal, and log-logistic) R packages. Base case distributions were selected according to fit statistics (Akaike information criterion [AIC] and Bayesian information criterion [BIC]), visual inspection, hazard functions, time-dependent HRs, diagnostic plots for treatment effects, and clinical plausibility.
Parametric survival curves were fitted for each health state (stable, CR/CRi, and post-HSCT) separately.
If the proportional hazards assumption was violated, individually fitted curves were applied; otherwise, survival curves with treatment as a covariate were used [25]. For PFS, the log-logistic and generalised gamma function with treatment as a covariate were used for stable and CR/CRi patients, respectively. For OS, a lognormal distribution was used for stable patients and a generalised gamma was used for CR/CRi patients (both using treatment as a covariate). For post-HSCT survival, generalised gamma and log-logistic distributions were fitted individually for the InO and SoC arms, respectively (the proportional hazards assumptions were violated).
Some patients that receive an HSCT will be long-term survivors [7, 9,10,11,12]. For patients who survived until the end of the maximum follow-up of the INO-VATE ALL trial (approximately 50 months), it was assumed that the HSCT was successful and therefore a lower mortality risk was applicable from 50 months onwards. This risk was estimated by applying a standardised mortality ratio (SMR) to general population mortality data. This way, the model reflects that patients surviving longer term after successful HSCT have survival prospects closer to, yet still lower than, the general population [26]. The use of a point in time post-transplant where the model’s probability of survival moves from using shorter-term clinical trial data to data adjusted from the general population (a ‘functionally cured’ point) has been accepted in other models in the literature [26,27,28,29,30]. To calculate longer-term survival, general population mortality for Norway and Sweden was taken from national life tables [31, 32]. An SMR of 4 from the study of Martin et al. [33] was then applied to elevate the general population mortality risk (implying a risk of death four times higher in any future year than a similarly aged person from the general population). This estimated SMR was preferred by NICE in the United Kingdom (UK) HTA of InO [34]. The model applies the same SMR for both treatment arms, implying an HR of 1 for InO versus SoC is applied from 50 months onwards, i.e. if a patient survived past this point, then they have had a successful HSCT and there was no assumed difference in their survival expectations going forwards whether they had received InO or SoC pre-HSCT.
Based on the PFS and OS extrapolations for stable, CR/CRi, and post-HSCT patients, the time in each health state was determined. The PFS and OS Kaplan-Meier data from the ITT population, the base case parametric survival curves, and parametrisation are summarised in Supplementary Table 1 and Supplementary Figures 1–3 (see the electronic supplementary material). In Supplementary Figures 4 and 5, the long-term modelled OS and PFS are compared to the Kaplan-Meier data from the INO-VATE ALL trial.
2.3 Clinical Inputs
Table 1 presents an overview of the applied clinical inputs and their corresponding uncertainty distributions. The SoC comparator was informed by the INO-VATE ALL trial, in which it was a choice of one of FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor), cytarabine plus mitoxantrone, or high-dose cytarabine (HIDAC), from which the majority received FLAG. Whilst SoC in our Norwegian base case mirrored the trial, the base case in Sweden assumed 50% FLAG-Asp (fludarabine, cytarabine, pegylated-asparaginase plus granulocyte colony-stimulating factor) and 50% MEA (mitoxantrone, etoposide and cytarabine) based on key opinion leader (KOL) opinion. Other inputs informed by the INO-VATE ALL trial included probabilities of response (CR/CRi) and HSCT, adverse event rates, hospitalisation days, and subsequent induction therapy after treatment with InO or SoC (chemotherapy regimens FLAG, HIDAC, and cytarabine plus mitoxantrone based, tyrosine kinase inhibitors [TKIs], or innovative anti-cancer treatments blinatumomab and InO) [16, 20]. Overall, patients in the InO arm used fewer subsequent induction therapies than those in the SoC arm.
Hospitalisation days were measured while patients were on their primary treatment in the INO-VATE ALL trial. For subsequent treatment with InO (which was relevant to the SoC arm), the same number of hospitalisation days as observed on primary induction therapy with InO were applied. For other subsequent treatments, the number of bed-days for SoC from the INO-VATE ALL trial was applied. This was assumed more reflective than using InO’s bed-days as InO can be administered in an outpatient setting. One exception applied for the Swedish subsequent chemotherapy costs, with these estimated using a national healthcare cost code that already included hospitalisation costs (as discussed in the “Cost Inputs” section below). Post-HSCT hospitalisation was assumed to be already captured in the follow-up costs applied after HSCT.
2.4 Utility Inputs
The utilities at baseline and in the stable and CR/CRi health states (see Table 1) were based on the safety population from the INO-VATE ALL trial [16, 20]. In the INO-VATE ALL trial, patients completed the EuroQoL 5 Dimensions Questionnaire (EQ-5D-3L) at baseline, on day 1 of each cycle, and at the end of treatment. The EQ-5D-3L UK value set was applied to determine the utility values [20]. At the time of TLV and NoMA’s appraisals of InO, the UK value set was the preferred set for HTAs in both countries due to the lack of a formally adopted Swedish or Norwegian value set. Given the baseline utility was similar in both arms, a pooled baseline utility of 0.69 was applied for InO and SoC. In the stable and CR/CRi health states, treatment specific utilities were used (stable: InO 0.51, SoC 0.47; CR/CRi: InO 0.73, SoC 0.75). As utilities were not measured past the end of treatment, post-HSCT utilities were not available from the INO-VATE ALL trial; hence, post-HSCT utilities (Table 1) were taken from a study by Kurosawa et al. [35], which estimated utilities of 0.59 for < 1 year after HSCT, 0.75 at 1–2 years after HSCT, 0.74 at 3–5 years after HSCT, and finally 0.76 at 5 + years post-HSCT. The utilities from Kurosawa et al. [35] were estimated from a cross-sectional questionnaire study that administered SF-36, FACT-Leukaemia, and EuroQOL5D to 524 acute leukaemia survivors in Japan [36]. Although they were not from a UK population, at the time of our analyses, these were deemed the best available data. For the progressed disease health state, a utility score of 0.30 was applied, taken from the study by Aristides et al. [37], in which utility values were assigned to R/R ALL health states from the perspective of the UK general population (n = 123) using the time trade-off method for preference elicitation. All utilities were decreased over time to reflect the aging of patients in the model [38].
2.5 Cost Inputs
Table 2 presents an overview of the applied country-specific costs and the corresponding uncertainty distributions. All costs were converted to 2017 Euros to make the results easy to compare, using 31-12-2017 conversion rates of 0.10185 for Sweden [39] and 0.10159 for Norway [40]. Drug costs for InO and SoC were determined by multiplying the average number of vials/tablets used with the costs per vial/tablet (wastage was included). For InO and SoC, the number of vials/tablets used were determined based on the actual doses in the INO-VATE ALL trial (dosing schedules were previously published [15]) and the average body surface area (1.88 m [2]) and weight (76.9 kg) of the INO-VATE ALL patients [20]. As the Summary of Product Characteristics (SmPC) restricted the use of InO for patients who either do not achieve a CR/CRi or were planning to bridge to an HSCT to a maximum of three cycles, an average of 8.9 InO vials was used for our models’ population, corresponding to these SmPC recommendations. Although the trial allowed treatment up to six cycles, all CR/CRi was first achieved during the first three cycles. Therefore, the CR/CRi rate and subsequent HSCT rate associated with only three cycles of InO can be assumed to be similar to that observed in the trial. The costs per vial/tablet were based on Swedish and Norwegian list prices [41, 42].
The costs per inpatient bed-day were higher for Norway (€4376) [43] than for Sweden (€2163) [44]. The costs of HSCT we applied were comparable for both countries (Norway €51,893 [45], Sweden €59,788 [46]); in Norway, our base case used a cost estimate based on reported cost for autologous HSCT, which was the most relevant cost we identified from the literature. Follow-up costs after HSCT were converted from the UK National Health Service Blood and Transplant study (NHSBT) [47] for Sweden as no Swedish data were available, and were based on studies conducted by public institutions for Norway [45].
For subsequent TKIs, drug costs were based on the national list prices of Sweden and Norway [41, 42, 48, 49] and a median treatment duration for ponatinib of 83 days [50]. Hospitalisation associated with TKIs was assumed to be equal to SoC [20]. For subsequent chemotherapy costs, in Sweden, a national healthcare cost code was applied that covered the costs of providing chemotherapy, including hospitalisation in acute leukaemia [51]. For Norway, the cost of subsequent chemotherapy consisted of standard chemotherapy drug costs based on national list prices [41] and hospitalisation costs (INO-VATE ALL SoC bed-days [16, 20] multiplied with the cost per inpatient bed-day in Norway [43]).
Adverse event costs (excluding veno-occlusive disease [VOD], cytokine release syndrome [CRS], and graft-versus-host disease [GVHD]) were sourced from national cost data for Sweden [52, 53] and Norway [54, 55]. For VOD, in Sweden, a weighted average of cost of defibrotide (47.8%) and ursodeoxycholic acid (52.2%) [49, 56] was applied, in line with VOD treatment in the INO-VATE ALL trial [16, 20]. In Norway, VOD was assumed to be treated with defibrotide only [41]. CRS costs were converted from a UK source for Sweden [26] and were assumed to be equal to haematological adverse events in Norway [54]. GVHD costs were estimated based on Espérou et al. in both countries as no country-specific data were available [57]. End-of-life costs were higher in Norway (€16,335) [58] versus Sweden (€8046) [59].
In both countries, costs from a societal perspective were included that reflected the cost of time lost due to travelling to, and staying in, the hospital. The time value per hour for ‘leisure time’ was higher in Norway (€28) [60] versus Sweden (€6) [61]. The travelling time was 1 h in Sweden and 37 h in Norway (average of 0 h for residents of the Oslo/Bergen regions [24%] and 48 h for all other residents [76%] [62]). For Sweden, the costs of lost productivity were included as well. Based on expert opinion from Swedish KOLs, 50% of the HSCT patients were assumed to go back to work after 12 months post-HSCT and the remaining 50% after 18 months post-HSCT. The production value per patient was estimated to be €4390 per month [63, 64], and the general employment rate was 81% [65].
2.6 Analyses
Four sets of analyses are presented: our modelled base cases from the Swedish and Norwegian perspectives, and TLV’s and NoMA’s base cases from their country’s perspective. All four analyses used the same model structure, core clinical inputs, and utility inputs (Table 1). Our two base cases and the two HTA authorities’ all used their respective country’s input costs and discount rates (Table 2). There were some differences between TLV’s and NoMA’s preferred assumptions versus our base case assumptions (Table 3). In summary, TLV’s preferred type of SoC chemotherapy differed slightly to our base case and the population assessed by TLV was more limited than in our base case (TLV selected a subgroup of the INO-VATE ALL trial [the no prior HSCT population], which represents 81% of the full INO-VATE ALL population). The extrapolation of long-term survival post-HSCT also varied in that there were slight differences in the time horizons used. Furthermore, societal costs were not included by TLV [4, 18]. NoMA chose to apply a higher cost for HSCT than our preferred cost, using an estimate based on a Diagnosis Related Group (DRG) code for allogeneic HSCT for patients older than 17 years [4].
Lifetime costs, life years (LYs), and quality-adjusted life years (QALYs) were estimated for each treatment. Scenario analyses were performed to estimate the impact of using the lower or upper limit of the 95% confidence interval of the PFS and OS curves and the impact of applying general population utilities beyond 5 years post-HSCT. Additionally, deterministic sensitivity analyses (DSAs) and probabilistic sensitivity analyses (PSAs) with 1000 simulations were conducted. Where data on distributions were unavailable, a ± 5% or ± 10% range was assumed for costs and utilities, respectively. The DSA findings are presented in a tornado diagram including the ten parameters with the largest influence on the outcomes. Results of the PSA were used to generate acceptability curves for InO versus SoC, showing the probability that InO would be preferred over SoC given a range of willingness-to-pay (WTP) thresholds. Approximate WTP for severe disease, such as R/R ALL, were estimated at €102,000 per QALY in Sweden (corresponding to 1,000,000 Swedish Krona) and €84,000 per QALY (corresponding to 825,000 Norwegian Krone) in Norway [66].
3 Results
Table 4 displays the incremental LYs, QALYs, costs, and resulting incremental cost-effectiveness ratio (ICERs) for our Swedish and Norwegian base cases, alongside the results when TLV’s and NoMA’s preferred assumptions were applied. Our base case ICERs for InO versus SoC were €16,219 and €44,405 per QALY gained for Sweden and Norway, respectively. If costs of lost productivity are excluded, the base case ICER for Sweden was €50,361 per QALY gained, closer to the Norwegian ICER.
Using our model but applying the preferred assumptions of TLV and NoMA, we estimated the ICERs for InO versus SoC to be €75,127 per QALY gained for Sweden (reported as 733,577 Swedish Krona [€74,715] in TLV’s documentation [18]) and €59,391 per QALY gained for Norway (reported as 584,615 Norwegian Krone [€59,391] in NoMA’s documentation [4]). These ICERs all remained below the approximated WTP thresholds.
The results of the scenario analyses are presented in Supplementary Tables 2 and 3 (see the electronic supplementary material). The ICERs did not change substantially for most of the scenarios and generally remained below the approximated WTP thresholds. Applying the lower and upper 95% confidence interval limits of the post-HSCT curve had the biggest impact on the ICERs, as it influenced the number of patients who would be long-term survivors after HSCT. ICERs decreased if general population utilities were applied beyond 5 years post-HSCT, as this increased the quality of life post-HSCT, with InO having bridged more patients to HSCT.
The results of the PSA are presented in Fig. 2. Probabilistic ICERs are presented in Supplementary Table 4. Convergence tests confirmed a sufficient number of PSA iterations were performed (Supplementary Figure 6). The cost-effectiveness acceptability curves showed that the probability of InO being cost-effective versus SoC at the approximated Swedish WTP threshold was 99% in our base case and 81% if TLV’s preferred assumptions were used (Fig. 3). At the approximated Norwegian WTP threshold, the probability of our base case being cost-effective was 93%, or 77% under NoMA’s preferred assumptions.
Cost-effectiveness planes. CE cost-effectiveness, ICER incremental cost-effectiveness ratio, InO inotuzumab ozogamicin, NoMA Norwegian Medicines Agency, PSA probabilistic sensitivity analysis, QALY quality-adjusted life year, SoC standard of care chemotherapy, TLV Tandvårds- och läkemedelsförmånsverket, WTP willingness to pay
The DSA for our base case (Fig. 4a) shows that, in Sweden, the three parameters with the highest influence on the ICER were the number of bed-days for InO, the HSCT rate for InO, and the proportion of patients receiving blinatumomab as a subsequent treatment after SoC. Under TLV’s preferences, the HSCT rate for InO, HSCT rate for SoC, and the number of bed-days for InO were the three main drivers (Fig. 4b). For Norway, in both our base case and NoMA’s, the number of bed-days for InO, the number of bed-days for SoC, and the proportion of patients receiving blinatumomab as subsequent treatment after SoC had the highest influence (Fig. 4c and d).
4 Discussion
The aim of this paper was to assess the cost-effectiveness of InO compared to SoC for treatment of B cell R/R ALL in Sweden and Norway, comparing our base case assumptions to those preferred by TLV and NoMA. We sought to present the model’s assumptions and justify our base case preferences. Table 3 presents where the perspectives of NoMA and TLV differed to ours (noting that assumptions not highlighted were implicitly agreed upon). In Table 4, the paper then aimed to present the differing estimates of cost-effectiveness side by side, allowing an examination of the impact of the differing perspectives.
Our base case and HTA ICERs were below the approximated WTP thresholds for both countries. Incremental QALYs and LYs were similar for InO versus either SoC. The difference in our base case ICERs for Sweden (€16,219 per QALY gained) and Norway (€44,405 per QALY gained) was mainly driven by the inclusion of productivity costs in Sweden, but higher bed-day costs in Norway, and the use of different discount rates also was a factor; removing these three differences would bring our base case ICERs for the two countries to within €200 of each other.
TLV’s preferred assumptions increased the Swedish ICER by €58,908 per QALY gained versus our base case. However, the majority of this difference is driven by TLV’s decision to exclude societal costs. TLV argued these should not be included as there is a risk that patients above the retirement age will be discriminated against for not being able to deliver the expected productivity gains related to treatment with InO [18]. Without societal costs, the difference between our Swedish base case and TLV’s preferred assumptions was €24,766 per QALY gained. An important driver in this difference was TLV’s preference to pool post-HSCT survival; this eliminated differences between arms upon the point of HSCT, which increased the SoC post-HSCT survival over the course of the model whilst reducing the InO survival versus our base case. No precise argumentation for this assumption was provided except for a general discussion on uncertainty around differences in long-term survival between the two treatment arms [18]. TLV also applied a more conservative SMR than our base case, which reduced long-term survival post-HSCT. While we considered an SMR of 4 to already be conservative as transplantation practices and related long-term survival have likely improved compared to the Martin et al. [33] cohort of 30 years ago, TLV argued an SMR of 9 was more applicable [18] as the median age of the INO-VATE ALL trial population was slightly higher than that of the Martin et al. patients [15, 16, 33].
NoMA’s preferred assumptions increased the Norwegian ICER by €14,986 per QALY gained versus our base case. The majority of this difference came from a higher cost used for an HSCT. Our base case used a cost per HSCT of €51,893 in Norway and €59,788 in Sweden. NoMA applied a cost of €105,686 per HSCT, just over twice that in our base case. As InO bridges significantly more patients to HSCT versus SoC, this increased the incremental cost for InO in the model. NoMA also preferred to use parametric curves to extrapolate long-term post-HSCT instead of an SMR as they felt there was insufficient evidence to justify applying an SMR to general population mortality [4]. However, this only had a small impact on the ICER versus our base case assumption to use an SMR of 4. Changing the time horizon from lifetime (60 years) in our base case to NoMA’s preference of 40 years also had minimal impact on the ICER.
This study had several limitations. Although long-term data from the INO-VATE ALL trial was available, there was still uncertainty on how to model post-HSCT survival as OS and PFS data were only collected up to a final point of follow-up and not beyond. Long-term data collected from R/R post-HSCT ALL patients across a multi-decade period is sparse in the literature; further, improvements in transplant practices may render older registry data inaccurate when predicting long-term survival for those who have had successful transplants. In order to account for future survival uncertainty, multiple differing scenarios informed the various results presented in Table 4. As set out in Table 3, the two HTA authorities applied differing assumptions for post-HSCT survival versus our base case, which, together with our base case, presents a range that helps account for this uncertainty. Although the HTA authorities’ assumptions were generally more conservative towards InO than our base case (leading to an increase in the ICERs), both authorities still found InO was associated with acceptable cost-effectiveness in both Norway and Sweden. A further limitation was the uncertainty around the time spent in the hospital while treated with InO or SoC. The hospitalisation duration is expected to vary in clinical practice, demonstrated through a recent study in the UK real-world setting showing InO patients had a vastly reduced hospitalisation duration in the real world (a median of 5 days) compared to that observed in the INO-VATE ALL trial [70]. Although the time spent in the hospital will likely vary between countries, lower real-world hospitalisation with InO has a strong downward effect on the ICER. Finally, an explanation for differences between our base case assumptions and those of the HTA authorities was sometimes difficult to provide as the HTA documentation did not always include a clear argumentation for deviating from our base case assumptions.
By way of external comparison, no previous R/R ALL models in Norway or Sweden were identified. In the UK, however, an adapted version of our described model was used for the NICE appraisal of InO (appraisal TA541) [19]. The model structure was similar to ours and was deemed appropriate for decision-making [19]. The final ICER versus SoC, upon which InO was recommended for use, was £37,497 per QALY gained (approximately €41,000) [19, 71]. The NICE ICER differed slightly from our base case because of confidential UK data on drug costs and a greater real-world hospitalisation advantage for InO over SoC than was observed in INO-VATE ALL; these data would be expected to lower all ICERs for InO versus SoC should it be incorporated into our model [70].
In conclusion, the results presented in this paper highlight our base cases in Sweden and Norway versus estimates informed by TLV and NoMA. All results use the same model structure, core clinical inputs, utility inputs, and similar country-specific input costs and discount rates. We have attempted to provide justification and rationale for our choices and preferences whilst acknowledging the differing preferences of the HTA authorities on specific assumptions. Key differences between our base case and those of the HTA authorities were preferences on the inclusion of societal costs, assumptions for survival post-HSCT, and the cost of HSCT. Based on all the different ICERs presented in this paper, InO is likely to be a cost-effective treatment versus SoC. The transparency reflected in this paper and the side-by-side examination of results will aid future decision makers in making informed choices on where and how to use InO for the treatment of R/R ALL.
References
Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–43. https://doi.org/10.1016/S0140-6736(08)60457-2.
Svenska Vuxen ALL-gruppen. Akut lymfatisk leukemi (ALL) Nationella riktlinjer; 2017. http://www.sfhem.se/Files.aspx?f_id=138838. Accessed 14 Oct 2020
Cancer Registry of Norway. Cancer in Norway 2016—cancer incidence, mortality, survival and prevalence in Norway. Oslo. 2017. https://www.kreftregisteret.no/globalassets/cancer-in-norway/2016/cin-2106.pdf. Accessed 14 Oct 2020
Statens legemiddelverk. Hurtig metodevurdering for legemidler finansiert i spesialisthelsetjeneste Inotuzumab ozogamicin til behandling av tilbakevendende eller behandlingsrefraktær akutt lymfoblastisk leukemi -som bro til allogen hematopoetisk stamcelletransplantasjon (HSCT): Vurdering av innsendt dokumentasjon. 2018. https://legemiddelverket.no/Documents/Offentlig%20finansiering%20og%20pris/Metodevurderinger/B/Besponsa_ALL_2018.pdf. Accessed 14 Oct 2020
Mathisen MS, Kantarjian H, Thomas D, O’Brien S, Jabbour E. Acute lymphoblastic leukemia in adults: encouraging developments on the way to higher cure rates. Leuk Lymphoma. 2013;54(12):2592–600. https://doi.org/10.3109/10428194.2013.789509.
Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av maligne blodsykdommer. 2016. https://helsedirektoratet.no/retningslinjer/nasjonalt-handlingsprogram-med-retningslinjer-for-diagnostikk-behandling-og-oppfolging-av-maligne-blodsykdommer. Accessed 14 Oct 2020
Cancer Research UK. Acute lymphoblastic leukaemia (ALL). https://www.cancerresearchuk.org/about-cancer/acute-lymphoblastic-leukaemia-all. Accessed 28 Feb 2021
Khaled SK, Thomas SH, Forman SJ. Allogeneic hematopoietic cell transplantation for acute lymphoblastic leukemia in adults. Curr Opin Oncol. 2012;24(2):182–90. https://doi.org/10.1097/CCO.0b013e32834f5c41.
Chaidos A, Kanfer E, Apperley JF. Risk assessment in haemotopoietic stem cell transplantation: disease and disease stage. Best Pract Res Clin Haematol. 2007;20(2):125–54. https://doi.org/10.1016/j.beha.2006.10.003.
Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. https://doi.org/10.1182/blood-2006-05-018192.
Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–41. https://doi.org/10.1182/blood-2011-12-399287.
Tavernier E, Boiron J-M, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907–14. https://doi.org/10.1038/sj.leu.2404824.
Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98(7):1337–54. https://doi.org/10.1002/cncr.11664.
European Medicines Agency. Besponsa—EMEA/H/C/004119—N/0002—Summary of product characteristics Besponsa. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004119/WC500231261.pdf. Accessed 23 Feb 2018
Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53. https://doi.org/10.1056/NEJMoa1509277.
Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–87. https://doi.org/10.1002/cncr.32116.
NT-rådet. Besponsa (inotuzumab ozogamicin) vid akut lymfatisk leukemi (ALL): NT-rådets yttrande till landstingen 2018-05-16. 2018. https://janusinfo.se/download/18.46d4de0e163638cbf6f105a8/1535626614334/Inotuzumab-ozogamicin-(Besponsa)-180516.pdf. Accessed 14 Oct 2020
Tandvårds- och läkemedelsförmånsverket. Underlag för beslut i landstingen Besponsa (Inotuzumab ozogamicin): Pulver till koncentrat till infusionsvätska, lösning. 2017. https://www.tlv.se/download/18.577a4290162f61625083cfde/1524645272062/bes180411__underlag_besponsa.pdf. Accessed 14 Oct 2020
National Institute for Health and Care Excellence. Technology appraisal guidance. Inotuzumab ozogamicin for treating relapsed or refractory B-cell acute lymphoblastic leukaemia [TA541]. 2018. https://www.nice.org.uk/guidance/ta541. Accessed 14 Jan 2019
Pfizer. Patient-level data of the INO-VATE ALL Phase III trial: data on file; 2017.
Tandvårds- och läkemedelsförmånsverket. Tandvårds- och läkemedelsförmånsverkets allmänna råd. 2017. https://www.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf. Accessed 5 Nov 2020
Statens legemiddelverk. Retningslinjer for dokumentasjonsgrunnlag for hurtig metodevurdering av legemidler: Gyldig fra 01.01.2018: Dokument nr. 17/08011-1. 2017. https://legemiddelverket.no/Documents/Andre%20temaer/H%C3%B8ringer/Retningslinjer%20metodevurderinger/H%C3%B8ringsutgave%20retningslinjer%20metodevurdering.pdf. Accessed 5 Nov 2020
Jackson CH. flexsurv: A platform for parametric survival modeling in R. J Stat Softw. 2016. https://doi.org/10.18637/jss.v070.i08.
Therneau T. Package ‘survival’: A Package for Survival Analysis in R: version 2.38. 2015. https://cran.r-project.org/web/packages/survival/survival.pdf. Accessed 10 Apr 2017
Latimer N. NICE DSU technical support document 14: survival analysis for cost-effectiveness analysis alongside clinical trials-extrapolation with patient-level data. 2017. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed 14 Oct 2020
Hettle RC, Corbett M, Hinde S, Hodgson R, Jones-Diette J, Woolacott N, Palmer S. Exploring the assessment and appraisal of regenerative medicines and cell therapy products. 2015. https://www.nice.org.uk/Media/Default/About/what-we-do/Science%20policy%20and%20research/final-york-report-march-16.pdf. Accessed 26 Oct 2020
Scottish Medicines Consortium. Blinatumomab, 38.5 micrograms powder for concentrate and solution for solution for infusion (Blincyto®). 2016. https://www.scottishmedicines.org.uk/medicines-advice/blinatumomab-blincyto-fullsubmission-114516/. Accessed 26 Oct 2020
All Wales Medicines Strategy Group. Final Appraisal Report: Clofarabine (Evoltra): Advice No: 0107—June 2007. 2007. https://awmsg.nhs.wales/medicines-appraisals-and-guidance/medicines-appraisals/clofarabine-evoltra/. Accessed 26 Oct 2020
All Wales Medicines Strategy Group. Final Appraisal Report Nelarabine (Atriance®T) for the treatment of T-cell acute lymphoblastic leukaemia and T-cell lymphoblastic lymphoma: Advice No: 0909—April 2009. 2009. https://awmsg.nhs.wales/medicines-appraisals-and-guidance/medicines-appraisals/nelarabine-atriance/. Accessed 26 Oct 2020
Costa V, McGregor M, Laneuville P, Brophy JM. The cost-effectiveness of stem cell transplantations from unrelated donors in adult patients with acute leukemia. Value Health. 2007;10(4):247–55. https://doi.org/10.1111/j.1524-4733.2007.00180.x.
Statistics Norway. Life tables, by sex and age 2016. 2017. https://www.ssb.no/en/statbank/table/07902. Accessed 5 Nov 2017
SCB. Swedish life table 2016. 2017. https://www.scb.se/. Accessed 16 Oct 2017
Martin PJ, Counts GW, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011–6. https://doi.org/10.1200/JCO.2009.25.6693.
National Institute for Health and Care Excellence. Single technology appraisal. Inotuzumab ozogamicin for treating relapsed or refractory B-cell acute lymphoblastic leukaemia [ID893] Committee papers 4. 2018. https://www.nice.org.uk/guidance/gid-ta10091/documents/committee-papers-4. Accessed 8 Aug 2018
Kurosawa S, Yamaguchi H, Yamaguchi T, et al. Decision analysis of postremission therapy in cytogenetically intermediate-risk acute myeloid leukemia: the impact of FLT3 internal tandem duplication, nucleophosmin, and CCAAT/enhancer binding protein alpha. Biol Blood Marrow Transplant. 2016;22(6):1125–32. https://doi.org/10.1016/j.bbmt.2016.03.015.
Kurosawa S, Yamaguchi T, Mori T, et al. Patient-reported quality of life after allogeneic hematopoietic cell transplantation or chemotherapy for acute leukemia. Bone Marrow Transplant. 2015;50(9):1241–9. https://doi.org/10.1038/bmt.2015.137.
Aristides M, Barlev A, Barber B, Gijsen M, Quinn C. Population preference values for health states in relapsed or refractory B-precursor acute lymphoblastic leukemia in the United Kingdom. Health Qual Life Outcomes. 2015;13:181. https://doi.org/10.1186/s12955-015-0377-3.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
xe.com. 31-12-2017 conversion rate SEK/EUR. 2017. https://www.xe.com/currencycharts/?from=SEK&to=EUR&view=5Y. Accessed 14 Oct 2020
xe.com. 31-12-2017 conversion rate NOK/EUR. 2017. https://www.xe.com/currencycharts/?from=NOK&to=EUR&view=5Y. Accessed 14 Oct 2020
SLV. Legemiddelsøk. 2017. https://www.legemiddelsok.no/. Accessed 15 Oct 2017
Swedish drug prices. 2017. www.apoteket.se. Accessed 15 Oct 2017
Regjeringen. 2014. https://www.regjeringen.no/no/tema/helse-og-omsorg/sykehus/innsikt/nokkeltall-og-fakta---ny/ofte-stilte-sporsmal/id534086/. Accessed 14 Oct 2020
Karolinska US. 2017. A47 Center for Allogen stemcell transplant (CAST), Price inpatient care, allgogen stemcell transplant adult transplant.
Kunnskapssenteret. Autolog hematopoietisk stamcelletransplantasjon ved multippel sklerose. 2015. https://www.fhi.no/globalassets/kss/filer/filer/publikasjoner/rapporter/20152/rapport_2015_23_ms_stamceller_v2.pdf. Accessed 15 Oct 2017
Region Skåne out of county price list; 2017. R20N. Allogen stamcellstransplantation.
NHS Blood and Transplant. Unrelated donor stem cell transplantation in the UK. 2014. https://studyres.com/doc/16711905/unrelated-donor-stem-cell-transplantation-in-the-uk. Accessed 8 Aug 2017
Swedish drug prices. 2017. www.medicinpriser.se. Accessed 15 Oct 2017
Swedish drug prices. 2017. www.tlv.se. Accessed 15 Oct 2017
European Medicines Agency. Iclusig—EMEA/H/C/002695—PSUSA/00010128/201612 EMA/MB/69923/2010—summary of product characteristics Iclusig. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002695/WC500145646.pdf. Accessed 3 Oct 2017
Region Skåne out of county price list; 2017. Chemotherapy treatment for acute leukemia as secondary diagnosis (R50N) two occations assumed
Region Skåne out of county price list; 2017, visit to hematologist (BLÄK01HÅ).
Socialstyrelsen. Huvuddiagnos: D709C. Bidiagnos: C910,C910A, C910B; 2017.
Innsatsstyrt finansiering. Poliklinisk konsultasjon vedrørende lymfom, leukemi, myelomatose og visse andre benmargssykdommer. DRG 917A. 2017. https://helsedirektoratet.no/Lists/Publikasjoner/Attachments/1265/Innsatsstyrt%20finansiering%202017%20IS-2568.pdf. Accessed 15 Oct 2017
Innsatsstyrt finansiering. Sepsis ved sykdommer i HDG18. DRG 416N. 2017. https://helsedirektoratet.no/Lists/Publikasjoner/Attachments/1265/Innsatsstyrt%20finansiering%202017%20IS-2568.pdf. Accessed 15 Oct 2017
Fass. Defibrotide and Ursodeoxycholic acid dosing October 2017. 2017. www.fass.se. Accessed 15 Oct 2017
Espérou H, Brunot A, Roudot-Thoraval F, et al. Predicting the costs of allogeneic sibling stem-cell transplantation: results from a prospective, multicentre. French study. Transplantation. 2004;77(12):1854–8. https://doi.org/10.1097/01.tp.0000129409.84087.62.
Innsatsstyrt finansiering. 2017. https://helsedirektoratet.no/Lists/Publikasjoner/Attachments/1265/Innsatsstyrt%20finansiering%202017%20IS-2568.pdf. Accessed 14 Oct 2020
Region Skåne out of county price list; 2017. Palliative care (ten care days are assumed).
Statistics Norway. Wage Index: hourly average wage in Norway 2016. 2017. https://www.ssb.no/en/lonnkvart/. Accessed 15 Nov 2017
Trafikverket. Samhällsekonomiska principer och kalkylvärden, ASEK 5. 2012. https://www.trafikverket.se/contentassets/4b21db8abbe14998a78b6edfe399a3cc/samhallsekonomiska_principer_och_kalkylvarden_for_transportsektorn_asek_5_kapitel_10_buller_2.pdf. Accessed 2 Oct 2017
Statistics Norway. Population statistics 2016. 2017. https://www.ssb.no/en/befolkning/statistikker/folkemengde/aar-per-1-januar. Accessed 16 Nov 2017
SCB. Salary in Sweden October 2017. 2017. www.scb.se. Accessed 12 Oct 2017
Ekonomifakta. Payroll tax Sweden October 2017. 2017. www.ekonomifakta.se. Accessed 12 Oct 2017
SCB. Employment rate Sweden 2017. 2017. www.scb.se. Accessed 12 Oct 2017
Zhang, K., Garau, M. International cost-effectiveness thresholds and modifiers for HTA decision making: OHE Consulting Report: London. 2020. https://www.ohe.org/publications/international-cost-effectiveness-thresholds-and-modifiers-hta-decision-making. Accessed 14 Oct 2020
Fass. FLAG-Asp and MEA dosing January 2017. 2017. www.fass.se. Accessed 16 Jan 2017
Pensionsskyddscentralen. Pensionsålder. 2017. https://www.etk.fi/sv/arbete-och-pension-utomlands/internationell-jamforelse/pensionsalder/. Accessed 12 Nov 2017
Innsatsstyrt finansiering. Allogen stamcelletransplantasjon >17 år. DRG 481Bl. 2017. https://helsedirektoratet.no/Lists/Publikasjoner/Attachments/1265/Innsatsstyrt%20finansiering%202017%20IS-2568.pdf. Accessed 15 Nov 2017
Lecat CSY, Besley C, Hough RE, et al. Inotuzumab ozogamicin versus FLAG-Ida in the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia—real-world resource use data. Leuk Lymphoma. 2020;61(2):491–3. https://doi.org/10.1080/10428194.2019.1672057.
Cox E, Wade R, Peron M, et al. The clinical and cost effectiveness of inotuzumab ozogamicin for the treatment of adult relapsed or refractory b-cell acute lymphoblastic leukaemia: an evidence review group evaluation of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(9):1081–91. https://doi.org/10.1007/s40273-019-00779-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was financially supported by Pfizer Inc.
Conflict of interest
IvO and BH were employees of Ingress-Health BV, who were paid consultants to Pfizer in connection with the development of this article. At the time of the study, TARS, JTO, and MJ were employees of Pfizer Inc.
Author contributions
All authors were involved in the conceptualisation and the design of the study and interpretation of the data. IvO and TARS analyzed the data and drafted the manuscript. All authors critically reviewed the article and made substantial contributions to the finalisation of the manuscript and have given their final approval of the version to be submitted.
Data availability statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files, and within the referenced studies.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have provided their consent for publication.
Code availability
An overview of the model structure has been published in this article to allow replication; however, the coded model itself has not been published. Further details are available via the corresponding author upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van Oostrum, I., Russell-Smith, T.A., Jakobsson, M. et al. Cost-Effectiveness of Inotuzumab Ozogamicin Compared to Standard of Care Chemotherapy for Treating Relapsed or Refractory Acute Lymphoblastic Leukaemia Patients in Norway and Sweden. PharmacoEconomics Open 6, 47–62 (2022). https://doi.org/10.1007/s41669-021-00287-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00287-2