Abstract
Background
There are no published studies on the costs of hospital-acquired neonatal bloodstream infection (BSI) in Ghana. Therefore, this study aims to calculate the cost and extra length of stay (LOS) of neonatal BSI. A prospective case–control study was undertaken at the neonatal intensive care unit (NICU) of Korle Bu Teaching Hospital (KBTH) in Ghana.
Methods
The clinical data of 357 neonates were prospectively analysed. Overall, 100 neonates with BSI and 100 control neonates without BSI were matched by weight, sex and type of delivery. The direct and indirect costs to neonates and their caregivers was obtained on a daily basis. The cost of drugs was confirmed with the Pharmacy Department at KBTH. A count data model, specifically negative binomial regression, was employed to estimate the extra LOS in the NICU due to neonatal BSI. The study analyzed the total, average and marginal costs of neonatal BSI for the case and control groups from the perspective of the patients/carers/providers.
Results
Fifty-four percent of the total sample were born with a low birth weight. Neonates with BSI recorded higher costs compared with neonates without BSI. The highest difference in direct costs was recorded among neonates with extremely low birth weight (US$732), which is 67% higher than similar neonates without BSI. The regression estimates show a significant correlation between neonatal BSI and LOS in the NICU (p < 0.001). Neonates with BSI stayed an additional 10 days in the NICU compared with their matched cohort. The LOS varies significantly depending on the neonate’s weight at birth. The extra days range from 1 day for neonates defined as macrosomia to 15 extra days for extremely low birth weight neonates.
Conclusions
Neonatal BSI was significantly associated with prolonged LOS. The continuous presence of experienced medical staff, as well as parents, to monitor newborns during their stay on the ward has enormous economic burden on both hospitals and caregivers.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Hospital-acquired neonatal bloodstream infections (BSIs) result in an excess length of hospital stay and extra costs for hospitals and caregivers. |
Healthcare providers bear a significant portion of the cost (69%), while caregivers of neonates bear the remaining 31% of costs. |
Policy makers have a role to play in terms of providing the right incentives for interventions against neonatal BSIs, and also absorbing the excess patient costs associated with neonatal BSI through the national health insurance scheme. |
1 Introduction
Despite the significant progress in reducing maternal and child mortality rates across regions, neonatal infection-related mortality remains high among hospitalized newborns in low- and middle-income countries (LMICs) [1]. Although LMICs account for 62% of the World’s newborns, they record over 82% of neonatal mortality cases [2]. Two studies have shown that more than one-quarter of the estimated neonatal deaths in hospitals are due to nosocomial or hospital-acquired infections (HAIs) [3, 4]. The incidence of nosocomial infections is estimated to be two- to fivefold higher in intensive care units (ICUs) than in general ward settings [5]. Most of the HAIs in ICUs are due to viral infection or systematic inflammatory response syndrome (SIRS) ensuing from a suspected or proven bacterial or fungal bloodstream infection (BSI). Although BSI is predominant at the extremes of age, specifically in neonates and the elderly, neonatal BSI has gained much recognition in modern microbiological studies since it remains a major cause of morbidity and mortality in children [6,7,8]. Depending on the onset of the infection, neonatal BSI can be either an early or late type. Early neonatal BSI, also known as maternal–fetal infection, is primarily caused by acquired infections before or during delivery, whereas late-onset BSI emanates from organisms within the environment after delivery [9, 10].
In sub-Saharan Africa, between 17 and 29% of neonatal deaths are attributable to BSI, compared with just 6% in high-income countries [11]. The incidence of clinically diagnosed neonatal BSI in the region also averages 170/1000 live births, equivalent to almost 2 million cases annually [11]. As sick newborns do not show exact signs and symptoms, clinical diagnosis and treatment of neonatal BSI is complicated and often leads to increased direct medical costs, prolonged hospital stay and indirect costs to parents [12]. A study that evaluated the cost of managing sick newborns in ICUs at a tertiary hospital in Enugu, Nigeria, shows that neonatal BSI results in an estimated average additional LOS of 12 days and an associated cost of US$223. The study found that approximately 97% of the cost of managing neonatal BSI is unbearable, in that it exceeds 10% of the neonate family’s monthly income [13].
Therefore, it is not surprising that there has been increasing interest by policymakers in investigating the relevance and cost effectiveness of clinical practices related to neonatal intensive care when allocating resources for medical interventions. Due to the increasing burden of healthcare costs worldwide, economic issues such as the cost of diseases, and the cost effectiveness of health interventions have become essential for the design and implementation of health policies. However, the biggest challenge of any cost analysis in healthcare is the difficulty in attaining the actual cost related to therapeutic interventions of a particular disease, and the lack of consistent methods for determining the direct and indirect costs of healthcare [14, 15]. This challenge also applies to the neonatal intensive care unit (NICU), where caregiving is extremely labour-intensive, requiring the continuous presence of experienced medical and nursing staff, as well as parents, to monitor newborns during their stay on the ward.
Although costing of diseases has a long history, empirical research on the economic burden of neonatal BSI has been relatively low in Africa. Most of the existing studies report on data from developed countries, particularly the US and Europe [16, 17]. In Ghana, the cost of healthcare is partly borne by both the government and the patient. Patients mostly pay the full expense of services, such as transport to and from a health centre, as well as drugs and laboratory services not fully or partially covered by the government. At the NICU where this study was conducted, the unit also runs a cost recovery system in which parents of neonates pay a fixed amount, as a consultation fee, into the unit’s revolving fund, either directly as an out-of-pocket payment or through a health insurance scheme; however, limited published information exists on the actual direct and indirect costs of neonatal BSI in ICU settings in Ghana. Therefore, the critical research question is to find the attributable cost of hospital-acquired neonatal BSI at a referral hospital in Ghana. Thus, this paper examines the cost of nosocomial neonatal BSI by analyzing the total, average and marginal costs borne by hospitals and caregivers of neonates with and without BSI at the NICU of the Korle Bu Teaching Hospital (KBTH) in Accra, Ghana. The intended outcome is to help healthcare providers and financiers assess the case for investment in this area in order to reduce the incidence and associated costs of neonatal BSI.
2 Methods
2.1 Setting
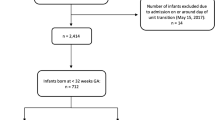
This study was conducted in the NICU in the KBTH. The choice regarding the NICU at the KBTH was due to the fact that this study is part of a wider project that selected KBTH for the study of clinical microbiology, interventional studies, cost analysis and ethnographic research of neonatal sepsis. Data from the hospital records show that the unit admits approximately 2400 neonates every year, accounting for more than 90% of all neonates in the hospital born with complications. From Ghana’s crude birth rate of approximately 30/1000 of the population in 2017 [18], the estimated annual admission capacity of the unit, compared with the number of newborns in 2017, was 0.26%. The unit is managed by the Department of Child Health and consists of three cubicles. Most neonates admitted to the NICU are admitted to cubicle 1 when they are most severely ill. When their health condition improves, they are moved to cubicle 2 and then to cubicle 3 prior to discharge. At most, each cubicle can accommodate 33 neonates, equivalent to 99 in total. Most surviving neonates transit to at least two different incubators or cots to accommodate new entrants with severe complications.
2.2 Study Design and Sampling
A matched pair design was adopted to assess the costs associated with neonatal BSI from the perspective of caregivers and providers. Neonates admitted to the NICU were enrolled prospectively and followed-up until discharge or death. An anticipated incidence of 10% and 25% was applied to the case and control groups, respectively, to estimate the total sample of 100 neonates for each group at 80% power and 0.05 alpha. The study was undertaken from October 2017 to January 2018. Patients with a birth weight > 750 g and aged < 48 h at the time of enrolment were included in the study. Not included in the study were neonates with severe congenital malformations (n = 2) and those who had undergone surgical procedures (n = 1). Sixty-seven percent of the excluded neonates (two-thirds) were likely to pick up nosocomial BSI. Overall, the number of neonates excluded was 62.5% less than the expected number (n = 8). The inclusion and exclusion criteria were contingent on clinical data from the NICU. Neonates were matched by sex, type of delivery, and birth weight, and were grouped into four categories: extremely low birth weight (< 1 kg), low birth weight (1–2.5 kg), normal birth weight (2.6–4.0 kg) and macrosomia (> 4 kg). A total of 357 neonates were included in the study. For the comparison of costs, 100 neonates with BSI and 100 control neonates without BSI were carefully matched on ± 0.1 kg birth weight. BSI neonates included those whose blood culture tested positive for pathogen and those infected with coagulase-negative staphylococci (CONS). Neonates with BSI were identified 48 h after admission, with the help of a medical microbiologist, following blood cultures. This study received ethical approval from the KBTH.
2.3 Outcomes
The primary outcome of this study was to assess the extra days of hospitalisation and the extra costs due to neonatal BSI. In both cases, we estimated the mean difference between neonates with BSI and their matched pair without BSI. Length of stay (LOS) in this study is defined as the number of days the neonate had been on admission prior to discharge. Those who were discharged the same day of admission were given an LOS value of ‘0’.
2.4 Data Collection
A data collection protocol was devised to obtain data on demographic characteristics (sex and age), birth weight, primary diagnosis at admission, and LOS. All identified caregivers of neonates were administered a structured questionnaire to capture the direct and indirect costs. Thirteen caregivers of neonates declined to participate in the study, the reasons for which include the relevance of the study (2), death of the neonate (7) and personal health conditions (4). The questionnaire completion rate was 100% in the matched pair sample (N = 200) and 95.7% in the full sample.
2.5 Data Analysis
The clinical data of 357 neonates were analysed prospectively. The additional direct and indirect costs and LOS were analysed by comparing all BSI cases with their matched controls. We also repeated the analysis by splitting the matched pairs by type of BSI (CONS and sepsis). Differences in the distribution of outcomes were tested using the Wilcoxon rank-sum test.
2.5.1 Patients’ Direct Costs
Direct costs were measured as a combination of the costs of pharmaceuticals such as antibiotics; laboratory tests; diagnostic tests; medical procedures; and medical supplies. Direct costs also included transportation costs incurred by caregivers, as well as the cost of outpatient visits to review the health status of neonates, overnight costs, and other costs related to hospitalization of the neonates. The cost of drugs was confirmed with the pharmacy at the teaching hospital.
2.5.2 Productivity Loss
Productivity loss (indirect cost) was measured as the opportunity cost to parents or caregivers of neonates related to hospital admission, i.e. loss of income during admission and the subsequent 30 days after discharge. Loss of income was limited to 30 days because epidemiological data from the NICU suggest the inpatient and outpatient days for BSI neonates sum up to 30 days on average. In addition, newborns cease to be neonates when they are 4 weeks of age (approximately 1 month) [9]. Loss of income was computed as the number of lost working days and the corresponding loss in daily wage. For employed caregivers with paid jobs, the average daily income was calculated from the 2017 national average monthly income specified by sex and type of employment and used to value time loss per day [19]. For caregivers aged 18–60 years engaged in informal sector work who could not provide a specific monthly income, their indirect cost was computed using the same procedure. The national average monthly income, rather than the actual income of the study participants, was used to estimate the indirect cost of productivity loss in order to enable generalisation of the result.
2.5.3 Hospital Costs
To estimate the institutional cost of neonatal BSI, the study used an activity-based composite cost approach rather than a disaggregated approach [20]. The former captures the sum of all recurrent and annualized capital expenditures incurred by the specific health facility or department. The capital cost includes the annualized cost of office space, incubators, and baby cots used by the Unit. The recurrent expenditure comprises staff-related costs, cost of clinical support, and the cost of all other consumable items used by the Unit within the 2017/2018 financial year. The cost of consumable items, i.e. baby wipes, toiletries and liquid antisepsis donated to the Unit and used in the review year were included in the analysis. These expenditure data were obtained from both the Human Resource Directorate and the Chief Accountant at the Child Health Department of the KBTH. The total number of admissions to the NICU was obtained and the total number of inpatient days estimated. The average cost per inpatient day was then calculated and was used to estimate the hospital cost incurred based on the LOS of neonates with and without BSI. Cost calculations were made in Ghana cedis (GH₵) and converted to 2017 purchasing power parity (PPP)-adjusted US dollars (US$) using a web-based PPP converter [21].
2.6 Statistical Analysis
Using the entire sample (n = 357), we estimated the extra number of days neonates with BSI spend at the NICU, using the negative binomial regression approach. Our approach was to control for overdispersion in the dataset, unlike the Poisson regression, which is limited by its equidispersion assumption [22, 23]. For multivariate analysis, LOS was treated as a continuous variable. Our objective was to assess whether BSI is significantly associated with LOS when adjusted for birth weight. Marginal effects of the negative binomial regression output were also analysed to examine the effect of BSI on LOS. All statistical analyses were performed using both Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and STATA analytical software version 13 (StataCorp LLC, College Station, TX, USA).
3 Results
3.1 Description of Participants
We recruited a total of 357 neonates admitted to the NICU. Approximately 57% of the total neonates were males. Those delivered through caesarean section constituted 86.3% of neonates, while those born with low birth weight accounted for 59.4% of neonates. Approximately 70% (249/357, 69.8%) of neonates admitted to the NICU were admitted to cubicle 1 (Table 1). Overall, 100 neonates had BSI, equivalent to 28% prevalence (100/357). Of the BSI neonates, 26% were infected with pathogens (sepsis), while 74% were infected with CONS.
3.2 Direct Patient Costs of Neonatal Bloodstream Infection (BSI)
In general, neonates with BSI recorded higher patient costs ($749; 95% confidence interval [CI] $729–$888) compared with those without BSI [$494; 95% CI $424–$471]. The highest difference in direct patient costs was recorded among neonates with extremely low birth weight (US$732), followed by US$403 for those with low birth weights (Table 2). Among BSI neonates, 72.9% ($546; 95% CI $497–$639) of the average direct patient costs were medically related, while the remaining 27.1% ($203; 95% CI $187–$249) were non-medically related. For non-BSI neonates, the direct medical patient costs were 84.8% ($419; 95% CI $371–$462) of the overall direct patient costs, while the non-medical direct patient costs were 15.2% ($75; 95% CI $64–$87). As the birth weight increases, the difference in direct patient costs decreases. The least difference was recorded among neonates who were classified as macrosomia (US$81).
3.3 Productivity Loss of Neonatal BSI
Table 3 shows the estimated productivity loss (indirect patient costs) to caregivers of the two groups of neonates. For almost all weight categories, caregivers of neonates with BSI reported higher productivity loss ($277; 95% CI $240–$357) compared with carers of neonates without BSI ($187; 95% CI $116–$289). The highest indirect patient cost difference was recorded among caregivers of neonates with extremely low birth weights.
3.4 Hospital Costs
Hospital cost was estimated from LOS and average departmental/administrative cost per day. The average LOS for BSI neonates was 20.1 days, compared with 9.9 days in the control group (Chi-square: p = 0.006). The extra LOS ranges from 1 day for neonates defined as macrosomia to 15 extra days for extremely low birth weight neonates (Table 4). In 2017, the total expenditures incurred by the NICU amounted to US$2,482,046 (Table 5). Of this amount, the cost of staffing accounted for 62.8%, while the remaining 37.2% covered the cost of medical supplies, capital equipment and other consumables. The NICU admitted a total of 1933 neonates in 2017. Assuming an average LOS of 13 days, as observed for the 357 neonates enrolled in the present study, this corresponds to 25,129 patient days; thus, the daily average cost per neonate in the NICU amounts to US$99. Taking into account Ghana’s consumer price index of 9.4%, with a 10% annual salary increase between 2017 and 2019 [24, 25], we adjusted the hospital costs of neonatal BSI from US$506,175 to US$612,472, equivalent to US$841,300 in total costs.
3.5 Cost and Length of Stay (LOS) by BSI Type
Table 6 shows there was a statistically significant difference in direct patient costs between neonates with sepsis and their control group (p = 0.001); likewise, for neonates diagnosed with CONS and their matched pair (p = 0.009).
Similarly, indirect patient costs were significantly different between neonates with sepsis and their matched pair (p = 0.001), and was the same for neonates with CONS and their control group (p = 0.002).
3.6 Neonatal BSI and LOS
We examined whether neonatal BSI is significantly associated with LOS in the NICU using the entire sample (N = 357). Tables 7 and 8 give the negative binomial regression estimates and the marginal effects, respectively. The results indicate a significant association between the two (p < 0.001). In Table 7, the results show, from the coefficient estimate, that the difference in the expected count is likely to be 0.7106 units higher for BSI neonates relative to non-BSI neonates. Furthermore, the results from the marginal effect analysis reported in Table 8 suggest that BSI neonates spend, on average, 10 days longer in the hospital compared with non-BSI neonates.
3.7 Summary
In summary, the estimated mean additional costs to society per neonate due to BSI is US$1359, of which 69% are institutional costs, 25% are direct patient costs paid by caregivers of neonates, and 6% productivity loss (indirect patient costs). With an estimated annual BSI prevalence rate of 28% (541/1933) in the NICU, the annual cost to society amounts to about US$735,126 (Table 9). Likewise, from the estimated average patient day costs, the additional institutional cost per neonate with BSI is US$936.
4 Discussion
This paper analyzed the cost of neonatal BSI from the perspective of patients/carers/providers in Ghana. Knowledge of patient and hospital costs of neonatal BSI is necessary for two reasons. First, to inform healthcare professionals about the need to reduce the incidence of hospital-acquired neonatal BSI at ICUs, not only for the sake of patient health but also for the consequential cost of treatment, and, second, to feed into assessments of the cost effectiveness of interventions to reduce neonatal BSI and to help prioritise such investments. In a resource-limited setting such as Ghana, the study is very insightful in that it draws the attention of the wider players in healthcare delivery, including pharmaceutical companies, to the growing interest and importance of the economic evaluation of healthcare interventions. Thus, studies such as this help to assess effectiveness and deficiencies in the health care sector.
This study has shown a significant relationship between neonatal BSI and LOS in the NICU. The results indicate that, on average, neonates with BSI spend approximately 10 additional days in the NICU compared with neonates without BSI. This implies that morbidities associated with BSI infection require prolonged days of close monitoring in order to restore good health. However, the continuous presence of experienced medical staff, as well as parents, to monitor newborns during their stay on the ward has enormous economic burden on both parents and the hospital facility. The extra cost attributable to BSI and borne by patients and caregivers is approximately twofold higher than the costs incurred by neonates without BSI and their caregivers. In addition, the difference in LOS between the two groups suggests that without BSI, the NICU can avoid approximately 8544 patient bed days annually, and, consequently, admit an additional 657 neonates.
Similarly, the results show that birth weight influences the additional number of days BSI neonates stay in the NICU. This is in tandem with other studies [4, 13] which found that the LOS in the NICU for premature infants is influenced by a number of factors, including birth weight. We found that a neonate with low birth weight spends at least four additional days on the ward compared with those with normal birth weight. Although not significant, macrosomic neonates stay at least 1 day less on the ward compared with their cohorts with normal birth weight.
In addition, neonates who were born through caesarean section stay an extra day longer in the NICU compared with those who were born through vaginal spontaneous delivery, although this was not significant. This concurs with the conclusion of Cheruiyot [26], who found that most neonates born through caesarean section are more likely to have a longer hospital stay compared with those born via vaginal delivery.
From the regression analysis, it came as a surprise that neonates admitted to cubicle 2 were at higher risk of BSI than those admitted to cubicle 1 although those admitted to cubicle 1 were supposed to be in a more severe health condition. This finding may have resulted in part from limited space to accommodate all severely ill neonates in cubicle 1, which results in some severely ill neonates being admitted to cubicle 2, where procedures may not have been geared towards these patients.
Nonetheless, generalization of the findings may be limited because the study utilized data from only one NICU setting in Ghana. Therefore, we cannot assume that the estimated patient and hospital costs of neonatal BSI in other hospitals across the country may be the same due to regional differences in costs of living and travel distance, as well as transportation costs to and from other hospitals.
4.1 Limitations
The strength of this study is the detailed cost information of good quality for the direct and indirect costs. Nevertheless, the study has some limitations. The institutional costs are based on the average cost per day at the department and thus disregard any variation in nursing time between neonates. As the neonates with BSI are likely to be among the most severely ill, requiring more than the average nursing time, this may imply that the costs associated with BSI are underestimated. In addition, the share of hospital overhead costs above the departmental level have not been included. Some confounding factors other than BSI, including the neonate’s comorbidities or daily severity of BSI, may affect the neonate’s LOS in the NICU. However, due to limited information in the data, this study did not analyse how comorbidities and the severity of BSI in neonates influenced their LOS in the NICU, but was limited to the association between the presence of BSI and LOS. This could limit the generalisability to other settings with different patterns of comorbidities and severity of BSI. Limitations in the data also made it difficult to adjust for days of admission prior to BSI.
Most economic evaluation studies have used the comparison of infected and uninfected pairs. Due to the lack of a perfect match, neonates in this study were carefully matched on ± 0.1 kg birth weight, although we acknowledge ± 0.1 kg in birth weight may have a negligible effect on LOS, especially for extremely low birth weight neonates.
5 Conclusion
This study indicates that BSI in neonates in the NICU at KBTH in Ghana is significantly associated with prolonged LOS and increased cost of hospitalisation. A reduction in BSI would not just improve the health of babies but would also free hospital beds and staff resources to the benefit of other patients. Neonatal BSI poses an economic burden on caregivers as well as society. Our findings have provided an idea of the extent of the economic burden regarding the cost of hospitalisation, as well as the additional LOS attributable to neonatal BSI. Interventions aimed at improving quality of care to reduce BSI in neonates can lead to savings in resource use that may outweigh the intervention costs. The extent to which such interventions will be economically advantageous will depend on the cost of the intervention and its effectiveness in reducing BSI.
Neonatal infections, including BSI, are well-known but have not been given much recognition as an important cause of morbidity and mortality in neonates in Ghana. There is a need for health policy makers to pay critical attention to interventions that will ensure the effective management of sicknesses in newborn babies, including the prevention of BSI acquired in the hospital. Furthermore, the attributable direct costs of hospital-acquired neonatal BSI must be considered by policymakers for inclusion in the national health insurance benefit to provide additional financial relief to carers of BSI neonates. Frequent mapping of the epidemiology of neonatal BSI is essential for detecting new trends and changes in hygienic standards, as well as the infant population at most risk, thereby allowing an immediate and targeted response.
The capacity of the NICUs at KBTH is very limited. This places incessant pressure on the few available facilities, and hence may jeopardize the quality of care received by neonates.
References
Rudan I, Theodoratou E, Nair H, Marušić A, Campbell H. Reducing the burden of maternal and neonatal infections in low income settings. J Glob Health. 2011;1(2):106–9.
World Health Organization. Every newborn: an action plan to end preventable deaths. Geneva: World Health Organization; 2014.
Edmond K, Zaidi A. New approaches to preventing, diagnosing, and treating neonatal sepsis. PLoS Med. 2010;7(3):e1000213.
Boia M, Ilie C, Ioanas L, Manea A, Iacob D, Cioboata D. Neonatal septicemia retrospective study on premature newborn. Jurnalul Pediatrului. 2010;8:49–50.
Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1(1):47.
Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;14(390):17701780.
Khan AM, Morris SK, Bhutta ZA. Neonatal and perinatal infections. Pediatr Clin N Am. 2017;64(4):785–98.
Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701.
Sorsa A. Epidemiology of neonatal sepsis and associated factors implicated: observational study at neonatal intensive care unit of Arsi University Teaching and Referral Hospital, South East Ethiopia. Ethiop J Health Sci. 2019;29(3):333–42.
Cortese F, Scicchitano P, Gesualdo M, DeGiorgi E, Schettini F, Laforgia N, et al. Early and late infections in newborn: where do we stand? A review. Pediatr Neonatol. 2016;57(4):265–73.
Ranjeva SL, Warf BC, Schiff SJ. Economics burden of neonatal sepsis in sub-Saharan Africa. BMC Glob Health. 2018;3:e000347. https://doi.org/10.1136/bmjgh-2017-000347.
Stevens W, Shih T, Incerti D, Ton TGN, Lee HC, Peneva D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–48.
Ekwochi U, Osuorah DC, Ndu IK, Ezenwosu OU, Amadi OF, Nwokkoye IC, et al. Out-of-pocket cost of managing sick newborns in Enugu, southeast Nigeria. Clinicoecon Outcomes Res. 2014;16(6):29–35.
Detoumay B. Economic evaluation in healthcare. Med Sci (Paris). 2014;20(5):584–7.
Huton J. Health economics and the evolution of economic evaluation of health technologies. Health Econ. 2012;21(1):13–8.
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10.
Reyna-Figueroa J, Ortiz-Ibarra FJ, Esteves JA, Reyna-Fiueroa J. Cost of therapeutic failure of ampicillin plus amikacin in the treatment of early neonatal sepsis. An Pediatr (Barc). 2009;71(1):54–9. https://doi.org/10.1016/j.anpedi.2009.03.013.
United Nations Population Division. World population prospects: 2019 revision. https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?locations=GH. Accessed 3 Sept 2020.
Ghana Statistical Service. 2015 Labour Force Report. Accra: Ghana Statistical Service; 2016.
Simoens S. Health economic assessment: a methodological primer. Int J Environ Res Public Health. 2009;7(4):1831–4.
Ian S, James T, Marcello M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy J Res Debate Pract. 2010;6(1):51–9.
Cameron AC, Trivedi PK. Microeconometrics using stata. College Station: Stata Press; 2010.
Gujarati DN. Basic econometrics. Noida: Tata McGraw-Hill Education; 2009.
Ghana Statistical Service. Statistical bulletin. Consumer price index (CPI). Accra: Ghana Statistical Service; 2018.
Ministry of Employment and Labour Relations. 2018 Statistical report. Accra; 2019. http://www.melr.gov.gh/wp-content/uploads/2020/02/2018-Statistical-Report-MELR.pdf. Accessed 3 Sept 2020.
Cheruiyot B. Factors influencing length of hospital stay of neonates admitted to the newborn unit at Kenyatta National Hospital [doctoral dissertation]. Nairobi: University of Nairobi; 2013.
Acknowledgements
The authors acknowledge the support of Prof. Christabel Enweronu-Laryea, Head of the Pedriatric Department, KBTH, as well as Kingsley Laar and Dziedzom Awalime who assisted in the data collection.
Author information
Authors and Affiliations
Contributions
APF, FAA and UE conceived and designed the study. FAA, UE and KL reviewed the data collection tools. APF, EO and KL undertook the data collection. APF, UE and EO analyzed the data. APF and EO wrote the manuscript. FAA, UE and KL critically reviewed and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received ethical approval from the KBTH. All caregivers of the neonates who were interviewed granted written consent prior to the interviews.
Consent for publication
All authors consent to the publication of this manuscript.
Conflict of interest
All authors declare no competing interests.
Funding
Financial support was provided by the Danish Ministry of Foreign Affairs as part of the HAI-Ghana project (DANIDA Grant number 16-PO1-GHA). The funder was not involved in the design, data collection, analysis and interpretation of data, or the decision to publish the results.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to ongoing analysis but are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fenny, A.P., Otieku, E., Labi, K.AK. et al. Costs and Extra Length of Stay because of Neonatal Bloodstream Infection at a Teaching Hospital in Ghana. PharmacoEconomics Open 5, 111–120 (2021). https://doi.org/10.1007/s41669-020-00230-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-020-00230-x