Abstract
Background
In several countries, the dolutegravir (DTG)-based regimen is generally preferred as first-line antiretroviral therapy (ART) over the efavirenz (EFV)-based regimen, but the evidence in low-income countries is limited.
Objective
Our study aimed to evaluate the cost effectiveness of DTG- versus EFV-based first-line human immunodeficiency virus (HIV) treatment in Ethiopia.
Methods
We developed a microsimulation model for the progression of HIV/acquired immune deficiency syndrome (AIDS) to examine the cost effectiveness of DTG-based first-line ART compared with an EFV-based regimen from a healthcare payer perspective. We used a lifetime horizon with a 1-month cycle length and a 3% annual discount rate. The primary outcomes were a lifetime cost in US dollars ($), quality-adjusted life-months (QALMs) that converted to QALYs using the formula QALY = QALM/12, and incremental cost-effectiveness ratio (ICER). Deterministic sensitivity analysis was conducted to account for parameter uncertainty.
Results
Compared with the EFV-based regimen, the DTG-based regimen was associated with an expected lifetime cost of $12,709 (vs. $12,701) and expected QALYs of 15.3 (vs. 14.7 QALYs) per patient, resulting in an ICER value of $13.33 per QALY. From an alternative analysis with a 5-year time horizon, DTG-based ART was found to be dominant, with expected gains of 0.17 QALYs at a lower cost of $1 per patient. The deterministic sensitivity analysis depicted that the maximum increase in ICER value was $72 per QALY, and all ICER values were below the estimated threshold value.
Conclusions
The DTG-based first-line regimen appears to be cost effective compared with the EFV-based regimen for the treatment of HIV/AIDS patients in an Ethiopian setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This economic analysis has shown that a dolutegravir (DTG)-based regimen appears to be a cost-effective first-line treatment option for HIV/AIDS patients in Ethiopia. |
The findings support the decision made by the Ministry of Health in Ethiopia to use a DTG-based regimen. |
This study included only antiretroviral therapy and laboratory costs and did not consider the impact of an adverse event associated with treatment regimens, suggesting further studies to strengthen the evidence. |
1 Introduction
Human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) is a chronic condition characterised by reduced immune function. Progression of the disease is characterised by a low cluster of differentiation 4 (CD4) count and high viral load [1, 2]. HIV/AIDS disproportionately affects the poorer segments of society, especially those in the already struggling economies of most African countries [3]. The disease is estimated to reduce the economic growth rate of African countries by up to 4% and this burden is expected to increase with an upsurge in disease prevalence [3, 4]. At the household level, HIV/AIDS poses a higher financial burden compared with other causes of illness, an effect that is even more daunting among poor households. HIV/AIDS also takes the largest share of the national health expenditure in poor countries such as Ethiopia [5].
To reduce the burden of the disease, the introduction of new and effective treatments has to be considered and treatment guidelines should be updated to accommodate advancements in the field of drug discovery. Until recently, combinations of two nucleoside reverse transcriptase inhibitors (NRTIs) as the backbone with non-NRTIs (NNRTIs) or boosted protease inhibitors have been the preferred regimens for the management of HIV/AIDS. Efavirenz (EFV) is a popular NNRTI used in combination therapy; however, all existing combination therapies have been challenged by resistance, adverse drug events (ADEs) and drug interactions [1, 6,7,8]. As a result, second-generation integrase strand transfer inhibitors (INSTIs) such as dolutegravir (DTG)-based regimens are becoming the preferred first-line treatment instead of regimens based on NNRTIs such as EFV [9]. The updated WHO 2019 guidelines recommend using a DTG-based regimen (50 mg) as the preferred first-line antiretroviral treatment and an EFV-based regimen (400 mg) as an alternative [10]. This is also the case for Ethiopia, as the national guideline indicated that “the preferred first-line regimen for adults and adolescents is tenofovir disoproxil fumarate (TDF) + lamivudine (3TC) + DTG or TDF + 3TC + EFV as a once-daily dose”. These guidelines also state that “TDF + 3TC + EFV400 (FDC) will replace the TDF + 3TC + EFV600 (FDC) for adults and adolescents (except for pregnant mothers, women of child bearing age and TB/HIV co-infected patients as there is no adequate data for this groups) up on availability” [6].
Although the DTG-based regimen is better in terms of treatment efficacy and rate of treatment failure, its current price is greater in comparison with the EFV-based regimen. Studies from developed countries such as Canada [11] and Russia [12] demonstrated the cost effectiveness of the DTG-based regimen. Consistent with this modelling, studies in the context of sub-Saharan countries revealed that using a DTG-based regimen as the preferred first-line treatment ensured efficient use of resources [13,14,15].
In Ethiopia, changes are being made to the treatment guidelines based on the evidence regarding treatment efficacy [6]. Such changes call for economic evaluations to investigate the cost effectiveness of the newly introduced regimen and to examine whether the added cost of therapy can be justified by the added benefit. However, the new regimen is being implemented without a proper economic evaluation of its costs and outcomes in the Ethiopian context. This study therefore aimed to evaluate the cost effectiveness of replacing an EFV-based regimen with a DTG-based regimen as a preferred first-line treatment for HIV/AIDS patients in the Ethiopian context.
2 Methods
A cost-utility analysis was conducted to compare the DTG-based regimen with the EFV-based regimen as first-line antiretroviral therapy (ART) from a health care payer perspective in the Ethiopian setting. We targeted adult populations (≥ 18 years) because they comprise more than 90% of the people who are living with HIV/AIDS in Ethiopia [16]. Costs were measured in US dollars ($), and effectiveness was measured in quality-adjusted life-months (QALMs) but converted to QALYs using the formula QALY = QALM/12. The incremental cost-effectiveness ratio (ICER) was calculated to compare the two strategies. Since Ethiopia has no discount rate recommendations, we used the WHO recommendations, and therefore both costs and outcomes were discounted at 3% annually. As Ethiopia lacks an established cost-effectiveness threshold value, we used the World Health Organization (WHO) recommendation, where an intervention with an ICER less than 1–3 × gross domestic product (GDP) per capita per QALY is considered a cost-effective strategy [17]. Ethiopia’s GDP per capita in 2019 was $951.1 [18].
2.1 Model Structure and Assumptions
We developed a microsimulation model to simulate the progression of the disease on HIV-infected adult patients over a lifetime horizon using Tree Age Pro 2019 [19]. Face validity with a clinician working at Tikur Anbessa Specialized Hospital (TASH) was conducted. Treatment algorithms followed in the model were crosschecked with Ethiopian HIV/AIDS treatment guidelines [6]. Details of the model structure are described in the following section.
2.1.1 Health States
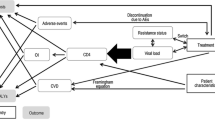
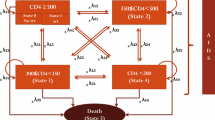
The WHO immunological classification of HIV/AIDS was used, with some modifications [20]. Accordingly, the model considers the disease in five health states based on the CD4 count range. As part of the modification, a health state with a CD4 count lower than 100 cells/mm3 was added to account for people with a lower CD4 count. As has been depicted in previous studies, the health state with a lower CD4 count is associated with a lower utility value [11]. Hence, inclusion of health states with a lower CD4 range has meaningful implications in the economic analysis of HIV/AIDS interventions. Starting health states for individuals were determined randomly by assuming a gamma distribution of CD4. We conducted a utility study to determine the utility value of different HIV/AIDS health states and enrolled 511 HIV/AIDS patients who were attending the outpatient clinic of TASH. The participants’ mean CD4 value and standard deviation (SD) were calculated to be 390.2 cells/mm3 and 232.6 cells/mm3, respectively [21]. A patient entering the model was defined according to their CD4 level, and tracked based on the treatment outcomes. Health state transition within a cycle length of 1 month was determined by treatment response and change in CD4 count. An increase in CD4 count for treatment success and a reduction in CD4 count for not responding to a regimen were expected. Movement to an adjacent state (improved or progressed health state) or staying in the same state was assumed within one cycle [22,23,24,25]. The markov state representing HIV progression based on CD4 counts is shown in Fig. 1. The model assumed a maximum CD4 limit of 1000 cells/mm3 for the responding regimen, which was reported as a maximum CD4 value in healthy Ethiopian individuals [26].
2.1.2 Treatment Algorithm
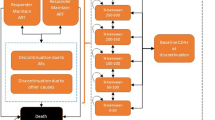
Five treatment regimens were considered based on the Ethiopian HIV treatment guidelines [7]. Patients in the DTG-based regimen arm were expected to start a fixed-dose combination therapy with two NRTIs (TDF/3TC) and DTG as the preferred first-line regimen followed by an alternative fixed-dose combination containing TDF, 3TC and EFV. Patients in the EFV-based regimen arm were expected to start with TDF/3TC/EFV as the preferred first-line followed by an alternative TDF/3TC/DTG regimen. Change in treatment regimen was determined based on treatment response and ADE. The treatment response is based on its impact on viral load. The patient responds to the treatment with probability p (hence, 1-p is the probability of non-response). Conditional on this outcome, CD4 count changes according to the parameters described in Table 1. As a result, the patient may stay in the same state or move to another state. Patients were expected to move to the second-line if they faced treatment failure. For both arms, preferred and alternative second-line regimens were zidovudine (AZT)/3TC/atazanavir (ATV)/ritonavir (r) and AZT/3TC/lopinavir (LPV)/r, respectively. The darunavir (DRV)/r-based regimen DRV/r + TDF/3TC/DTG was considered as the third-line treatment. Discontinuation related to ADEs of the preferred regimen was assumed to result in the use of the alternative regimen. For discontinuation of an alternative regimen, the model assumed the change in the treatment line as being similar to treatment failure. All patients in this model were expected to start with first-line treatment, i.e. either DTG- or EFV-based regimens. The treatment algorithm used in the model is described in Fig. 2.
Prescription algorithm for HIV/AIDS patients in Ethiopia. For the DTG-based arm: preferred first-line, TDF/3TC/DTG; alternative first line, TDF/3TC/EFV. For the EFV-based arm: preferred first-line, TDF/3TC/EFV; alternative first line, TDF/3TC/DTG. For both arms: preferred second-line, AZT/3TC/ATV/r; alternative second-line, AZT/3TC/LPV/r. The DRV/r-based regimen was considered as the third-line treatment. Rx prescription, DTG dolutegravir, TDF tenofovir disoproxil fumarate, 3TC lamivudine, EFV efavirenz, AZT zidovudine, ATV/r atazanavir/ritonavir, LPV/r lopinavir/ritonavir, DRV/r darunavir/ritonavir
2.2 Model Input
The parameter inputs for the modelling were abstracted from primary and secondary data sources (Table 1) and all parameters were estimated on a monthly basis.
2.2.1 Treatment Outcomes
The probabilities of treatment response were estimated based on a published systematic review [23]. Measuring viral load is the commonly used criterion for considering treatment response for ART, and a viral suppression below 50 copies/mL was considered a treatment success [29]. The annual probability of treatment response was estimated as 0.84 and 0.75 for DTG- and EFV-based regimens, respectively [23]. A probability of treatment failure was estimated based on treatment success (1-probability of treatment success), which is the probability of having a viral load above 50 copies/mL. A published meta-analysis reported that the odds of DTG-emergent ADEs in comparison with EFV was 0.84, and the odds of discontinuation due to ADEs in the DTG-based regimen compared with the EFV-based regimen was estimated to be 0.26 [24]. These values were converted into probabilities using built-in Tree Age functions [19].
In addition to probabilities, the microsimulation model included a change in CD4 count, which determines the change in the health state of the patient. The change in CD4 count could be an increase or decrease in CD4 count for treatment response or treatment failure, respectively. The change in CD4 values was mainly taken from a systematic review, which estimated an increase of 226.8 cells/mm3 and 179.1 cells/mm3 in CD4 count from baseline after 48 weeks of treatment initiation for DTG- and EFV-based regimens, respectively [23]. This figure was transformed into monthly CD4 change. The combination of probabilities (probabilities of treatment failure/success and adverse events) and change in CD4 count (increase/decrease) were included in the model in order to be able to accommodate predictors of the disease progression.
The starting CD4 count of an individual in the model was determined randomly from the established gamma distribution, and an equation was developed to decide the maximum limit of the CD4 count. A rapid increase in CD4 count was expected at the initial phase of treatment, followed by gradual changes. Three phases of changes in CD4 count were assumed for the responding regimen [12]. Using a time tracker, we counted the number of cycles that the patient stayed in a given state after treatment response or failure. An equation was built to accommodate an event including an increase or decrease in CD4 count. For treatment failure, monthly decline in CD4 count without ART intervention was considered [28].
2.2.2 Utility Data
The health states of HIV/AIDS patients based on CD4 range were commonly used to determine the utility score and health-related utilities expected to reduce with a decline in CD4 count [11, 12, 30]. This study used the utility value obtained from patients who had a follow-up in the ART clinic at a tertiary teaching hospital in Ethiopia using the EQ-5D-5L questionnaire [21]. The utility scores were calculated based on the preference of the general population of Ethiopia [31] and the reported median utility values were used as the base-case utility inputs. The median utility value from the EQ-5D index, with confidence intervals, were reported as 1 (0.94–1), 0.94 (0.88–1), 0.94 (0.86–0.97), 0.88 (0.76–0.95) and 0.88 (0.66–0.96) for patients with a CD4 range of > 500 cells/mm3, 350–500 cells/mm3, 200–350 cells/mm3, 100–200 cells/ mm3 and < 100 cells/mm3, respectively.
2.2.3 Cost Data
The cost data of different ART regimens were obtained from the Ethiopian Pharmaceutical Supply Agency (EPSA) and TASH. The EPSA is an agency established for supplying pharmaceuticals for all public health facilities in Ethiopia. The programme medicines, including ART, antimalarial and antitubercular medicines, are exclusively supplied by this agency. The costs of the DTG- and EFV-based regimens per patient per month were calculated to be $6.87 and $6.24, respectively. Furthermore, the costs of routine laboratory investigation for patients were obtained from TASH and were expected to be similar at any health facility within the country. Expert interviews were used to estimate the average cost for laboratory investigations, and invoices for payment and Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) documents were referred from the EPSA and TASH. The May 2019 price estimate was used. The cost data were gathered in Ethiopian Birr (ETB) and converted to US dollars [US$1 = 29.03ETB] using the May 2019 conversion rate [32].
2.3 Analysis
2.3.1 Base-Case Analysis
For the microsimulation analysis, sequencing the analysis results of different sample sizes was performed to determine the sample size that gives the most stable ICER value. We ran 100,000 simulations to determine the outcome parameters. Base-case analysis with a lifetime horizon was conducted to estimate the mean cost, QALY and ICER values per individual for the proposed regimens.
2.3.2 Alternative Analysis
Policymakers may sometimes be interested in the short-term outcome of the interventions [33], therefore we performed analysis for alternative scenarios by changing the time horizon to 5 years, while keeping other input parameters the same.
2.3.3 Sensitivity Analysis
Deterministic sensitivity analysis was performed using the range of values presented in Table 1, and plausible intervals were obtained from the relevant literature [23, 24]. Variations in price and treatment outcomes of the two regimens were considered as critical parameters. Furthermore, variations in the patients’ utility values were considered as sensitive variables that could influence the ICER.
2.4 Model Debugging
Tree Age Pro provides debugging options to display the internal calculations performed as a model is analysed, and we reviewed the calculation details in the Calculation Trace Console [19].
3 Results
3.1 Base-Case Analysis
The base-case analysis showed that the DTG-based regimen as first-line ART was associated with a lifetime cost of $12,709 and expected QALYs of 15.3, while the EFV-based regimen incurred a lifetime cost of $12,701 and expected QALYs of 14.7 per patient. Compared with patients receiving the EFV-based regimen, patients who were started on the DTG-based regimen gained 0.6 extra QALYs for an extra cost of $8, resulting in an ICER of $13.33 per QALYs gained per patient. Undiscounted analysis revealed that the DTG-based regimen was dominant compared with the EFV-based regimen (Table 2).
3.2 Alternative Analysis
From the alternative analysis, the DTG-based regimen was found to be dominant, with expected gains of 0.17 QALYs at a lower cost (5-year cost savings of $1 per patient). The undiscounted analysis also depicted that the DTG-based regimen was associated with more QALYs and lesser cost than the EFV-based regimen (Table 2).
3.3 Sensitivity Analysis
Deterministic sensitivity analysis showed that the probability of ADEs, treatment response or price of both regimens (DTG- and EFV-based), and utility value of different health states were found to be influential parameters. The maximum change in ICER was $6/QALM (equivalent to $72/QALY) for the highest value of the probability of ADEs of DTG (range of probability of ADEs of DTG: 0.03–0.07); however, all ICER values were below the threshold values and did not change the conclusion (Fig. 3).
4 Discussion
The present investigation is the first of its kind to perform a full economic analysis of alternative first-line treatment regimens for HIV/AIDS patients in the context of Ethiopia. Despite the absence of national guidelines for the conduct of pharmacoeconomic studies, the study established that compared with the EFV-based regimen, the DTG-based regimen was cost effective as a first-line treatment, from the health payer perspective. The results were consistent with earlier studies in Canada and Spain that reported better clinical outcomes with lower costs for the DTG-based regimen [11, 34]. The findings from this study are also consistent with a modelling analysis from sub-Saharan Africa that revealed the DTG-based regimen was a cost effective first-line treatment option in low-income settings [13,14,15]. According to the current analysis, it is possible to support the decision to include the DTG-based regimen as a preferred first-line treatment option in the Ethiopian treatment guidelines [6]. Apart from justifying the study from an economic perspective, the study was an important step in encouraging similar economic evaluations in the Ethiopian healthcare system. This is essential because consideration of economic evaluations for the introduction of new public health or clinical interventions has a vital role to manage the risk in the future and to utilise available domestic resources efficiently [17, 33].
At a 3% discount rate, it was found that the DTG-based regimen was associated with an incremental cost of $13.33 with each QALY gain. With the GDP per capita of the Ethiopian population being $951.1 in 2019, the ICER value was far below the recommended threshold values recommended by WHO (up to 3 × GDP per capita) and Woods et al. (1–51% of GDP per capita) [17, 35]. Therefore, replacing the EFV-based regimen with the DTG-based regimen appears to be a cost-effective investment in the health care system of Ethiopia. The ICER value depicted that the DTG-based regimen incurs a cost of $13.33 for each QALY gained. Findings from Spain depicted that for every QALY gained, DTG saves around €250,000 [34]. This discrepancy could be explained by the expensive nature of ART medicine in high-income countries in comparison with Africa [36]. The low cost of medicine in low-income countries is the result of global support and the use of generic products. However, there is a continuous decline in support for HIV/AIDS programmes from high- to low-income countries, therefore low-income countries should be prepared to handle the programmes using domestic resources [37]. Similar to other low-income countries, Ethiopia has received huge support from global donors for HIV/AIDS care (more than 80% of the total fund for the programme); however, there has been a decline in the external fund, which has resulted in a financial gap in the running of the programme. Ethiopia needs to increase domestic funds to offset the decline in financial support from external funders [38, 39].
The DTG-based regimen is cost-incurring in the base case but it produces better health. From the analysis, it has been depicted that the DTG-based regimen is associated with a total of 15.3 QALYs, while the EFV-based regimen is expected to yield 14.7 QALYs for an adult patient with an average age of 42 years and a life expectancy of 66 years [40]. An incremental QALY of 0.6 was observed in the DTG-based regimen, and the findings from this modelling analysis are consistent with a previous study that reported 6 months more life expectancy from the DTG-based regimen than the EFV-based regimen [11]. Ethiopia has a population of around 110 million and the adult prevalence rate of HIV is estimated to be 1% [40]. Extrapolation of the extra QALY gain of DTG at the country level has profound implications.
Policymakers may sometimes be interested in short-term outcomes [33], therefore this analysis tried to explore an alternative scenario with an analytic horizon of 5 years. Accordingly, the DTG-based regimen was found to be the dominant strategy in both discounted and undiscounted analysis and was associated with an extra gain of 0.17 QALYs and savings of $1 over a 5-year time horizon. Although the DTG-based regimen is cost-incurring in the base case, the ICER value associated with this regimen is below the threshold value of the society; therefore, introducing the DTG-based regimen appears to have humanistic and economic benefits in the Ethiopian context. Furthermore, sensitivity analysis has been conducted to accommodate parameter uncertainity. The deterministic sensitivity analysis has shown that the finding that the DTG-based regimen is a cost-effective strategy over the EFV-based regimen as a first-line treatment is robust.
4.1 Limitations
Treatment outcome data were taken from the systematic review and meta-analysis of randomised clinical trials, which may present variation in real-life settings. With the exception of treatment discontinuation due to AEs, we did not consider the impact of AEs on the patient, including the impact of weight gain associated with DTG. The treatment algorithm was mainly established based on the treatment guideline and did not accommodate variations in real practice. Costs other than ART and laboratory costs were not included, such as outpatient and hospital visits, and the impact that this might have had on the findings. However, this study used a microsimulation model for economic analysis that enabled consideration of patient heterogeneity. Economic evaluations of pharmaceuticals prior to making a decision is an unusual practice in Ethiopia, and therefore this study encourages the application of pharmacoeconomic evaluation in decision making.
5 Conclusion
Our study showed that the DTG-based first-line regimen appears to be a cost-effective strategy to treat adult HIV patients in Ethiopia. Although this treatment regimen has already been included in the Ethiopian treatment guidelines as the preferred first-line treatment, we generate evidence that supports the recommendations in the guidelines and encourages scaling-up of DTG-based first-line ART throughout the Ethiopian health care system.
References
Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2(4):a007161.
Vlahov D, Graham N, Hoover D, Flynn C, Bartlett JG, Margolick JB, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. JAMA. 1998;279(1):35–40.
Essig A, Kang S, Sellers R. The relationship between HIV infection rates and GDP Per Capita in African Countries. Atlanta: Georgia Institute of Technology; 2015.
Dixon S, McDonald S, Roberts J. The impact of HIV and AIDS on Africa’s economic development. BMJ. 2002;324(7331):232–4.
Tekola F, Reniers G, Haile Mariam D, Araya T, Davey G. The economic impact of HIV/AIDS morbidity and mortality on households in Addis Ababa, Ethiopia. AIDS Care. 2008;20(8):995–1001.
Federal Ministry of Health (FMoH). National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. 2018. https://www.afro.who.int/sites/default/files/201904/National%20Comprehensive%20HIV%20Care%20%20Guideline%202018.pdf. Accessed 11 Jan 2019.
World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 11 Jan 2019.
Katlama C, Murphy R. Dolutegravir for the treatment of HIV. Expert Opin Investig Drugs. 2012;21(4):523–30. https://doi.org/10.1517/13543784.2012.661713.
World Health Organization (WHO). Policy brief update on antiretroviral regimens for treating and preventing HIV infection and update on early infant diagnosis of HIV. Interim guidance (No WHO/CDS/HIV/1819). Geneva: World Health Organization; July 2018.
World Health Organization. Update of recommendations on first-and second-line antiretroviral regimens. Report No.: WHO/CDS/HIV/19.15. Geneva: World Health Organization; 2019.
Despiégel N, Anger D, Martin M, Monga N, Cui Q, Rocchi A, et al. Cost-effectiveness of dolutegravir in HIV-1 treatment-naive and treatment-experienced patients in Canada. Infect Dis Ther. 2015;4(3):337–53.
Tremblay G, Chounta V, Piercy J, Holbrook T, Garib SA, Bukin EK, et al. Cost effectiveness of dolutegravir as a first-line treatment option in the HIV-1–infected treatment-naive patients in Russia. ViHRI. 2018;16:74–80.
Phillips AN, Cambiano V, Nakagawa F, Revill P, Jordan MR, Hallett TB, Doherty M, De Luca A, Lundgren JD, Mhangara M, Apollo T. Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. Lancet HIV. 2018;5(3):e146–54.
Phillips AN, Venter F, Havlir D, Pozniak A, Kuritzkes D, Wensing A, Lundgren JD, De Luca A, Pillay D, Mellors J, Cambiano V. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6(2):e116–27.
Nishimwe ML, Tovar-Sanchez T, Wandji ML, Mpoudi-Etame M, Maradan G, Bassega PO, Varloteaux M, Montoyo A, Kouanfack C, Delaporte E, Boyer S. Cost-utility analysis of a dolutegravir-based versus low-dose efavirenz-based regimen for the initial treatment of HIV-infected patients in Cameroon (NAMSAL ANRS 12313 trial). Pharmacoeconomics. 2021;39(3):331–42.
Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90–90-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):320.
World Health Organization (WHO). WHO Guide to cost-effectiveness analysis? 2003. https://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed 11 Jan 2019.
International Monetary Fund (IMF). World Economic Outlook (October 2019)—GDP per capita, current prices. https://www.imf.org/external/datamapper/NGDPDPC@WEO. Accessed 11 Jan 2019.
Tree Age Pro 2019, R1—Tree Age Software. TreeAge Software. 2020. http://www.treeage.com/software-download/treeage-pro-2019-r1/. Accessed 16 May 2020.
World Health Organization (WHO). WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization; 2007.
Belay YB, Ali EE, Sander B, Gebretekle GB. Health-related quality of life of patients with HIV/AIDS at a tertiary care teaching hospital in Ethiopia. Health Qual Life Outcomes. 2021;19(1):1–1.
Kryst J, Kawalec P, Pilc A. Efavirenz-based regimens in antiretroviral-naive HIV-infected patients: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2015;10(5):e0124279.
Patel DA, Snedecor SJ, Tang WY, Sudharshan L, Lim JW, Cuffe R, et al. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1–infected patients: a systematic review and network meta-analysis. PLoS ONE. 2014;9(9):e105653.
Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV. 2016;3(11):e510–20.
Stellbrink H-J, Reynes J, Lazzarin A, Voronin E, Pulido F, Felizarta F, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013;27(11):1771.
Tsegaye A, Messele T, Tilahun T, Hailu E, Sahlu T, Doorly R, et al. Immunohematological reference ranges for adult Ethiopians. Clin Diagn Lab Immunol. 1999;6(3):410–4.
Indexmundi. Ethiopia Demographics Profile. https://www.indexmundi.com/ethiopia/demographics_profile.html. Accessed 11 Jan 2019.
Patrikar S, Basannar DR, Bhatti VK, Kotwal A, Gupta RM, Grewal RS. Rate of decline in CD4 count in HIV patients not on antiretroviral therapy. Med J Armed Forces India. 2014;70(2):134–8.
Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19(1):169.
Kauf TL, Roskell N, Shearer A, Gazzard B, Mauskopf J, Davis EA, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health. 2008;11(7):1144–53.
Welie A, Gebretkle G, Stolk E, Mukuria C, Krahn M, Enquoselassi F, Fenta T. Valuing Health State: An EQ-5D-5L Value Set for Ethiopians. Value in Health. 2020;22:7–14.
National Bank of Ethiopia (NBE). Commercial Banks Exchange Rate. https://nbebank.com/commercial-banks-exchange-rate/. Accessed 11 Nov 2019.
Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. In: Guidelines for the economic evaluation of health technologies: Canada, 3rd ed. Ottawa: CADTH; 2006.
Guillén SM, García JE, Berenguer JB, Sesmero JM, Gomis SC, Graefehain R, Sánchez-Cambronero DL, García FJ. Cost-utility analysis of the fixed-dose combination of dolutegravir/abacavir/lamivudine as initial treatment of HIV+ patients in Spain. Farm Hosp. 2017;41(5):601–10.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35.
Rosenberg. H.I.V. Drugs Cost $75 in Africa, $39,000 in the U.S. Does It Matter? https://www.nytimes.com/2018/09/18/opinion/pricing-hiv-drugs-america.html. Accessed 11 Nov 2019.
Avert. Funding for HIV and AIDS. https://www.avert.org/professionals/hiv-around-world/global-response/funding. Accessed 20 Sep 2019.
President’s Emergency Plan for AIDS Relief (PEPFAR). Ethiopia Country Operational Plan 2018. https://www.pepfar.gov/documents/organization/285863.pdf. Accessed 11 Nov 2019.
Kassahun WM. HIV Prevalence and Donor Funding in Ethiopia 2019. Walden Dissertations and Doctoral Studies. 6446. https://scholarworks.waldenu.edu/dissertations/6446. Accessed 11 Nov 2019.
Centers for Disease Control and Prevention (CDC). Ethiopia Country Profile. https://www.cdc.gov/globalhivtb/where-we-work/ethiopia/ethiopia.html. Accessed 20 Sep 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by Addis Ababa University and, in part, by a Canada Research Chair in Economics of Infectious Diseases held by Beate Sander (CRC-950-232429).
Conflict of interest/Competing interest
The project design, data collection, and analysis and interpretation of the results were conducted without any interference from funding organisations. Yared Belete Belay, Eskinder Eshetu Ali, Karen Y. Chung, Gebremedhin Beedemariam Gebretekle and Beate Sander declare they have no competing interests.
Ethics approval
Ethical clearance was obtained from the Ethical Review Board of the School of Pharmacy, Addis Ababa University (ERB/SOP/53/03/2019). For gathering cost data, we received permission from the Pharmacy Directorate of TASH and the EPSA.
Consent to participate
Prior to data collection, participants were informed of the purpose and procedure of the study by verbal means and informed consent was requested.
Consent for publication
Not applicable.
Availability of data and material
The Tree Age Pro model is available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Author contributions
YBB, BS and GBG conceived the project. YBB collected the data, and GBG and BS were involved in the verification of the collected data. YBB, EEA, KYC, GBG, and BS developed the model and performed the analysis. YBB prepared the manuscript. All authors provided critical feedback on the analysis and reviewed and approved the submitted manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Belay, Y.B., Ali, E.E., Chung, K.Y. et al. Cost-Utility Analysis of Dolutegravir- Versus Efavirenz-Based Regimens as a First-Line Treatment in Adult HIV/AIDS Patients in Ethiopia. PharmacoEconomics Open 5, 655–664 (2021). https://doi.org/10.1007/s41669-021-00275-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00275-6