Abstract
Background
Mantle cell lymphoma (MCL) is a rare and aggressive subtype of non-Hodgkin’s lymphoma. While treatment of patients with MCL and their outcomes are previously published, the availability of heath economics evidence is unclear.
Objective
The aim of this paper was to conduct a comprehensive review of studies relating to economic evaluations, costs and resource use, and health-related quality of life (HRQoL) in patients with MCL.
Methods
Search strategies were designed to capture studies reporting economic or HRQoL outcomes published in the previous 11 years (2007–2018). The following electronic databases were searched: MEDLINE, Embase, NHS Economic Evaluation Database (NHS EED), and EconLit. In addition, we reviewed congress abstracts presented over the previous 2 years (2015 and 2016; where 2017 proceedings had occurred, these were searched instead of 2015). Publications were screened in duplicate by two reviewers and supplementary searches were carried out on health technology assessment websites. Searches were first conducted in October 2017 and updated in March 2018.
Findings
The systematic literature review identified 11 economic evaluations (in 16 publications), seven studies reporting data relating to costs or resource use, and five relating to HRQoL. Four economic evaluations presented results for patients with MCL modelled in the first-line setting, while seven modelled patients in the relapsed/refractory setting. The majority of economic evaluations were conducted using a Markov model with three to five health states. Seven studies assessed resource use and reported adverse events as key drivers of increased costs and resource use. Across the five studies reporting HRQoL, disparate measures were used. Two studies reported improvement in Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) total scores following treatment and found that clinical response to treatment was associated with improvement in overall HRQoL.
Conclusions and Relevance
The published economic and HRQoL evidence in MCL, although scarce, reveals that the economic and HRQoL burden associated with MCL is substantial. In highlighting this evidence, this analysis underlines a critical unmet need for more effective treatments with improved outcomes in MCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This systematic literature review highlights the paucity of peer-reviewed cost-effectiveness assessments in mantle cell lymphoma, particularly given further differentiation between first-line and relapsed/refractory analyses. |
Where health resource utilization data have been available, they suggest high-dose chemotherapy is more resource intensive than chemo-immunotherapy. |
Despite the paucity of evidence, the available evidence highlights a critical unmet need for new agents that improve outcomes in this patient population. |
1 Introduction
Mantle cell lymphoma (MCL) is an aggressive malignancy that accounts for approximately 5–7% of incident non-Hodgkin’s lymphoma cases in Western Europe and the United States (US) [1, 2], and has an annual incidence of 1–2 cases per 100,000 people. It occurs mainly in individuals aged over 60 years, is three times more common in men than in women [1, 3], and is associated with poor prognosis [4, 5].
For younger and fitter patients, aggressive chemo-immunotherapy containing rituximab followed by autologous stem cell transplantation (ASCT) is the treatment of choice [1, 6]. However, a large proportion of patients are not eligible for intensive treatment and are commonly treated with conventional chemo-immunotherapy followed by rituximab maintenance [1, 4, 6]. In the relapsed/refractory (R/R) setting, patients are treated with chemo-immunotherapy or targeted agents (ibrutinib, acalabrutinib, lenalidomide, temsirolimus, bortezomib or venetoclax) [1, 6].
It is important to understand not only the clinical burden of MCL [5] but also the economic burden and health-related quality of life (HRQoL) impact, which have been scarcely reported in published literature. To our knowledge, no recent review of the evidence relating to the economic or HRQoL burden in patients with MCL has been published. The objective of this systematic literature review (SLR) was to evaluate the evidence relating to economic evaluations, costs and resource use, and HRQoL in patients with MCL.
2 Methods
Three separate SLRs were undertaken to identify economic evaluations, costs and resource use, and HRQoL. Methodology for the SLRs was consistent with recommendations published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, the Centre for Reviews and Dissemination, and the Cochrane Collaboration [7, 8].
2.1 Identification of Eligible Publications
For each review, the MEDLINE, Embase, NHS Economic Evaluation Database (NHS EED), and EconLit databases were searched using published, validated search filters and were limited to publication dates of 2007–2018. Search strategies are presented in the Electronic Supplementary Material (ESM). Supplementary searches included searches for congress abstracts and health technology assessment (HTA) submissions. Abstract searches were limited to those published in the 2015 and 2016 (however, where the 2017 proceedings had occurred, these were searched instead of 2015) key congresses: ASCO (American Society of Clinical Oncology), ASH (American Society of Hematology), ISPOR (International and European meetings), AMCP (Academy of Managed Care Pharmacy), EHA (European Hematology Association), and ESMO (European Society for Medical Oncology). The Tufts Cost Effectiveness Analysis Registry was searched for economic evaluations submitted as part of HTA submissions. Publicly available information from National Institute for Health and Care Excellence (NICE), Scottish Medicines Consortium (SMC), Canadian Agency for Drugs and Technologies in Health (CADTH), and Pharmaceutical Benefit Advisory Committee (PBAC) was also searched. All searches were conducted in October 2017 and updated in March 2018.
Titles and abstracts were reviewed by two systematic reviewers (PO’D and KW). Abstracts were included if they studied a patient population of adults (≥ 18 years) with MCL treated with pharmacological interventions (or no treatment for costs and resource use or HRQoL studies). For the economic review, only cost-utility, cost-effectiveness, cost–benefit, cost-minimization analyses or budget impact models were included. For the costs and resource use review, studies reporting data for direct medical or non-medical costs, indirect costs, or resource use were included. For the HRQoL review, studies reporting scores or utilities for specified HRQoL instruments (EuroQol Five Dimension [EQ-5D; 3L or 5L], Short Form [SF]-6D, SF-36, Health Utilities Index-3 [HUI3], European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire [EORTC QLQ-C30], Functional Assessment of Cancer Therapy-General [FACT-G], Functional Assessment of Cancer Therapy-Lymphoma [FACT-Lym], Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT fatigue], or Functional Assessment of Cancer Therapy-Biologic Response Modifier [FACT-BRM]) were included. These instruments were selected as they include both widely used generic HRQoL instruments (EQ-5D, SF-6D, etc.) and also a number of cancer-specific instruments (FACT-Lym, EORTC QLQ-C30, etc.). Queries were referred to a third reviewer (IF) and a consensus reached. The same process was applied to the subsequent review of full papers.
3 Results
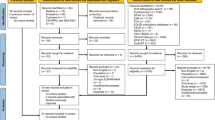
After removal of duplicates, and including both the original and updated searches, a total of 459 abstracts were reviewed for all components. For economic evaluations, 11 studies were included in the SLR. For cost and resource use, seven studies were included. For HRQoL, five studies were included. PRISMA flow diagrams indicating the number of studies included and excluded at each stage are shown in the ESM.
3.1 Economic Evaluations
Eleven economic evaluations were identified, reported in 16 publications. One was a full paper publication [9], and the remainder were identified from submissions to HTA bodies or conference abstracts. Most presented cost-effectiveness analyses (CEAs) and/or or cost-utility analyses (CUAs) [9,10,11,12,13,14,15,16,17], one presented a cost-minimization analysis (CMA) [18], and one presented a budget impact model (BIM) [19]. Australia, Canada, and the United Kingdom (UK) were the most frequently represented countries. Characteristics of the evaluations are summarized in Table 1.
Four of the economic evaluations focused on first-line treatments [9,10,11,12], and seven [13,14,15,16,17,18,19] presented models for the R/R setting. Many of the modelling studies were country-specific adaptations of the same model with amendments and results relevant to the submission country. Due to the differing inputs and results of these adapted models, they are identified here as separate studies. The interventions modelled for each setting are described in Table 1.
3.1.1 Model/Analyses Design Overview
All first-line studies used a Markov approach, with three to five health states as described in Table 1. The time horizon was 20 years for three of the models [9, 11, 12], whereas Aw et al., ASH 2016, which took the perspective of the healthcare payer in Canada, had a 24-year time horizon [10].
Among the R/R studies, two used a Markov approach [14, 17], two were partitioned survival models [13, 15], one was designated as a de-novo model but provided no further details [16], and one (the only included study that was not from Australia, Canada, UK, or Scotland) presented a cost-minimization model only [18]. Of the three studies that reported details of the health states, all were three-state models with states of pre-progression, post-progression, and death [13,14,15]. Time horizons were shorter than for the first-line setting, ranging from eight [17] to 15 years [14,15,16]. In addition, one BIM was identified [19].
3.1.2 Model/Analyses Inputs: Direct Versus Indirect Treatment Comparisons
Clinical efficacy data from specific trials were used to inform the majority of models in both treatment settings. However, two second-line models incorporated estimates of clinical efficacy derived from indirect treatment comparisons (ITCs); both were evaluations of ibrutinib submitted to UK HTA agencies [14, 16]. In the base case of the cost-effectiveness model submitted to NICE [14], the efficacy of rituximab (R)-chemo was based on the efficacy of the physician’s choice arm from an ITC conducted using data from a trial by Hess et al. [20] of temsirolimus versus physician’s choice of chemotherapy. As the physician’s choice arm was composed of single chemotherapy agents, the treatment effect from the ITC was adjusted to account for the addition of rituximab based on information on the benefit of R-chemo versus single-agent chemotherapy derived from an audit by the Haematological Malignancy Research Network (HMRN). The model for ibrutinib submitted to the SMC [16] incorporated both trial data from MCL-3001 and treatment effect estimates derived from an ITC comparing ibrutinib with physician’s choice, using temsirolimus as a common comparator (taking data from the Hess trial [20]).
3.1.3 Model/Analyses Results
In the first-line setting, incremental cost-effectiveness ratios (ICERs) were reported for bendamustine and rituximab (BR) versus rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) and bortezomib, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (VR-CAP) versus R-CHOP (Table 1). BR versus R-CHOP resulted in ICERs of 2016 Canadian dollars (CAD) $8924 [10] and Australian dollars (AUD) $15,000–45,000 [11] (cost year not reported [NR]) per quality-adjusted life-year (QALY) gained. Neither total costs nor total QALYs were reported in these publications.
For VR-CAP versus R-CHOP, Janssen Bortezomib SMC 2015 reported an ICER of Great Britain pound (£) 23,020 per QALY gained, and a 78% probability of being cost effective at a £30,000 willingness-to-pay (WTP) threshold [12]. Total costs for the VR-CAP arm were £45,453 per patient, compared with £26,291 per patient for R-CHOP. Total QALYs were 4.05 and 3.31, respectively, resulting in an incremental QALY gain of 0.75. van Keep et al. [9] used the same trial data taking a UK payer perspective and reported an ICER of £20,043 (2013/2014) per QALY gained and an 88.9% probability of being cost effective at the £30,000 WTP threshold. Costs and QALYs were similar to the Janssen Bortezomib SMC 2015 model, at £45,842 and £29,630 and 4.1 and 3.29 for the intervention and reference arms, respectively, and an incremental QALY gain with VR-CAP of 0.81.
In the R/R setting, all CEA/CUA studies reported ICERs (Table 1), but few reported the results as total QALY or life-year gain, and none reported total costs. ICERs for ibrutinib were presented in four studies, each with a different comparator. Janssen Ibrutinib CADTH 2016 presented an ICER of CAD $201,671 (2015) per QALY gained for ibrutinib versus standard of care (incremental QALY gain was 0.86, at an incremental cost of CAD $173,687) [13]. The Janssen Ibrutinib SMC 2016 submission comparing ibrutinib with physician’s choice reported a final ICER of £41,798 per QALY gained, based on a patient access scheme [16]. In the NICE appraisal of ibrutinib, the “…committee concluded that the most plausible ICER for the one previous therapy subgroup is likely to be lower than the company’s estimate of £49,848 per QALY gained” [14]. In Janssen Ibrutinib PBAC 2016, the ICERs for ibrutinib versus R-CHOP (with a temsirolimus proxy) were AUD $75,000–$105,000 per QALY gained [15]. The CMA for ibrutinib versus bortezomib, reported by Carlos et al. [18], ISPOR International 2016, reported incremental costs of United States dollars (USD) $3115 for ibrutinib.
Celgene Lenalidomide PBAC 2016 reported ICERs for lenalidomide versus mixed therapies (fludarabine, gemcitabine, chlorambucil, cytarabine, or rituximab monotherapies) of AUD $45,000–75,000 per QALY gained [17]. The incremental QALY gain with lenalidomide was lower than those modelled for ibrutinib, at 0.37; total costs were not reported.
Adding acalabrutinib as a treatment option for MCL patients who had received one previous therapy led to an incremental total cost saving of USD $128,437 over 1 year, comprising savings of USD $110,392 and USD $18,046 in relation to therapy acquisition and adverse event (AE) management, respectively, and reflecting an overall saving of USD $0.0107 per patient per month and USD $790.4 per-treated month [19]. Cost savings were also observed from the commercial (USD $41,544) and Medicaid (USD $30,371) perspectives.
3.2 Costs and Resource Use
A total of seven studies (all retrospective analyses) assessed costs and resource use associated with patients with MCL. All studies reported on resource use and three also reported cost data. Five were performed in the US, one in Switzerland, and one in Australia. A variety of costs and resource use data were reported, including treatment-specific and non-treatment-specific data. Findings are summarized in Table 2.
AEs were reported as key drivers of increased costs [21] and resource use [22]. Karve et al. reported that mean monthly costs varied considerably by treatment regimen and care setting. Inpatient admissions and office visits were reported to be the main cost drivers for patients treated with R-CHOP, BR, and rituximab. For those treated with ibrutinib, drug costs were the largest contributor to total costs [21]. Feinberg et al. [22] reported that AEs, supportive care, and treatment duration were associated with increases in hospitalizations and ER visits. The Ratnasingam et al. [23] study reported increased hospitalization for patients treated with R-HyperCVAD/R-MA compared with R-CHO/R-DHAC due to greater toxicity. The Widmer et al. [24] study reported that hospitalization and hematological toxicity rates were significantly higher in the R-hyper-CVAD therapy group versus the R-CHOP/HD-ASCT group.
3.3 Health-Related Quality of Life
Only five HRQoL studies were identified for inclusion in the review: three phase II single-arm trials [28,29,30], one publication of patient-reported outcomes in a phase III randomized controlled trial (RCT) (MCL-3001) [31], and one retrospective observational study [24]. Two studies were conducted in the US [28, 29], one each in the Republic of Korea [30] and Switzerland [24], and one was multinational [31]. All five used cancer-specific (EORTC QLQ-C30 or FACT-G) or lymphoma-specific (FACT-Lym) instruments. Only the RCT reported results from a standardized generic measure (EQ-5D-5L), and this study was also the only one to report a preference-based utility measure [31]. HRQoL data are summarized in Table 3.
3.3.1 First-Line Setting
Two studies reported HRQoL outcomes in a first-line setting [24, 28]. Ruan et al. [28] reported FACT-Lym scores from a single-arm phase II study of lenalidomide plus rituximab (induction followed by maintenance) carried out in the US. Among patients who completed the questionnaire at both baseline and at 12 months, mean FACT-Lym total score increased from 133.6 (SD 22.8) at baseline to 139.1 (SD 19.1) at 12 months (higher scores indicate better HRQoL) [28]. This did not meet the threshold for clinical importance, which was defined as a change of 6.5–11.2 in FACT-Lym total score. Mean FACT-Lym trial outcome index increased from 93 (SD 17.9) to 98.2 (SD 14), an improvement that was close to the threshold for clinical significance, which was defined as a change of 5.5–11. The Widmer et al. [24] study reported that patients treated with R-HyperCVAD/MTX-AraC had a significantly higher EORTC QLQ-C30 Global health/QoL score 6 months after treatment termination versus R-CHOP/HD-ASCT (81.6 vs 60.5).
3.3.2 Relapsed/Refractory Setting
Hess et al. [31] reported data from the FACT-Lym, FACT-G, and EQ-5D-5L (utilities and visual analog scale [VAS]) instruments for patients treated with ibrutinib or temsirolimus in the multinational MCL-3001 trial (N = 280). Among the ibrutinib-treated patients, the mean FACT-Lym score at baseline was 121.9 (SD 22.2), and following all cycles of ibrutinib therapy, 66% of patients had a 5-point improvement in score, which was selected by the study as a conservative estimate of the threshold for a clinically meaningful change in lymphoma symptoms. The study found superior improvement to patients treated with temsirolimus, for which mean FACT-Lym score at baseline was 121.5 (SD 20.3), and 48% of patients had a 5-point improvement. The mean baseline EQ-5D-5L utility value was 0.7 (SD 0.2) in both groups.
Ruan et al. [29] reported a mean baseline FACT-G score of 83.3 (SD 18.8) in a phase II study from the US (N = 22) of treatment with RT-PEPC (rituximab, thalidomide, prednisone, etoposide, procarbazine, and cyclophosphamide; induction followed by maintenance). This increased to a mean score whilst on treatment of 89.4 (SD 5.5), indicating a small improvement; clinical significance was not discussed.
Shin et al. [30] reported EORTC QLQ-C30 Global health/QoL scores in a single-arm phase II study (N = 20) of vorinostat, fludarabine, mitoxantrone, dexamethasone (V-FND) plus either ASCT or maintenance with vorinostat conducted in the Republic of Korea. Baseline score was 61.8 (SD 26.4). Scores fell during treatment (lower score indicates lower HRQoL), but the differences were not statistically significant and clinical significance was not discussed.
4 Discussion
These SLRs highlight the limited amount of published economic and HRQoL evidence relating to MCL.
Although a reasonably high number of economic model publications were found, the identification of only one peer-reviewed full paper highlights the paucity of evidence. This limitation is compounded by the fact that direct comparisons across the economic models was hindered by the different settings (first-line vs R/R) used in the models. The evidence is further limited by the fact that UK HTAs provide more details about the models used in the submissions than other HTA bodies; this analysis would have been enhanced if the same granularity were provided by all HTA bodies. An example of this can be found in the studies reporting ICERs where the HTA body has published their WTP threshold; two studies, both of which were conducted in the UK, reported these data. However, for other studies reported, there is no published WTP threshold and therefore conclusions cannot be drawn about cost effectiveness of the product.
The most frequently used model structure was a Markov model, with three to five health states. In the first-line setting, R-CHOP was the most common comparator. ICERs in the first-line setting were within WTP thresholds, where such thresholds are established. In the R/R setting, ibrutinib was the most common treatment appraised. ICERs were generally higher in the R/R setting. There was insufficient information to comment with confidence on key drivers within models. For example, total costs were rarely reported. However, in two of the three studies where it was reported, the incremental QALY gain was similar to those seen in the first-line setting [13, 14]. Although chemo-immunotherapy was more cost effective than novel agents overall, it resulted in significantly higher resource use (e.g., use relating to hospitalization duration and additional unplanned hospitalization duration, etc.) [24].
Interestingly, there are more economic models than published cost/resource use studies relating to MCL, which suggests a need for further research on the true economic burden of MCL. The study by Karve et al. [21] claimed that their study was the largest series of MCL patients for whom real-world cost data have been reported, and this assertion was borne out by this review. Their study highlights the substantial economic burden associated with MCL. AEs were identified as a key driver in increasing both costs and resource use in this study, with cost for patients with no AEs (USD $4298) substantially lower than for patients with six or more AEs (USD $10,355). These results indicate a need for additional research comparing costs for treatments that have demonstrated fewer AEs versus standard treatment options [21, 24].
The most notable paucity of data was found in the HRQoL SLR. As an example of such paucity, only the Ibrutinib MCL3001 paper reported preference-based utilities in patients with MCL [31], despite the importance of these data in CUAs. The instrument most commonly reported as an HRQoL measure in the identified studies was FACT-Lym. Baseline FACT-Lym values in an R/R population in MCL 3001 [31] were lower than those reported by Ruan et al. [28] for first-line patients, although cross-trial comparisons should be treated with caution. Mean baseline EQ-5D-5L utility value was 0.7 (SD 0.2) in both treatment arms in MCL 3001 [31]. One study [31] found that patients on ibrutinib had HRQoL improvement in R/R MCL, which was correlated with clinical response, indicating that better HRQoL was associated with decreased disease burden.
To our knowledge, this is the first SLR that identifies previous economic models of existing and new treatments in all treatment lines, and explores costs/resource use and HRQoL impact in patients with MCL. Our search strategies were robust and used the recommended Scottish Intercollegiate Guideline Network (SIGN) and CADTH study design filters [32, 33]. In addition to searches in key electronic databases and conference proceedings, hand searches of HTA bodies and publicly available information were also undertaken in this comprehensive approach.
A major limitation of this review arose due to the lack of published literature available for this disease area. Only one full peer-reviewed paper was identified that reported a previous cost-effectiveness model, with the majority of cost-effectiveness studies being identified in HTA sources, where the full HTA submissions were not available and only limited information was reported in the identified summary documents. For the costs/resource use SLR, two studies reporting costs and seven conference proceedings that reported resource-use data were identified. The SLR of utilities only identified non-preference-based HRQoL measures without mapping to preference-based measures such as EQ-5D, use of which is recommended by HTA organizations (including NICE). This is a significant gap in the evidence base, where further research is required.
5 Conclusions
Overall, this SLR highlights the scarcity of CUA models in the current literature. Where available, health resource utilization data has shown high-dose chemotherapy uses a large amount of resources in comparison with chemo-immunotherapy [25]. There was limited HRQoL data was reported scarcely with studies overall across studies, however novel agents were shown to provide the best evidence (preference-based utilities) for patient-reported outcome benefit, with either an improvement or maintenance in HRQoL [31]. This SLR only identified two studies that reported direct health care costs; this highlights a significant gap in the literature. The evidence relating to MCL in the published literature is scarce; however, the available evidence highlights a critical unmet need for new agents that improve outcomes in this patient population. Specifically, new agents that reduce healthcare costs, thereby easing the burden on healthcare systems, while improving or maintaining HRQoL is needed for patients with MCL.
References
Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Supplement 4):iv62–71.
Project TN-HsLC. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909–18.
Society AC. Mantle cell lymphoma. 2019. https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/b-cell-lymphoma.html. Accessed Mar 2019.
Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132(16):1647–56.
Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125(1):48–55.
NCCN. Clinical Guidelines in Oncology V2.2019: B cell lymphomas. 2019.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0; 2011. https://handbook-5-1.cochrane.org. Accessed Oct 2017.
van Keep M, Gairy K, Seshagiri D, Thilakarathne P, Lee D. Cost-effectiveness analysis of bortezomib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (VR-CAP) in patients with previously untreated mantle cell lymphoma. BMC Cancer. 2016;16:598.
Aw A, Coyle K, Bence-Bruckler I, Bredeson C, Coyle D. Bendamustine and rituximab versus conventional chemoimmunotherapy as a frontline treatment for patients with indolent B-cell lymphoma: a cost-effectiveness analysis. In: Paper presented at: 58th annual meeting of the American Society of Hematology, ASH2016.
PBAC. BENDAMUSTINE, powder for injection 100 mg vial, 1powder for injection 25 mg vial, 1, Ribomustin®, Jansen-CilagPty Ltd. Public Summary document March 2015. 2015.
SMC. Bortezomib 3.5 mg powder for solution for injection (Velcade)SMC No. (1075/15). Advice Summary Report. 2015.
CADTH. Imbruvica for mantle cell lymphoma (relapsed/refractory). Economic Guidance Report. 2016.
NICE. NICE single technology appraisal ID753: ibrutinib for treating relapsed or refractory mantle cell lymphoma: company submission; 2016. https://www.nice.org.uk/guidance/ta502/resources/ibrutinib-for-treating-relapsed-or-refractory-mantle-cell-lymphoma-pdf-82606716182725. Accessed Oct 2017.
PBAC. IBRUTINIB Oral capsule, 140 mg, Imbruvica®, Janssen-Cilag Pty Ltd. Public Summary Document - March 2016 PBAC Meeting. https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2016-03/files/ibrutinib-psd-march-2016.pdf. Accessed Oct 2017.
SMC. Ibrutinib 140 mg hard capsule (Imbruvica®) SMC No. (1150/16). Advice Summary Report. 2016.
PBAC. Lenalidomide, oral capsules, 5 mg, 10 mg, 15 mg, 25 mg, Revlimid®, Celgene PTY LTD. Public Summary Document. 2016.
Carlos F, Herrera M, Salazar A, Aguirre A, Gonzalez-Michaca L. Economic evaluation of ibrutinib for the treatment of patients with relapsed or refractory mantle-cell lymphoma in Mexico. Value Health. 2016;19:A249.
Kabadi S, Suri G, Augusto M, Telford C. Budget impact analysis for acalabrutinib in the treatment of adult patients with mantle cell lymphoma who have received at least 1 prior therapy: U.S. payer perspectives. In: AMCP managed care & specialty pharmacy annual meeting 2018. 2018;24(4-a):S31.
Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–9.
Karve S, Nagar S, Goyal R, Kaye J, Mato A. Adverse events, resource use, and economic burden in patients with mantle cell lymphoma in the United States. Blood. 2017;130(Suppl 1):3442.
Feinberg B, Schenkel B, McBride A, Ellis L, Bhor M, Radtchenko J, Lal LS. Predictors of emergency room (ER) visits and hospitalizations in patients with mantle cell lymphoma (MCL) treated with chemotherapy. Blood. 2015;126(23):4526.
Ratnasingam S, Gilbertson M, McQuilten Z, Htun KT, Grigoriadis G, Swe MH, Campbell P, Chai KL, Quach H, Patil SS, Shortt J, Opat S. Improved survival of older patients with mantle cell lymphoma (MCL) with front-line cytarabine-based immunochemotherapy. Blood. 2016;128(22):2965.
Widmer F, Balabanov S, Soldini D, et al. R-hyper-CVAD versus R-CHOP/cytarabine with high-dose therapy and autologous haematopoietic stem cell support in fit patients with mantle cell lymphoma: 20 years of single-center experience. Ann Hematol. 2018;97(2):277–87.
Goyal R, Karve S, Kaye JA, Mato AR. Real-world treatment patterns and associated adverse events among patients with mantle cell lymphoma (MCL) in the United States. Blood. 2017;130:5650.
Sawalha Y, Radivoyevitch T, Tullio K, Dean RM, Pohlman B, Hill BT, Kalaycio M, Majhail NS, Jagadeesh D. The role of upfront autologous hematopoietic cell transplantation in the treatment of mantle cell lymphoma, a population based study using the National Cancer Data Base (NCDB). Blood. 2017;130:2009.
Sharman JP, Black-Shinn JL, Clark J, Karve S. MCL treatment patterns and outcomes: a community oncology practice experience. Blood. 2017;130:4684.
Ruan J, Martin P, Shah B, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373(19):1835–44.
Ruan J, Martin P, Coleman M, et al. Durable responses with the metronomic rituximab and thalidomide plus prednisone, etoposide, procarbazine, and cyclophosphamide regimen in elderly patients with recurrent mantle cell lymphoma. Cancer. 2010;116(11):2655–64.
Shin DY, Kim SJ, Yoon DH, et al. Results of a phase II study of vorinostat in combination with intravenous fludarabine, mitoxantrone, and dexamethasone in patients with relapsed or refractory mantle cell lymphoma: an interim analysis. Cancer Chemother Pharmacol. 2016;77(4):865–73.
Hess G, Rule S, Jurczak W, et al. Health-related quality of life data from a phase 3, international, randomized, open-label, multicenter study in patients with previously treated mantle cell lymphoma treated with ibrutinib versus temsirolimus. Leuk Lymphoma. 2017;58(12):2824–32.
Scottish Intercollegiate Guidelines Network (SIGN). http://www.sign.ac.uk/methodology/filters.html#econ. Accessed Oct 2017.
Canadian Agency for Drugs and Technologies in Health (CADTH). https://www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#health. Accessed Oct 2017.
Acknowledgements
Medical writing support was provided by Atish De (ICON Plc) and Jo Whelan (funded by ICON Plc).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this work was provided by Janssen.
Conflict of interest
Neerav Monga, Lori Parisi, and Christoph Tapprich are employees of Janssen. Jamie Garside was an employee of Janssen when this work was carried out. Matthew Davids and Constantine Tam received research support/honoraria from Janssen. Katherine Ward, Iain Fotheringham and Peter O’Donovan are employees of ICON plc and have received funding from Janssen to conduct/support this research.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors declare that the data supporting the findings of this study are available within the article. All data were identified and assessed from the references listed in the study.
Code availability
Not applicable.
Authors’ contributions
Neerav Monga and Jamie Garside provided writing and technical support on SLR development and manuscript. Katherine Ward conducted evidence synthesis including search strategy development, abstract and full text screening, data validation and SLR report writing and provided writing and technical support on the development of the manuscript. Iain Fotheringham conducted evidence synthesis including search strategy development, abstract and full text screening consolidation and also provided technical support on SLR report. He provided writing and technical support on the development of the manuscript. Peter O’Donovan conducted evidence synthesis including search strategy development, abstract and full text screening consolidation and also provided technical support on SLR report. He provided writing and technical support on the development of the manuscript. Matthew Davids, Constantine Tam, Lori Parisi, and Christoph Tapprich provided writing and technical support on SLR development and manuscript.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Monga, N., Garside, J., Davids, M.S. et al. Systematic Literature Review of Economic Evaluations, Costs/Resource Use, and Quality of Life in Patients with Mantle Cell Lymphoma. PharmacoEconomics Open 5, 175–186 (2021). https://doi.org/10.1007/s41669-020-00231-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-020-00231-w