Abstract
Introduction
Cystic fibrosis (CF) is a life-limiting, hereditable condition, with the highest prevalence in Europe. CF treatments have led to improvements in clinical symptoms, disease management and decelerated disease progression. However, little is known about the health state utility (HSU) associated with CF disease states, adverse events, and changes in disease severity. Although HSU data have contributed to existing health economic modelling studies, a lack of such data have been highlighted. This systematic review aims to provide a summary of HSU-related research in CF and highlight related research gaps.
Methods
Online searches were performed in six databases and studies in any of the following categories were included: (1) estimation of HSUs in CF; (2) mapping studies between patient-reported outcome measures (PROMs) and HSUs; (3) economic evaluations on the management of CF that report primary HSU data; and (4) any CF clinical trial that reported HSU as an outcome.
Results
A total of 17 studies were reviewed, of which 12 provided HSU values for specific CF populations. The remaining five articles provided HSU data that were broken down by CF relevant health states, including lung transplantations, pulmonary exacerbation (PEx) events and forced expiratory volume in 1 s (FEV1).
Conclusion
Current HSU data in CF are limited and there is considerable scope for further research, both in providing HSU values for CF and in investigating methods for HSU elicitation/evaluation in CF populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cystic fibrosis (CF) is a life-limiting condition. In the UK, an increase in survival among people with CF leads to higher demands for treatment, with one author suggesting a rise in the population from 9400 in 2010 to 14,440 in 2025 [1]. The average annual cost for treatment from a societal perspective was €49,000 per CF patient, doubling to €76,000Footnote 1 for those with CF receiving additional caregiver support [2]. As a result, even though CF has a low incidence, it results in substantial economic burden [2]. Treatments received by CF patients lead to improvements in clinical outcomes [3,4,5,6]. However, estimates of the cost effectiveness of new treatments are important components of the decision-making framework for adoption decisions made by governing bodies such as the National Institute for Health and Care Excellence (NICE) in the UK [7]. Health state utility (HSU) values play a central role in summarising health-related quality of life (HRQOL) to support economic evaluations and can be elicited or evaluated through direct or indirect methods, respectively [8].
Direct methods such as time trade-off (TTO) and standard gamble (SG) elicit values directly from individuals, either in relation to their own health or to a range of hypothetical scenarios; however, these exercises can be onerous and difficult for individuals to complete. HSU generated by these exercises are anchored at 0 (dead) and 1 (full health) [8]. Indirect methods use questionnaires, such as the EQ-5D, to determine the perceived health states of respondents, across a number of domains such as mobility or pain. Subsequently, the EQ-5D health states scores are converted to country-specific utility values through the use of country-specific value sets. The importance of using country-specific value sets has recently been studied by Gerlinger et al. [9]. In summary, the study has highlighted that misappropriation of value sets for calculating utility can lead to significant differences in utility values.
Indirect methods have the advantage that they are relatively straightforward to complete and can be easily included in many studies, such as randomised controlled trials. Indirect measures are required or suggested for inclusion in economic evaluations in some countries, including England, Wales, Spain, France, Finland, Poland, New Zealand and The Netherlands [10]. HSUs, particularly those generated through generic questionnaires such as the EQ-5D [7], are required by regulatory bodies such as NICE.
In a health economist’s ideal world, all clinical trials conducted on healthcare interventions would include some form of valuation of HSUs; however, often this does not occur. In these cases, it may be possible to provide estimates of HSUs through mapping [8]. ‘Mapping’ allows conversion of outcomes from a patient-reported outcome measure (PROM), which does not provide HSUs, to a generic preference-based measure (PBM) that allows estimation of HSUs [10], which in turn can be used for health economic modelling.
We undertook a systematic review that aimed to identify all studies that determine HSUs in CF, as well as studies that provide HSU data for specific populations of CF patients. Our main goal was to inform future health economic models by presenting the available evidence.
2 Methodology
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11] for reporting systematic reviews. Our review is not aimed at identifying HSU values for a particular treatment or intervention of interest; however, we follow guidelines set out by Ara et al. [12] on how to conduct systematic reviews for published HSU values.
2.1 Inclusion Criteria
The PRISMA guidelines do not relate directly to an HSU systematic review [13], however we still consider these useful in this case as they outline the boundaries of the review in a format familiar to readers. The HSU values we seek pertain to patients of any age with CF and the health states associated with these patients. Studies that reported HSU values gained through proxy responses are also included. Studies solely utilising HSUs, or sections of studies providing additional information on HSUs based on rating scales such as the visual analogue scale (VAS), were excluded. Such scales are not considered HSU values unless anchored by ‘full health’ and ‘dead’, and also risk scaling biases such as the end-of-scale bias [14].
Studies included in this review were assigned to one of three categories during the title and abstract screening process. These categories included (1) measuring HSU in CF patients; (2) mapping between PROMs and PBMs (e.g. the Cystic Fibrosis Questionnaire–Revised [CFQ-R] and the Short-Form 6-Dimension [SF-6D]); and (3) any CF clinical trial that reported HSU as an outcome. Economic evaluation studies were included to ensure the search for primary research around utility-based articles/research was more robust; however, they were only included if they presented primary utility data. If these economic evaluation studies referred to secondary utility data from the literature, the original source was retrieved and was considered for inclusion in the review.
2.2 Search Strategies and Study Selection
Search strategies were designed in order to identify the appropriate original published studies for this review. Text words, phrases, synonyms and indexing terms were selected through the Medical Subject Heading (MeSH) thesaurus. Preselected search strategies were also utilised from a previous study [15]. These met the recommendations reported by Ara et al. [12], a study detailing the identification, review and synthesis of HSU values from the literature. Appropriate changes were made to the search strategies in order to tailor them to different subject heading terms in alternative databases (see the electronic supplementary material). Databases utilised in this review were MEDLINE, Ovid, PubMed (PubMed + PubMed Central), PsycINFO, Web of Science, Cochrane Library (National Health Service Economic Evaluation Database [NHS EED] only), School of Health and Related Research Health Utilities Database (ScHARRHUD), and Cumulative Index to Nursing and Allied Healthcare Literature (CINAHL). The Google search engine was also used by selecting key search terms, as the search algorithm for this database changes frequently, with the first 50 results reviewed for inclusion. No date restrictions were applied, although we restricted the language to English only. Studies were not included if they were books, editorials, conference abstracts or theses.
Forward citation searching was undertaken using the Web of Science (ISI) to find further evidence that could be incorporated. Additionally, the bibliography of articles (backward citation searching) selected for full-text review, particularly the economic evaluations, were hand-searched for relevant literature. The last date for conducting searches in the databases was 15 March 2019.
Two rounds of selection were carried out by two authors (BM and AB) based on the inclusion criteria, and any disagreements were adjudicated by a third author (JW). Data were extracted using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), in which the initial form was piloted using a few studies, subsequent to which a final template for data extraction was selected.
2.3 Quality Assessment of Studies
Quality assessment of the studies evaluated in this review was not conducted. There were no instruments or checklists that focused on assessing HRQOL studies that allow definitive categorisation of study quality.
3 Results
3.1 Search Results and Study Selection
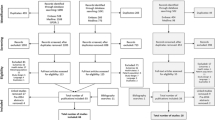
A total of 3480 articles were found through our electronic searches, reducing to 2632 articles after removing 848 duplicates. A further 2385 articles were excluded at the title and abstract screening stage, leaving 247 articles. Of these, 204 were removed after full-text review. Finally, a further 26 articles were excluded because they were study protocols, conference abstracts, not written in English, presented a VAS only, or were economic evaluations. The economic evaluations identified did not contain primary data on utilities, and were therefore removed from the review at this stage subsequent to checking references for primary utility research articles. A total of 17 articles were included in our review and were processed for data extraction in Microsoft® Excel by two authors (BM and AB). A PRISMA diagram demonstrating the process of study selection is presented in Fig. 1.
PRISMA diagram showing the study selection process (adapted from Moher et al. [11]). PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, CF cystic fibrosis, VAS visual analogue scale
3.2 Study Characteristics
Table 1 summarises the key study characteristics. Included studies were published from 1990 onwards. The most recent publication was 2018, with more than 20% being conducted in 2015. The length of each study period varied. Most studies undertook only a cross-sectional measurement, while others included longitudinal follow up, up to 5 years. Studies were undertaken within many different countries in and outside of Europe, with one study [21] covering multiple countries that were part of the same BURQOL-RD research network study. The most common countries were the US and the UK, with eight and three studies, respectively. Two studies were from Australia [25, 28].
In Table 2, we have categorised studies into three broad types since no studies of the fourth type specified in our inclusion criteria—economic evaluations presenting primary utility data—were identified for inclusion. Studies focusing on determining HSUs were categorised as HSU elicitation/evaluation studies, while studies that elicited/evaluated HSU as a secondary outcome in a clinical trial were categorised as randomised clinical or crossover trials, as appropriate. Finally, studies focusing on deriving HSU values from one instrument based on outcomes from another were labelled as mapping studies.
Patients in the studies included children, adolescents, adults, or mixed-age populations. The studies in this review included 2859 CF patients, with sample sizes ranging from 6 to 920. The largest sample came from a study looking at HRQOL across multiple European countries, conducted as part of the BURQOL-RD research network study [19]. Participant age varied across studies, with the youngest mean age being approximately 9 years [30] and the oldest mean age being 30 years [22].
In some cases, proxy HSU values were obtained for CF patients from their carers [2, 19,20,21, 30]. Dewitt et al. [24] only utilised a proxy when patients with CF were under a particular age, i.e. < 14 years. Some studies also provided values for the carer’s own HSU [2, 19,20,21].
Two studies were ambiguous about how the questionnaires were completed [25, 28], and one study interviewed the participants and subsequently allowed them to complete the questionnaire at home [30]. Lastly, one study collected information through face-to-face interviews [26].
3.3 Health State Utility (HSU) Elicitation/Evaluation
Table 2 provides a summary of HSU elicitation/evaluation procedures, value sets used and interventions considered.
From the 17 studies evaluated in this review, 13 reported HSUs derived using indirect evaluation. The most common instrument used to derive HSU was the EQ-5D [2, 16,17,18,19,20,21,22,23]. This included different versions of the EQ-5D—the 3-Level (3L) and the 5-Level (5L). Studies that utilised the EQ-5D-5L version of the instrument [2, 19, 21] mapped their results to the 3L instrument due to the lack of a value set at that time, which is what NICE recommends [7]. However, a single study [16] stated that they used the 5L value set to obtain EQ-5D-5L-related index values, but the source showed a 3L-related value set. Other instruments used included the Quality of Well-Being (QWB) instrument [25, 27, 28, 30, 31], and the Health Utilities Index (HUI), [24, 26]. Direct HSU elicitation via TTO and SG was used by two studies [26, 29].
3.4 Scoring Algorithms for Indirect Evaluation
In order to obtain HSU data from indirect evaluation instruments, the health state described by the patient or proxy is scored using a valuation set. A value set based on the UK general population, described by Dolan [33], was commonly used to calculate HSU values for studies using the EQ-5D-3L instrument, although it was not used exclusively for UK studies. Different value sets were utilised for the EQ-5D-3L on only two other occasions, i.e. by Chevreul et al. [21], who used a French value set [34] for a French study, and Chevreul et al. [19], who utilised multiple value sets for different European countries. Chevreul et al. [19] also applied value sets from different countries to the PBM scores in cases where value sets were not available for that particular country.
Five studies were investigated to understand which value sets they had utilised to convert QWB scores into HSUs [25, 27, 28, 30, 31]. There was no clear information about the value set in any study; however, we are aware that the HSU scoring algorithm is available from the developers of the instrument [8].
Finally, two studies utilised the HUI, versions 2 and 3 [24, 26]. Neither study provided information around the value sets that were used to calculate their respective HSUs.
3.5 Mapping between Instruments
In many instances, a study does not use an instrument capable of generating HSUs, but does report PROMs. Mapping is a technique that allows an estimate of HSU data for instances where no such data are directly available. In this review, we identified a single study that undertook mapping from the CFQ-R disease-specific instrument to the EQ-5D-3L [22]. Regression modelling approaches were utilised in order to determine the most appropriate prediction model [22].
3.6 Health State-Specific HSU
Of the 17 studies included in this review, only seven provided data that were broken down in some form by CF disease-relevant interventions or health states. These included health states related to lung transplantation [17, 29], pulmonary exacerbation (PEx) events [16, 18, 23] and forced expiratory volume in 1 s (FEV1) [18, 22, 26].
3.6.1 Lung Transplantation
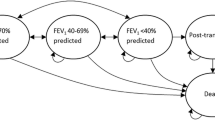
Lung transplantation HSU data reported by Busschbach et al. [29] were separated by time points prior to, during and after the transplant, where the utilities were 0.8, 0.4 and 0.9, respectively [29]. The treatment sample in the paper by Busschbach et al. [29] was small, and, as a result, no significance testing was undertaken. Additionally, these CF patients were hypothetically put into different lung transplantation health states and were described as overestimating their HSU. Overestimation of HSU was linked to coping mechanisms [29]. For these reasons, HSU data derived from these CF patients should be used with caution in health economic modelling.
In contrast, a US-based study by Singer et al. [17], prospectively evaluated HSUs in 19 CF patients aged between 18 and 49 years as part of a larger cohort of 211 patients with advanced lung disease. The cohort consisted of those subjects being admitted to hospital after donor offer and those staying at home. The study evaluated the HSU of patients from the point of entering a transplantation waiting list until 3 years post-surgery. At baseline, younger and older patients reported similar HSUs (0.60, interquartile range [IQR] 0.44–0.78). After initial improvement in HSUs up to 3 months post-surgery (+ 0.32, 95% confidence interval [CI] 0.23–0.41) for those who survived the remainder of the 3-year follow-up (those who died were assigned an HSU of 0), HSU declined by clinically negligible amounts (− 0.01, 95% CI − 0.03 to − 0.001). After adjusting for age, sex, body mass index (BMI) and FEV1, a significant difference was observed (p < 0.0003) in HSU post-surgery (0.30, 95% CI 0.22–0.39). There was no significant difference in HSU for those in hospital versus those at home in the run-up to surgery. Post-transplantation, improvements in HSUs experienced by younger and older patients were not significantly different. Overall, transplant was associated with a three/four times minimum clinically important difference in HSU of 0.06.
3.6.2 Pulmonary Exacerbations
The PEx HSU was separated by PEx requiring/not requiring hospitalisation and the time periods prior to and after the events [18], no/moderate/severe PEx [23], and index day, day 7 and end of PEx treatment (day 28) HSUs [16]. From the data reported by Bradley et al. [23], it is evident that the increasing severity of PEx events is associated with a decrease in the EQ-5D HSU score. HSU values and 95% CIs were 0.85 (0.80–0.89), 0.79 (0.67–0.91) and 0.60 (0.44–0.76) for no, mild and severe PEx events, respectively [23], for a patient sample size of 94.
HSU data relating to PEx were investigated by Solem et al. [18], who obtained data at various time points before and after the PEx. Their study, with a total of 161 patients, was based on whether the patient required hospitalisation or not. For PEx events that required hospital admission, HSU was the lowest through a 1-week period during the build-up to a PEx event (0.76, standard error [SE] 0.033). HSU up to 8 weeks prior to PEx was highest (0.9, SE 0.020, p < 0.001) compared with time periods up to 8 weeks after the event (0.86, SE 0.021, p = 0.002). Compared with the reference period, these HSUs 8 weeks prior and post were significantly different (p < 0.001). This change suggests a clinically important minimum difference in HSU according to a value of 0.074 (range − 0.011 to 0.140) [37]. This pattern for the EQ-5D HSU score does not exist in the non-hospitalised PEx events group. The HSU value for the non-hospitalised group during the build-up to a PEx was lowest (0.88, SE 0.029) compared with the HSU score 1–4 weeks after the PEx (0.92, SE 0.029), with differences in these values compared with the reference period being non-significant (p = 0.7). When looking at the association of EQ-5D to PEx events and FEV1 through multivariate analysis, lower FEV1 and PEx (any, hospitalisation/no hospitalisation) had a significant (p < 0.05) negative impact on EQ-5D index scores. However, when PEx status (requiring hospitalisation or not) was added to the multivariate analysis, the results showed that experiencing a PEx that required hospitalisation, as well as having a low FEV1, were significant (p < 0.05) predictors of low EQ-5D index scores (− 0.002, p = 0.013) [18], whereas, when looking at those who were not hospitalised and had a low FEV1, there was a negligible positive effect (0.001, p = 0.016) [18].
Lastly, Gold et al. [16] evaluated PEx HSU at three time points. Patients were stratified by those younger and older than 18 years of age. Both patient groups experienced significant improvements in HSU at the end of treatment compared with index day (< 18: index day 0.84, standard deviation [SD] 0.10; end of treatment 0.92, SD 0.09; > 18: index day 0.79, SD 0.13; end of treatment 0.89, SD 0.13).
In summary, PEx event data presented covered a 4- to 48-week period [16, 18, 23] and had limited application for this particular health state due to the nature of the populations, treatments being investigated, and the duration of the studies. Solem et al. [18] evaluated the impact of Ivacaftor on PEx events; Bradley et al. [23] examined the HSU of those who were taking oral or inhaled antibiotics; and Gold et al. [16] investigated the impact of PEx and subsequent intravenous treatment in hospital. Therefore, HSU values are only available in CF patients receiving those treatments or in those settings.
3.6.3 Forced Expiratory Volume in 1 s
FEV1-derived HSUs were investigated in three studies [18, 22, 26]. Acaster et al. [22] categorised FEV1-derived HSU into three states: mild, moderate and severe. Their HSU was self-reported in a cohort of self-diagnosed CF patients. Yi et al. [26] and Solem et al. [18] reported and categorised FEV1-derived HSU into four states.
The studies undertook FEV1 evaluation using different approaches. Acaster et al. [22], with surveys completed by 401 participants, mapped the CFQ-R instrument to the EQ-5D-3L by three FEV1 severity levels, while Yi et al. [26] used a combination of a direct elicitation approach of TTO and SG, in addition to the HUI2 PBM instrument, in 65 adolescents aged between 12 and 18 years, to determine HSU and categorise FEV1 by four severity levels. Solem et al. [18] reported HSU from a cohort of 161 CF patients who were being treated with Ivacator.
The calculated HSU data in the study by Acaster et al. [22] shows a decrease in HSU scores with increasing severity according to the EQ-5D-3L (0.74 [SD 0.27] to 0.54 [SD 0.29]). Similarly, Solem et al. [18] presented such a relationship with no, mild, moderate and severe lung dysfunction, i.e. 0.931 (SE 0.023), 0.923 (SE 0.021), 0.904 (SE 0.018), 0.870 (SE 0.020), respectively. Through multivariate analysis, Solem et al. [18] showed that better FEV1 was associated with a higher EQ-5D score [18]. This is contrary to the relationship between FEV1 and EQ-5D that was identified by Gold et al. [16]. They did not identify a significant association between change in FEV1 and EQ-5D-5L over treatment time. This relationship was further evaluated through a generalised linear regression model that indicated that FEV1 was not significantly associated with changes in EQ-5D-5L index scores.
The above relationship is not so evident in some cases reported by Yi et al. [26]. For instance, the HUI2 score was higher for those in the 60–70% predicted class (0.85, SD 0.15) than those in the > 79% predicted class (0.82, SD 0.15). Similarly, for the SG, there was variation across different FEV1 severities, with HSU for 40–59% FEV1 (0.96, SD 0.08) being better than that of > 79% FEV1 (0.92, SD 0.16). The same is evident in the TTO data, with those in both the 60–70% predicted class (0.97, SD 0.06) and the 40–59% predicted class (0.98, SD 0.03) having similar HSUs to those in the > 79% predicted class (0.96, SD 0.08).
3.7 Mean Population-Based HSU
Of the 15 studies included in this review, 10 provided mean HSUs for specific CF populations. The studies cover populations on the following treatments/interventions: recombinant human DNase (rhDNase) [25, 27], antibiotics [31], aerobic versus resistance training [28], education [30] and chloride channel activator [24]. These studies particularly focus on characterising change in HSU pre- and post-intervention over time. A range of issues with HSU reporting were highlighted for these articles, including non-significant differences in HSUs [24, 27, 31], poor data presentation [25, 28] and no post-treatment HSU [27].
3.7.1 Recombinant Human DNase
rhDNase was evaluated in two clinical trials [25, 27]. Each study targeted different population groups, i.e. children only [27] or children and adults [25]. Both studies used the QWB instrument. In their study, Suri et al. [27] did not provide HSU data post-treatment with rhDNase, and only included a baseline QWB score of 0.61 for their CF study population. Suri et al. [27] also evaluated two different rhDNase treatment regimens—once daily or alternative days of rhDNase against twice-daily hypertonic saline. The article simply states that QWB scores following the 12-week trial showed no significant differences between the treatment options.
Fitzgerald et al. [25] evaluated the impact of administering rhDNase before or after physiotherapy treatment as part of a clinical trial. The results showed significant differences in QWB between the two treatment periods—0.778 vs. 0.752 (p < 0.05); however, it is not clear from the article what period represents which treatment option.
3.7.2 Chloride Channel Activator
Dewitt et al. evaluated the impact of denufosol, a chloride channel activator, on CF patients with mild impairment in lung function, over a 48-week period [24]. The study utilised the HUI2/3 to evaluate the HSU of treatment; the baseline value was high at 0.90 (SD 0.14), but there were no significant changes in the HSU of the treatment period in either instrument (0.01, 95% CI − 0.013 to 0.031).
3.7.3 Aerobic Training Compared with Resistance Training
Selvadurai et al. [28] evaluated the impact of aerobic versus resistance training on QWB subsequent to a pulmonary infection. Significant changes (p < 0.05) in quality of life were only seen in the aerobic training group; however, graphically, this is presented poorly and as a result is difficult to quantify.
3.7.4 Education Intervention
Czyzewski et al. measured HSU using the QWB, as part of a study investigating the effect of a clinical education intervention [30]. The interdependent respondent agreement between parent/caregiver and adolescent CF patient in terms of HSU was evaluated. Scores were 0.79 (SD 0.09) and 0.76 (SD 0.08) for caregivers and adolescents, respectively, and they were poorly correlated (r = 0.39, < 1, which shows a perfect positive relationship). Pearson’s correlation coefficient showed poor correlations between adolescents and caregivers (p = 0.05) in terms of inter-respondent agreement. There was also no significant relationship between disease severity (FEV1) and QWB scores for either parents or patients (p = not given).
3.7.5 Antibiotics
The QWB instrument was applied to CF patients being treated with oral ciprofloxacin for PEx [31]. Change in QWB was scored in the patient sample subsequent to treatment and showed a mean change of 0.104 (SD 0.122), but the worse and best changes in QWB were −0.201 and 0.209, respectively.
3.8 Cohort Studies
Finally, four studies [2, 19,20,21] evaluated HSU in a range of European countries as part of the BURQOL-RD Research Network. The overall population covered within the individual countries was based on the same criteria—CF patient centre or its equivalent in different countries, and CF Trust registries. The instrument used to calculate the HSU was the EQ-5D. Three studies presented novel data [2, 20, 21], with a fourth study presenting a summary of these studies, with some additional countries being evaluated as part of the project [19], including Germany, Hungary, Italy, Spain and Sweden.
The summary article by Chevreul et al. [19] evaluated HSU in 649 adults and children. For the adults, the mean HSU assessed through the EQ-5D in eight countries was between 0.640 and 0.870, and showed a statistically significant negative relationship with increasing age associated with decreasing HSU (p < 0.0172) [19]. HSU values for children were obtained by means of proxy responses obtained from caregivers. HSU values were between 0.647 and 0.919, but the relationship between age and HSU was not evident in this group, although there was a clear relationship between decreasing HSU for those children with high dependency (p < 0.0023) [19].
The HSU of CF patients in France was evaluated in a total of 166 patients, 54% of whom were children [21]. The study showed an average HSU of 0.73 (SD 0.231). No gender-based differences in HSU were found, although patients with longer disease duration had significantly worse HSU (0–9 years: 0.738 vs. 0.356; after 30 years, p = 0.0498) [21]. Compared with children, adults had a lower HSU (0.783 vs. 0.667, p = 0.0015), although a majority of the children’s HSU data were calculated through carer proxy (89%) [21]. In Bulgaria, the HSU of 33 CF patients was evaluated, but only represented 19% of those treated in an outpatient setting [20]. Of the 33 patients, 23 provided HSU data with a median value of 0.592, but values ranged from worse than death (− 0.385, more than 25% of patients) to 0.768 [20]. The study found that HSU was worse in children compared with adults [20], which is the opposite of what one may expect based on the worsening FEV1 of CF over time.
Evaluation of the individually published cohort studies showed discrepancies in the data. Not all the data in the study by Chevreul et al. [19] matched those figures provided within the studies by Chevreul et al. [21], Angelis et al. [2] or Iskrov et al. [20]. Further evaluation of the number of patients utilised to reflect the EQ-5D-3L data showed, for example in the study by Angelis et al. [2], that not all of the 37 adult patients filled in an HRQOL questionnaire, as stated in Table 2 of the study by Chevreul et al. [19]. The reported HSU values in the study by Angelis et al. [2] were based only on adult patients, even though Table 1 also states patient characteristics for children. More detailed reporting on children or proxy HSUs is available in the study by Chevreul et al. [19]; however, Angelis et al. [2] also state that the article is intended to be a descriptive summary, not a quantitative summary.
A similar case is evident in the other two publications [20, 21]. Chevreul et al. [21] state that of the total 240 patients, only 166 provided HSU data. Additionally, the HSU data values presented for children in the study by Chevreul et al. [21] are different to those presented by Chevreul et al. [19]; we are unsure why this is the case. Similarly, in the study by Iskrov et al. [20], it is stated that 23 patients were evaluated for their HSU, whereas the study by Chevreul et al. [19] states 33 patients. Iskrov et al. [20] presented HSU data as a median value with an IQR, however Chevreul et al. [19] presented HSU data as a mean value, which makes cross-checking of the results difficult. Consistent reporting or reporting in a single article would prove more beneficial for readers interested in using HSUs for health economic modelling of CF.
4 Discussion
Health economic modelling has become a key component of healthcare decision making and its use is recommended by NICE for technology appraisals [7]. However, in order to undertake health economic modelling, there needs to be sufficient data to populate the model, which in turn should reflect disease progression [38]. Previous research [39] and models have highlighted a lack of HSU evidence to inform CF health economic models [15, 40].
This is the first systematic review to describe the HSU data available in the literature for CF. We found that HSU values for specific CF health states were only available for seven studies [16,17,18, 22, 23, 26, 29] that focused on lung transplantation, PEx events and FEV1. These studies have substantial limitations in their application. A total of 10 studies evaluated HSU in a range of different CF populations, and these studies provided mean values at cross-sectional time points, i.e. every 12 weeks for up to 1.5 years. The majority of the HSU information was gathered using the EQ-5D (3L/5L). These studies are of particular interest in the UK as the EQ-5D is the reference-case instrument recommended by NICE for use in all Health Technology Appraisals (HTA) [7]. From the studies that evaluated HSU using the EQ-5D, we can understand that the population samples are quite different from each other, as well as the possible application of the HSU data obtained from the studies.
As the first study to review the literature for information around HSU in CF, we have identified the small number of studies that focus their attention on deriving HSU data for CF patients for the health states that may be needed to model the cost effectiveness of interventions for CF. Considering the improvements in CF mortality and morbidity over the last 50 years, which are largely related to improvements in screening [41, 42] and treatment of the condition [1, 43], this finding comes as a surprise, especially since health economic models currently exist that examine the cost effectiveness of a range of interventions available to CF patients [15, 40, 44,45,46,47,48]. For this dearth of evidence to come to light at this time suggests that CF research around health utilities has been slow.
HSU values found in this review have limited application due to the treatments being considered. Such studies do not allow for the generalisability of the HSU data to CF patients as the studies have selectively picked certain CF patients for inclusion into their clinical trials.
Future work should look at HSU elicitation/evaluation, longitudinal HSU measurement, and mapping studies. However, priority should be given to eliciting/evaluating HSU through use of the aforementioned direct and indirect measures, rather than mapping. Health state preference elicitation/evaluation could focus on significant adverse events such as PEx, CF-related diabetes (CFRD), CF-related liver disease (CFLD) and other lifelong complications such as distal intestinal obstruction syndrome. Attempts should be made to measure HSU as close to the event as possible. Similarly, the HSU of adults with differing FEV1 could be assessed multiple times annually, or collected on the encounter of complications or adverse events. Such longitudinal measurements will allow for more reflective health economic evaluation of interventions. Another avenue for elicitation/evaluation of HSU data could be the CF Trust registry, which recently launched a study looking at quality of life in CF adults [49, 50]. Studies on HSUs using the EQ-5D would also allow research to further explore problems around the ceiling effects of the instrument that have been mentioned in NICE appraisals of Orkambi® [51] as well as the published literature [18]. This in turn would provide evidence of the appropriateness of the EQ-5D as a PBM measure in CF.
HSU derived from the EQ-5D is advantageous as it is considered to be the most appropriate measure for HTA by NICE in most circumstances [7]. When studies use different measures, other than the EQ-5D, to determine HSU, this inherently prevents cross-comparison against findings from cost-effectiveness studies that use the EQ-5D outside of CF. However, the sensitivity and responsiveness of the EQ-5D is not proven in CF and therefore use of a conditions-specific measure in addition to the EQ-5D may be appropriate. This review indicates that a number of different methods have been used to determine HSUs, but who decides which measure is the best or most appropriate? No current consensus exists as to which instruments are the most appropriate for use in CF. Studies conducted in the past, in a range of disease areas, around the comparison of HSU data obtained from different instruments showed that there was poor to moderate agreement between instruments [8]. These differences can subsequently impact the cost per quality-adjusted life-year (QALY) ratio [8].
From the review, it is evident there is only one study considering mapping for a CF-specific instrument to the generic EQ-5D [22]. Currently, many instruments that measure PROMs exist that do not have an associated preference-based scoring system (such as the CFQ-R and the Cystic Fibrosis Quality of Life [CFQoL]), and therefore do not allow for HSU and subsequent (QALY) measurement. Although generally considered inferior to evaluation through PBMs as an outcome measure, future mapping studies between PROMs and PBMs could allow for better availability of HSU and QALY data. This could prove useful for health economic modelling in CF. An added incentive to undertake such studies, especially in the UK, could be the fact that NICE recommend undertaking mapping in the absence of EQ-5D data in clinical trials [7].
Recently, the James Lind Alliance (JLA) showed quality-of-life evaluation, particularly for the long-term effects of CF transmembrane receptor (CFTR) modulators [52], was a top research priority. This is based on what patients, clinicians, nurses and other healthcare staff consider to be priorities of research in CF.
5 Limitations of this Review
This review only considered full-text articles. Abstracts identified in this review included a study by Choyce et al. [53] that evaluated the impact of home monitoring in reducing hospital admission and HSU through the EQ-5D-5L; Giron et al. [54] evaluated EQ-5D-3L-derived HSUs in Spanish patients who had mild or moderate PEx events; L’abbe et al. [55] evaluated HSUs in CF lung transplantation patients; and Yarlas et al. [56] evaluated HSUs in CF patients in Europe and the US. These studies would prove useful additions to this review if/when a future update is available to expand on these abstracts. A total of five studies were excluded from this review as they were in languages other than English. Incorporation of these articles could have contributed towards better understanding of general country- and population-specific HSUs.
6 Conclusions
This review aimed to determine the level of available HSU information around CF, particularly in relation to various health states. The studies identified were cross-sectional, with little application on longitudinal evaluations without the use of assumptions. Research on eliciting health state preferences, particularly for FEV1, PEx events (by severity) and lung transplantation, still has some way to go. Research could focus on eliciting representable HSU values ascertained based on large samples sizes over time. However, new studies on HSU data are also warranted for CFRD, liver disease (CFLD) and intestinal obstructive syndrome. Further research on determining HSU data is needed for decision modelling of CF treatments. This would prove beneficial for the health economic modelling of CF-related treatments in order to aid future decision making in CF.
Notes
Cost year 2012.
References
Burgel P-R, Bellis G, Olesen HV, Viviani L, Zolin A, Blasi F, et al. Future trends in cystic fibrosis demography in 34 European countries. Eur Respir J. 2015;46(1):133.
Angelis A, Kanavos P, López-Bastida J, Linertová R, Nicod E, Serrano-Aguilar P. Social and economic costs and health-related quality of life in non-institutionalised patients with cystic fibrosis in the United Kingdom. BMC Health Serv Res. 2015;15(1):428.
McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med. 2014;2(11):902–10.
Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187(11):1219–25.
Konstan MW, Wagener JS, Pasta DJ, Millar SJ, Jacobs JR, Yegin A, et al. Clinical use of dornase alpha is associated with a slower rate of FEV1 decline in cystic fibrosis. Pediatr Pulmonol. 2011;46(6):545–53.
Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340(1):23–30.
NICE. National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013 (PMG9). https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed Jan 2017.
Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and valuing health benefits for economic evaluation. Oxford: Oxford University Press; 2017.
Gerlinger C, Bamber L, Leverkus F, Schwenke C, Haberland C, Schmidt G, et al. Comparing the EQ-5D-5L utility index based on value sets of different countries: impact on the interpretation of clinical study results. BMC Res Notes. 2019;12(1):18.
Wailoo AJ, Hernandez-Alava M, Manca A, Mejia A, Ray J, Crawford B, et al. Mapping to estimate health-state utility from non–preference-based outcome measures: an ispor good practices for outcomes research task force report. Value Health. 2017;20(1):18–27.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Ara R, Brazier J, Peasgood T, Paisley S. The identification, review and synthesis of health state utility values from the literature. PharmacoEconomics. 2017;35(Suppl 1):43–55.
Sampson CJ, Tosh JC, Cheyne CP, Broadbent D, James M. Health state utility values for diabetic retinopathy: protocol for a systematic review and meta-analysis. Syst Rev. 2015;4(1):15.
Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21.
Whiting P, Al M, Burgers L, Westwood M, Ryder S, Hoogendoorn M, et al. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18(18):1–106.
Gold LS, Patrick DL, Hansen RN, Beckett V, Goss CH, Kessler L. Correspondence between symptoms and preference-based health status measures in the STOP study. J Cyst Fibros. 2019;18(2):251–64.
Singer JP, Katz PP, Soong A, Shrestha P, Huang D, Ho J, et al. Effect of lung transplantation on health-related quality of life in the era of the lung allocation score: a US prospective cohort study. Am J Transpl. 2017;17(5):1334–45.
Solem CT, Vera-Llonch M, Liu S, Botteman M, Castiglione B. Impact of pulmonary exacerbations and lung function on generic health-related quality of life in patients with cystic fibrosis. Health Qual Life Outcomes. 2016;14(1):63.
Chevreul K, Michel M, Brigham KB, Lopez-Bastida J, Linertova R, Oliva-Moreno J, et al. Social/economic costs and health-related quality of life in patients with cystic fibrosis in Europe. Eur J Health Econ. 2016;17(Suppl 1):7–18.
Iskrov GG, Stefanov RS, López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P. Economic burden and health-related quality of life of patients with cystic fibrosis in Bulgaria. Folia Medica (PlovDiv). 2015;57(1):56–64.
Chevreul K, Berg Brigham K, Michel M, Rault G. Costs and health-related quality of life of patients with cystic fibrosis and their carers in France. J Cyst Fibros. 2015;14(3):384–91.
Acaster S, Pinder B, Mukuria C, Copans A. Mapping the EQ-5D index from the cystic fibrosis questionnaire-revised using multiple modelling approaches. Health Qual Life Outcomes. 2015;13(1):33.
Bradley JM, Blume SW, Balp M-M, Honeybourne D, Elborn JS. Quality of life and healthcare utilisation in cystic fibrosis: a multicentre study. Eur Respir J. 2013;41(3):571.
Dewitt EM, Grussemeyer CA, Friedman JY, Dinan MA, Lin L, Schulman KA, et al. Resource use, costs, and utility estimates for patients with cystic fibrosis with mild impairment in lung function: analysis of data collected alongside a 48-week multicenter clinical trial. Value Health. 2012;15(2):277–83.
Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics. 2005;116(4):e549–54.
Yi MS, Britto MT, Wilmott RW, Kotagal UR, Eckman MH, Nielson DW, et al. Health values of adolescents with cystic fibrosis. J Pediatr. 2003;142(2):133–40.
Suri R, Metcalfe C, Lees B, Grieve R, Flather M, Normand C, et al. Comparison of hypertonic saline and alternate-day or daily recombinant human deoxyribonuclease in children with cystic fibrosis: a randomised trial. Lancet. 2001;358(9290):1316–21.
Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol. 2002;33(3):194–200.
Busschbach JJ, Horikx PE, van den Bosch JM, Brutel de la Riviere A, de Charro FT. Measuring the quality of life before and after bilateral lung transplantation in patients with cystic fibrosis. Chest. 1994;105(3):911–7.
Czyzewski DI, Mariotto MJ, Bartholomew LK, LeCompte SH, Sockrider MM. Measurement of quality of well being in a child and adolescent cystic fibrosis population. Med Care. 1994;32(9):965–72.
Orenstein DM, Pattishall EN, Nixon PA, Ross EA, Kaplan RM. Quality of well-being before and after antibiotic treatment of pulmonary exacerbation in patients with cystic fibrosis. Chest. 1990;98(5):1081–4.
Agency for Healthcare Research and Qualtiy. Calculating the US Population-based EQ-5D™Index Score. 2005. https://archive.ahrq.gov/professionals/clinicians-providers/resources/rice/EQ5Dscore.html. Accessed 25 Mar 2019.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
Perneger TV, Combescure C, Courvoisier DS. General Population reference values for the french version of the EuroQol EQ-5D health utility instrument. Value Health. 2010;13(5):631–5.
Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
MVP Group. The measurement and valuation of health. Final report on the modelling of valuation tariffs. Centre for Health Economics. 1995. https://www.york.ac.uk/media/che/documents/reports/MVH%20Final%20Report.pdf. Accessed Nov 2017.
Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–32.
Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M, et al. Conceptualizing a model: a report of the ISPOR-SMDM modeling good research practices task force-2. Value Health. 2012;15(6):804–11.
Mohindru B, Turner D, Sach T, Bilton D, Carr S, Archangelidi O et al. Health economic modelling in Cystic Fibrosis: a systematic review. J Cystic Fibrosis. https://doi.org/10.1016/j.jcf.2019.01.007.
Tappenden P, Harnan S, Uttley L, Mildred M, Carroll C, Cantrell A. Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: systematic review and economic model. Health Technol Assess. 2013;17(56):v–xvii, 1–181.
Heinemann Mitja L, Hentschel J, Becker S, Prenzel F, Henn C, Kiess W et al. Einführung des deutschlandweiten Neugeborenenscreenings für Mukoviszidose. Laboratoriums Medizin; 2016:373.
Wang L, Freedman SD. Laboratory tests for the diagnosis of cystic fibrosis. Am J Clin Pathol. 2002;117(Suppl):S109–15.
European Respiratory Society. European Lung White Book: Chapter 14. In: European Lung White Book. European Respiratory Society. 2014. https://www.erswhitebook.org/chapters/cystic-fibrosis/. Accessed Oct 2016.
Schechter MS, Trueman D, Farquharson R, Higuchi K, Daines CL. Inhaled aztreonam lysine versus inhaled tobramycin in cystic fibrosis. An economic evaluation. Ann Am Thorac Soc. 2015;12(7):1030–8.
McGirr AA, Schwartz KL, Allen U, Solomon M, Sander B. The cost-effectiveness of palivizumab in infants with cystic fibrosis in the Canadian setting: a decision analysis model. Hum Vaccines Immunother. 2017;13(3):599–606.
Dilokthornsakul P, Hansen RN, Campbell JD. Forecasting US ivacaftor outcomes and cost in cystic fibrosis patients with the G551D mutation. Eur Respir J. 2016;47(6):1697–705.
Panguluri S, Gunda P, Debonnett L, Hamed K. Economic evaluation of tobramycin inhalation powder for the treatment of chronic pulmonary pseudomonas aeruginosa infection in patients with cystic fibrosis. Clin Drug Investig. 2017;37(8):795–805.
Tappenden P, Sadler S, Wildman M. An early health economic analysis of the potential cost effectiveness of an adherence intervention to improve outcomes for patients with cystic fibrosis. Pharmacoeconomics. 2017;35(6):647–59.
Taylor-Robinson D, Archangelidi O, Carr B, Siobhán B, Cosgriff R, Gunn E, Keogh RH et al. Data resource profile: the UK cystic fibrosis registry. Int J Epidemiol. 2017;47(1):9–10e.
Cystic Fibrosis Trust. Annual Registry Report. 2016. https://www.cysticfibrosis.org.uk/~/media/documents/the-work-we-do/uk-cf-registry/2016-registry-annual-data-report.ashx?la=en. Accessed Jan 2018.
National Institute for Health and Care Excellence (NICE). Lumacaftor–ivacaftor for treating cystic fibrosis homozygous for the F508del mutation. Technology appraisal guidance [TA398]. 2016. https://www.nice.org.uk/guidance/ta398/chapter/3-Evidence#cost-effectiveness. Accessed Jan 2017.
James Lind Alliance. Cystic Fibrosis Top 10. 2018. http://www.jla.nihr.ac.uk/priority-setting-partnerships/cystic-fibrosis/top-10-priorities.htm. Accessed Jan 2018.
Choyce J, Shaw KL, Sitch AJ, Mistry H, Whitehouse JL, Nash EF. A prospective pilot study of home monitoring in adults with cystic fibrosis (HOME-CF): protocol for a randomised controlled trial. BMC Pulmonary Med. 2017;17(1):22.
Giron R, Reig JP, Olveira C, Pastor MD, Prados C, Quintana-Gallego E et al. 125 Influence of pulmonary exacerbations on health status of cystic fibrosis patients. Health UTIlities and Quality of Life Study (HUTIQOL). J Cystic Fibrosis. 2016;15(Suppl 1):S82–83.
L’Abbe JM, Loadman Joyce M, Bentley MJ, Lien DC. Quantifying health status and functional outcomes following lung transplant. J Heart Lung Transpl. 2004;23(2):S72.
Yarlas A, O’Callaghan L, Lopes V, Suthoff E, Wagener J. PRS55—measuring generic health-related quality of life and impact of health resource utilization in adults with cystic fibrosis. Value Health. 2015;18(7):A503.
Author information
Authors and Affiliations
Contributions
The systematic review and preparation of the draft manuscript of this paper was carried out by Bishal Mohindru, and Arjun Bhadhuri provided support as the second reviewer for study selection. All authors provided academic input to the study design, reviewed and critically revised the manuscript, and approved the final version.
Corresponding author
Ethics declarations
This review was undertaken as part of a PhD funded by the Cystic Fibrosis Trust in Collaboration with the Epi-Net group.
Conflicts of Interest
Bishal Mohindru, David Turner, Tracey Sach, Diana Bilton, Siobhan Carr, Olga Archangelidi, Arjun Bhadhuri and Jennifer A. Whitty have no conflicts of interest to declare.
Data Availability
The search strategies utilised in this review are available in the electronic supplementary material.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mohindru, B., Turner, D., Sach, T. et al. Health State Utility Data in Cystic Fibrosis: A Systematic Review. PharmacoEconomics Open 4, 13–25 (2020). https://doi.org/10.1007/s41669-019-0144-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-019-0144-1