Abstract
Background
Costs associated with an ACS incident are most pronounced in the acute phase but are also considerably long after the initial hospitalisation, partly due to considerable productivity losses, which constitute a substantial part of the economic burden of the disease. Studies suggest that person-centred care may improve health-related quality of life and reduce the costs associated with the disease.
Objective
The aim of this study was to calculate the cost-effectiveness of a person-centred care intervention compared with usual care in patients with acute coronary syndrome (ACS), in a Swedish setting.
Methods
Primary data from a randomised controlled trial of a person-centred intervention in patients with ACS was used. The person-centred intervention involved co-creation of a health plan between the patient and healthcare professionals, based on the patient’s narrative. Thereafter, goals for the recovery period were set and followed-up continuously throughout the intervention. The clinical data, collected during the randomised controlled trial, was complemented with data from national health registers and the Swedish Social Insurance Agency. The study was conducted at two hospitals situated in a Swedish municipality. Patients were enrolled between June 2011 and February 2014 (192 patients were included in this study; 89 in the intervention group and 103 in the control group). Incremental cost-effectiveness ratios were calculated separately for the age groups < 65 years and ≥ 65 years in order to account for the age of retirement in Sweden. The cost-effectiveness ratios were calculated using health-related quality of life (EQ-5D) and costs associated with healthcare and pharmaceutical utilisation, and productivity losses.
Results
Treatment effects and costs differed between those below and those above the age of 65 years. The base-case calculations showed that person-centred care was more effective and less costly compared with usual care for patients under 65 years of age, while usual care was more effective and less costly in the older age group. Probabilistic sensitivity analyses resulted in a 90% likelihood that person-centred care is cost-effective compared with usual care for patients with ACS under the age of 65 years.
Conclusions
Person-centred care was found to be cost-effective compared with usual care for patients with acute coronary syndrome under the age of 65 years. This clinical trial is registered at Researchweb (ID 65791).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Person-centred care was found to be cost-effective (less costly and more effective) compared with usual care for patients with acute coronary syndrome under the age of 65 years. |
Person-centred care for patients with acute coronary syndrome was less effective and more costly compared with usual care for patients 65 years and older. |
1 Introduction
Cardiovascular disease is the leading cause of death in Europe and the associated healthcare expenditures constitute a significant part of total European healthcare expenditures [1]. Globally, the burden of disease attributable to acute coronary syndrome (ACS) is increasing due to large ageing populations [2]. Among patients hospitalised with ACS, approximately 12% are projected to die from the disease and 62% will be re-hospitalised within a year [3, 4].
The costs resulting from an ACS incident are most pronounced in the acute phase but are also considerable long after the initial hospitalisation [5, 6]. In Sweden, cardiovascular diseases cause seven publicly reimbursed sick-leave spells (duration > 14 days) annually per 1000 employees (four among men and three among women). In the Swedish social security system, the employer reimburses the first 14 days of sick leave in each spell, while days of absenteeism beyond the 14th day are reimbursed by the social security system. Thus, total productivity losses associated with seven publicly reimbursed sick-leave spells are considerable [7]. Indeed, ACS has been shown to induce larger employer costs than other common diseases due to large productivity losses [8], and the productivity losses have been found to constitute a substantial part of the economic burden of the disease [9]. Furthermore, ACS morbidities, occurring after hospitalisation, induce the need for informal care [10]. Since costs associated with informal care are rarely included in calculations of the burden of disease [11, 12], published estimates of the economic impact of ACS typically underestimate the true costs.
Several medical interventions have been reported to reduce the need for recurrent healthcare treatments among ACS patients, for instance, drugs, early percutaneous coronary intervention and stenting, and the establishment of specific follow-up facilities for chest pain [13]. The interest in non-medical interventions, such as person-centred care, that aim for long-term disease management has increased during the last decade [14, 15]. Person-centred care is based on ethical principles, inspired by the capability approach by Sen [16], and aims at establishing a partnership between the patient and healthcare professionals in the planning of care and treatment, emphasising the patient´s capabilities and resources [17]. In contrast, usual care typically focuses on the disease itself and is conducted mainly from a medical perspective [17]. Person-centred care has been found to increase general self-efficacy, to improve both subjective and objective health outcomes, to increase health-related quality of life and to reduce healthcare utilisation for a number of medical conditions [18,19,20,21,22,23], such as chronic heart failure [24]. However, the cost-effectiveness of complex interventions (e.g. person-centred interventions) has only been examined in a limited number of studies [23, 24]. Thus, there is a need for improved knowledge regarding the costs and benefits and the cost-effectiveness of complex interventions in general and of person-centred care in particular [20]. In this study, we employed primary data from a randomised clinical trial in order to study the cost-effectiveness of person-centred care compared with usual care for patients with ACS, in a Swedish setting [26].
2 Methods
This study refers to a randomised controlled trial (RCT) that evaluated the effects of person-centred care after an event of ACS. The primary outcome was a composite score comprising general self-efficacy and return to prior activity level (e.g. work), and conditional on no re-hospitalisation due to cardiovascular events (or death). At the 6-month follow-up, patients receiving person-centred care together with usual care had an improved composite score compared with patients receiving usual care alone [26]. In the present study, data collected during the RCT was complemented with register data. In this section, we provide an account of the data used, and summaries of the health economic methods employed and the RCT.
2.1 Patients
The RCT was conducted at two hospitals in Sweden and patients were enrolled and randomised between June 2011 and February 2014. The following inclusion and exclusion criteria were used [26]. Inclusion criteria: (1) age < 75 years, and (2) were hospitalised for myocardial infarction (ICD-10 I21) or unstable angina pectoris (I20.0, I20.9). Exclusion criteria: patients who, at the time of randomisation, (1) expected to survive < 1 year; (2) suffered from severe disability prohibiting study participation; (3) had ongoing alcohol and/or drug abuse; (4) were planned for heart surgery, such as coronary artery bypass grafting; (5) had no current address; (6) attended private primary care; (7) participated in a conflicting study. After randomisation, patients were also excluded if they had been misdiagnosed with ACS (myocardial infarction or unstable angina pectoris) or if they had an anticipated hospital stay exceeding 14 days.
Fors et al. [26] provides a comprehensive description of the RCT from which the data utilised in this study was collected but a short summary is given below.
Both the intervention group and the control group received usual care according to national guidelines for cardiac care [27]. The care was initiated at the hospital and then continued with follow-up visits in outpatient and primary care. In addition to usual care the intervention group received person-centred care according to the framework developed by the Gothenburg Centre for Person-Centred Care (GPCC), which comprises routines for establishment of a partnership between patients and healthcare professionals. The intervention was provided by designated healthcare professionals (physicians and registered nurses), at each care level, who had received training through lectures, seminars and workshops on how to apply the intervention. The starting point of the intervention was to listen carefully to the patient’s narrative in order to include his or her needs and intrinsic personal resources relevant for the treatment and care process. Based on this narrative, a health plan was co-created, which reflects both the perspective of the patient and the expertise of the healthcare professionals. The health plan also contained agreed goals for the recovery period, which were followed-up and revised by the patient together with the designated healthcare professionals at each care level when necessary. In this study, the health professionals were particularly focused on supporting patients’ wishes to return to work or other preferred activities. A barrier to reach such goals could be, for example, uncertainty about the health condition and worries about daily life demands in relation to the event. Active listening and comforting as well as making both short- and long-terms goals together with the patients was part of the person-centred care strategy. Information about quality of life was collected at baseline, 1 month, 2 months, 6 months (clinical endpoint) and 1 year after the initial hospital discharge. Information on total healthcare utilisation, sickness absenteeism and drug prescriptions was collected for the 1-year period [26]. Figure 1 illustrates the design of the RCT and the process of the analysis performed in this study.
Design of the clinical trial and process of the analysis. ACS acute coronary syndrome, CABG coronary artery bypass grafting, EQ5D1 EQ-5D measured at first follow-up, EQ5D2 EQ-5D measured at second follow-up, EQ5D3 EQ-5D measured at third follow-up, EQ5D4 EQ-5D measured at fourth follow-up, EQ5D5 EQ-5D measured at fifth follow-up, LOS length of stay
2.2 Study Population
Previous studies suggest that person-centred care does not influence mortality (although mortality has not been the primary outcome). Expected survival < 1 year was an exclusion criterion within the RCT, since the intervention was not designed for very ill patients [26, 28]. Therefore, deaths occurring during the first year after randomisation were considered random and were excluded from this study. The final data set used for the cost-effectiveness calculations was obtained by (1) excluding the patients who died during the trial (5 in the intervention group and 2 in the control group), and (2) imputing missing quality-of-life observations (EQ-5D). The imputation of missing observations, for both the control and the intervention group, was performed following the approach developed by Roderick, which employs observed data in order to generate values that replace missing observations [29]. First, missing baseline values for EQ-5D were imputed using mean imputation [30, 31]. Thereafter, assuming that missing data occurred randomly, we employed multiple imputation with chained equation using predictive mean matching (PMM) [30, 32]. Values for EQ-5D were imputed at index level and not for each dimension [33]. Then, QALYs for each individual were computed using the area under curve method [34]. Finally, the incremental cost-effectiveness ratio (ICER) was calculated using mean QALYs for the intervention and comparison groups. We provide a more detailed explanation of this method in the electronic supplementary material.
2.3 Quality of Life
Quality-of-life information was collected using the EQ-5D-3L questionnaire [35]. The EQ-5D scores were translated to index values using the Swedish experience-based health-state value set [36].
2.4 Direct Costs and Productivity Losses
Individual-level direct costs were calculated as follows. First, Diagnosis Related Groups (DRGs) were collected from the regional patient register (VEGA; Region Västra Götaland, Sweden) for each in- and outpatient visit. National DRG weights [37] and the associated cost per DRG were then used to calculate (provider) costs [38]. Outpatient care visits for which no DRG weight was reported and primary care visits, respectively, were assigned unit (provider) costs based on 2015 national statistics on healthcare use and costs [39]. Second, pharmaceutical (both provider and patient) costs were collected from the Swedish National Pharmacy Register (the Swedish National Board of Health and Welfare). Third, we included productivity losses (indirect costs) associated with temporary and permanent illness, valued according to the human capital method; that is, time units of lost production were valued at their market value. Information on temporary work absenteeism was collected from the Micro Data for the Analysis of Social Insurance (MiDAS) database (the Swedish Social Insurance Agency). The market value of lost working time was measured by average wages for the general population, collected from national wage statistics (Statistics Sweden) [40], including employer contributions to social security. Productivity losses associated with permanent illness were calculated for the 1-year follow-up period.
2.5 Health Economic Evaluation and Cost-Effectiveness
We used the most commonly used cost-effectiveness measure, the ICER. The ICER is defined as
where \(\Delta C\) is the incremental costs and \(\Delta {\text{QALY}}\) the incremental benefits associated with the compared treatment alternatives (when the effects are measured, as in terms of utility, this ratio is sometimes named the incremental cost-utility ratio, ICUR). The number of quality-adjusted life-years (QALYs) associated with a specific treatment over a given time period is defined as the sum of units of time multiplied by quality of life associated with each time unit; that is, as the area under the curve obtained by plotting quality of life against time [34]. In our case, the curve was obtained by connecting the different quality-of-life observations linearly.
ICERs were calculated for all patients and separately for those under the age of 65 years (at enrolment) and for those aged 65 years and above, since the majority of people in Sweden exit the workforce at the age of 65 [41]. Since we have access to individual-level data, the uncertainty of the cost-effectiveness ratios was inferred by repeated imputing of missing quality-of-life observations and resampling of the data (bootstrapping) [42]. The cost-effectiveness plane shows pairs of incremental costs and incremental effects based on the bootstrapping procedure. The cost-effectiveness acceptability curve shows the percentage of ICERs falling beneath a given willingness-to-pay threshold (the informal threshold in Sweden is 500,000 Swedish krona (SEK)/QALY) [43, 44]. Calculations were performed from both a societal perspective (including productivity losses) and from a healthcare system perspective (excluding productivity losses) [45]. All calculations were performed for a 1-year time horizon and, hence, costs and effects were not discounted. All statistical analyses were performed using Stata Statistical Software: Release 13 [46].
3 Results
The base-case cost-effectiveness results are provided in Table 1 (descriptive statistics are provided in the supplementary material). When considering both age groups, the person-centred care intervention was found to be less costly and less effective compared with usual care. The uncertainty of the ICER is illustrated in the bootstrap-generated scatter plot (1000 iterations) in Fig. 2, and in the cost-effectiveness acceptability curve, Fig. 3. For a 500,000 SEK/QALY willingness-to-pay threshold, the likelihood that person-centred care is cost-effective compared with usual care was estimated at 55% (including both direct and indirect costs).
3.1 Subgroup Analysis
The cost-effectiveness of the intervention was found to differ between the two age groups (< 65 years with 117 patients and ≥ 65 years with 75 patients). In the younger age group, the intervention induced lower total costs and higher quality of life, while the opposite was true in the older age group. Thus, the person-centred care intervention was the cost-effective alternative when compared with usual care for those under the age of 65 years, while usual care was the cost-effective alternative in the older age group.
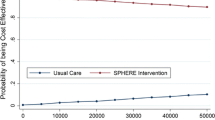
The uncertainty of the ICER for patients under the age of 65 years is illustrated in Fig. 4. The corresponding scatter plot for patients 65 years of age and above is illustrated in Fig. 5 and the cost-effectiveness acceptability graphs for the age groups are provided in Figs. 6 and 7. For a 500,000 SEK/QALY willingness-to-pay threshold, the likelihood that person-centred care is cost-effective compared with usual care in the < 65 age group was estimated at 89% (including direct and indirect costs). The corresponding likelihood when only healthcare costs were included was estimated at 86%. A sensitivity analysis including all patients (living and deceased) showed that the likelihood that person-centred care is cost-effective compared with usual care was 50%. The corresponding scatter plot and cost-effectiveness acceptability curve can be found in the electronic supplementary material.
4 Discussion
In this study, we estimated the cost-effectiveness of person-centred care compared with usual care for patients with ACS, in a Swedish setting, using primary data from a RCT. Our findings suggest that the person-centred care is both more effective and less costly than usual care for patients under the age of 65 years. In contrast, person-centred care was found to be less effective and more costly than usual care for patients aged 65 years and above. These results hold, qualitatively, when calculations are performed from a healthcare rather than a societal perspective. Thus, person-centred care induces both lower productivity losses and less healthcare utilisation for patients under the age of 65 years. Further, the simulated probability distribution of the societal perspective ICER for the younger age group implies that the likelihood that person-centred care is cost-effective compared with usual care is 89%, using a 500,000 SEK/QALY willingness-to-pay threshold. By comparison, the corresponding likelihood that person-centred care is cost-effective compared with usual care in the ≥ 65 years age group was estimated at 2%. Based only on the cost-effectiveness results obtained here, the policy implication would be to provide person-centred care to ACS patients under the age of 65 years, and usual care to patients aged 65 years and above. However, these findings should be considered in relation to the primary outcome analysis of the RCT showing that, regardless of age, general self-efficacy improved combined with return to prior activity level (e.g. work) without jeopardising clinical outcomes [26]. These effects were sustained at the 2-year follow-up and were even more pronounced in patients without post-secondary education [47]. Because self-efficacy is a valuable concept in person-centred care, it is likely that it reflects the outcome of a person-centred intervention at least as sufficiently as EQ-5D and should be considered as a measurement of effect in future cost-effectiveness studies. Moreover, the Swedish Health and Medical Services Act adopted by the Swedish parliament in 1997 mandates that prioritisation in the healthcare sector shall be governed by three ethical principles, of which cost-effectiveness is but one [48]. The other two ethical principles are the principle of human dignity and the principle of needs and solidarity. These three principles should be adopted using a joint ranking placing the human dignity principle first, and the cost-effectiveness principle last. In practice, this means that whenever human dignity values or needs and solidarity considerations are at risk, cost-effectiveness should not be used as the guiding principle for how to allocate scarce resources.
These results add to a small but growing literature on the cost-effectiveness of person-centred care. Previous studies have, for example, found that person-centred care is cost-effective compared with usual care when provided to patients with chronic heart failure [24]. Studies on patient-centred care suggest that it improves clinical outcomes and reduces the cost of care [49, 50]. Our results corroborate these earlier findings regarding the cost-effectiveness of person-centred care and adds to the stock of knowledge directly applicable for healthcare organisational decision makers. In our study, we evaluated the cost-effectiveness of person-centred care from a societal perspective employing indirect costs in the calculations of the ICERs and using data from a RCT. This differs from the previously mentioned studies on person-centred care that utilised data from controlled but non-randomised trials and where a healthcare perspective was used.
Cost-effectiveness analysis of complex interventions is more demanding than, for instance, cost-effectiveness analysis of competing drug treatments. This is due to a number of reasons pertaining to the typical characteristics of a complex intervention: there are several different healthcare professions involved, patients are seeking different amounts of care and the intervention affects different health-related dimensions for different patients. Thus, applying the standard method for computing the cost-effectiveness measure, which does not take the heterogeneity among participants regarding costs and effects associated with treatment into account, may lead to flawed results and less than optimal policy recommendations [25]. Such heterogeneity may be approached by latent class analysis, which, somewhat simplified, identifies homogenous groups among a study population for which separate cost-effectiveness analyses can be executed [25]. Even though we did not do a structured latent class analysis, we identified two age groups in our data, for which costs and effects differed. Of course, there may be numerous explanations for this finding, ranging from a genuine difference in the mechanism that transforms care into increments in health or quality of life, to systematically different degrees of engagement in care decisions, between the age groups. The findings in previous studies suggest that the latter explanation may have some significance [51,52,53]. Further, patients who had a < 1-year life expectancy at randomisation were excluded from the RCT analysed in this study. However, due to formal regulations and ethical considerations, person-centred care will be provided to all patients when implemented in practice. Assuming that the person-centred care intervention is unrelated to mortality, the cost-effectiveness of the person-centred intervention when implemented in practice will not differ from the cost-effectiveness calculated in this study.
4.1 Limitations
Finally, a number of caveats should be addressed. First, DRG weights as a measure of healthcare utilisation reflects not only true resource utilisation (opportunity cost) but also administrative decisions in the healthcare organisation concerning the distribution of fixed costs per operational part of the organisation. Thus, cost-effectiveness measures based on DRG weights may either over- or underestimate the true cost-effectiveness. Second, the relatively short observational period—1 year—disregards future quality-of-life improvements (or impairments), which tends to underestimate (or overestimate) the cost-effectiveness of the intervention. Third, the methods used for imputing missing quality-of-life values may bias our results [54, 55]. Fourth, the data on other types of productivity losses were not collected, therefore such losses among people above the age of 65 years were not accounted for in this study. Any potential effects of the intervention on indirect costs in that patient group could thus not be considered in the analyses. Aside from these facts, this study is a much-needed contribution to the scarce research on cost-effectiveness of person-centred care, and complex interventions.
5 Conclusions
The treatment effects and costs differed for patients below and patients above the age of 65 years. The results from the base-case calculations showed that person-centred care was more effective and less costly compared with usual care for patients under 65 years of age. In comparison, usual care was more effective and less costly in the older age group. Probabilistic sensitivity analyses resulted in a 90% likelihood that person-centred care dominates usual care for patients with ACS under the age of 65 years.
References
Taylor MJ, Scuffham PA, McCollam PL, Newby DE. Acute coronary syndromes in Europe: 1-year costs and outcomes. Curr Med Res Opin. 2007;23(3):495–503.
Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1493–501.
Santos IS, Goulart AC, Brandao RM, et al. One-year mortality after an acute coronary event and its clinical predictors: the ERICO study. Arq Bras Cardiol. 2015;105(1):53–64.
Southern DA, Ngo J, Martin B-J, et al. Characterizing types of readmission after acute coronary syndrome hospitalization: implications for quality reporting. J Am Heart Assoc. 2014;3(5):e001046.
Eisenstein EL, Shaw LK, Anstrom KJ, et al. Assessing the clinical and economic burden of coronary artery disease: 1986–1998. Med Care. 2001;39(8):824–35.
Menzin J, Wygant G, Hauch O, Jackel J, Friedman M. One-year costs of ischemic heart disease among patients with acute coronary syndromes: findings from a multi-employer claims database. Curr Med Res Opin. 2008;24(2):461–8.
Försäkringskassan. Social Insurance Report 2011;17. https://www.forsakringskassan.se/wps/wcm/connect/84cb4254-0889-4a51-9601-e4bc82931872/socialforsakringsrapport_2011_17.pdf?MOD=AJPERES. Accessed 2 May 2017.
Johnston SS, Curkendall S, Makenbaeva D, et al. The direct and indirect cost burden of acute coronary syndrome. J Occup Environ Med. 2011;53(1):2–7.
Zhao Z, Winget M. Economic burden of illness of acute coronary syndromes: medical and productivity costs. BMC Health Serv Res. 2011;11:35.
Fox KA, Carruthers KF, Dunbar DR, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J. 2010;31(22):2755–64.
Persson J, Ferraz-Nunes J, Karlberg I. Economic burden of stroke in a large county in Sweden. BMC Health Serv Res. 2012;12:341.
Goodrich K, Kaambwa B, Al-Janabi H. The inclusion of informal care in applied economic evaluation: a review. Value Health. 2012;15(6):975–81.
Turpie AG. Burden of disease: medical and economic impact of acute coronary syndromes. Am J Manag Care. 2006;12(16 Suppl):S430–4.
Washburn AM, Grossman M. Being with a person in our care: person-centered social work practice that is authentically person-centered. J Gerontol Soc Work. 2017;60(5):408–23.
Health Foundation. Person-centred care made simple. London, UK: Health Foundation; 2016. ISBN 978-1-906461-56-0.
Sen A. Capability and well-being. In: Nussbaum MSA, editor. The quality of life. Oxford: Clarendon; 1993. p. 30–53.
Ekman I, Swedberg K, Taft C, et al. Person-centered care—ready for prime time. Eur J Cardiovasc Nurs. 2011;10(4):248–51.
Olsson LE, Jakobsson Ung E, Swedberg K, Ekman I. Efficacy of person-centred care as an intervention in controlled trials—a systematic review. J Clin Nurs. 2013;22(3–4):456–65.
Hansson E, Carlstrom E, Olsson LE, Nyman J, Koinberg I. Can a person-centred-care intervention improve health-related quality of life in patients with head and neck cancer? A randomized, controlled study. BMC Nurs. 2017;16:9.
Pirhonen L, Olofsson EH, Fors A, Ekman I, Bolin K. Effects of person-centred care on health outcomes—a randomized controlled trial in patients with acute coronary syndrome. Health Policy. 2017;121(2):169–79.
Fors A, Taft C, Ulin K, Ekman I. Person-centred care improves self-efficacy to control symptoms after acute coronary syndrome: a randomized controlled trial. Eur J Cardiovasc Nurs. 2016;15(2):186–94.
Brannstrom M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail. 2014;16(10):1142–51.
Olsson LE, Hansson E, Ekman I, Karlsson J. A cost-effectiveness study of a patient-centred integrated care pathway. J Adv Nurs. 2009;65(8):1626–35.
Hansson E, Ekman I, Swedberg K, et al. Person-centred care for patients with chronic heart failure—a cost-utility analysis. Eur J Cardiovasc Nurs. 2016;15(4):276–84.
Sørensen SS, Jensen MB, Pedersen KM, Ehlers L. Examining the heterogeneity and cost-effectiveness of a complex intervention by segmentation of patients with chronic obstructive pulmonary disease. Value Health. 2017;21:239–47.
Fors A, Ekman I, Taft C, et al. Person-centred care after acute coronary syndrome, from hospital to primary care—a randomised controlled trial. Int J Cardiol. 2015;187:693–9.
The National Board of Health and Welfare. National Guidelines for Cardiac Care. https://www.socialstyrelsen.se/nationalguidelines/nationalguidelinesforcardiaccare. Accessed 2 Dec 2017.
Ekman I, Wolf A, Olsson LE, et al. Effects of person-centred care in patients with chronic heart failure: the PCC-HF study. Eur Heart J. 2012;33(9):1112–9.
Roderick JAL. Missing-data adjustments in large surveys. J Bus Econ Stat. 1988;6(3):287–96.
Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–70.
White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med. 2005;24(7):993–1007.
Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol. 2014;14(1):75.
Simons C, Rivero-Arias O, Yu L-M, Simon J. Missing data in the health related quality of life EQ-5D-3L instrument—should we impute individual domains or the actual index? In: Research paper presented at the 83rd Health Economists’ Study Group Meeting. 26th to 28th June 2013: Hosted by University of Warwick.
Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–5.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72.
Burström K, Sun S, Gerdtham U-G, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res. 2014;23(2):431–42.
The National Board of Health and Welfare. Klassificering och koder—Norddrg. http://www.socialstyrelsen.se/klassificeringochkoder/norddrg/vikter. Accessed 20 Aug 2017.
SKL. Vårdkostnader KPP 2016. 2016. https://skl.se/download/18.537af174160d51cb85ad2847/1516116941275/Vårdkostnader_KPP_2016.pdf.
Sveriges Kommuner och Landsting. Statistik om hälso- och sjukvård samt regional utveckling 2015. https://webbutik.skl.se/bilder/artiklar/pdf/7585-337-6.pdf?issuusl=ignore. Accessed 5 Sept 2017.
SCB. Lönestrukturstatistik i Sverige. 2016.
Olsson, H., Pensionsåldern, in Statistik & Utvärdering 2011-01-11. Pensionsmyndigheten; 2011.
Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37:2252–66.
Ryen L, Svensson M. The willingness to pay for a quality adjusted life year: a review of the empirical literature. Health Econ. 2015;24(10):1289–301.
Socialstyrelsen. Nationella riktlinjer för sjukdomsförebyggande metoder. Stockholm; 2011.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programme. 3rd ed. Oxford: Oxford University Press; 2005.
StataCrop LP. Stata Statistical software: release 13. StataCorp: College Station; 2013.
Fors A, Swedberg K, Ulin K, Wolf A, Ekman I. Effects of person-centred care after an event of acute coronary syndrome: two-year follow-up of a randomised controlled trial. Int J Cardiol. 2017;249:42–7.
Broqvist M, Branting Elgstrand M, Carlsson P, Eklund K, Jakobsson A. National model for transparent prioritisation in Swedish Health Care: reviderad version. Linköping: Linköping University Electronic Press; 2011.
Stone S. A retrospective evaluation of the impact of the Planetree patient-centered model of care on inpatient quality outcomes. Herd. 2008;1(4):55–69.
Pelzang R. Time to learn: understanding patient-centred care. Br J Nurs. 2010;19(14):912–7.
Beisecker AE. Aging and the desire for information and input in medical decisions: patient consumerism in medical encounters. Gerontologist. 1988;28(3):330–5.
Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20(6):531–5.
Rodriguez KL, Appelt CJ, Switzer GE, Sonel AF, Arnold RM. Veterans’ decision-making preferences and perceived involvement in care for chronic heart failure. Heart Lung. 2008;37(6):440–8.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99.
Sterne J, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:157–60.
Acknowledgements
We would like to thank the patients, healthcare professionals and managers involved in this RCT.
Data availability statement
The data used in this study cannot be made public. According to the General Data Protection Regulation, the Swedish law SFS 2018:218, the Swedish Data Protection Act, the Swedish Ethical Review Act, and the Public Access to Information and Secrecy Act, this type of sensitive data can only be made available, after legal review, for researchers who meet the criteria for access. Readers may contact the corresponding author regarding the data.
Author information
Authors and Affiliations
Contributions
Laura Pirhonen (LP) and Hanna Gyllensten (HG) conducted the study and performed the analyses. LP led the writing of the article and Kristian Bolin (KB) revised the text. KB and HG helped with the statistical methods as well as supervised the study. KB and HG helped to interpret the results. Andreas Fors (AF), Inger Ekman (IE) and Karl Swedberg (KS) designed the RCT, collected the data for the RCT and revised the manuscript from a person-centred care perspective. Elisabeth Hansson (EH) commented on the text and revised the manuscript from a person-centred perspective. All authors assisted in critical revision of the manuscript and have read and approved the final version of the article.
Corresponding author
Ethics declarations
Funding
This work was supported by the Centre for Person-Centred Care at the University of Gothenburg (GPCC), Sweden. GPCC is funded by the Swedish Government’s grant for Strategic Research Areas, Care Sciences (no. 2009-1088) and co-funded by the University of Gothenburg, Sweden. The Swedish Research Council (reference number 521-2013-2723), the Swedish agreement between the government and the county councils concerning economic support for providing an infrastructure for research and education of doctors (ALFGBG-444681); and Research and Development Unit, Primary Health Care, Region Västra Götaland also contributed to the funding of the study.
Conflict of interest
Laura Pirhonen, Kristian Bolin, Elisabeth Hansson-Olofsson, Andreas Fors, Inger Ekman, Karl Swedberg and Hanna Gyllensten declare that they have no conflict of interest.
Ethical approval
The Regional Ethical Review Board in Gothenburg has approved the clinical study. The study complies with the Declaration of Helsinki. This clinical trial is registered at Researchweb (ID 65791).
Informed consent
The randomisation was based on a computer-generated list, stratified for hospital site and employment status. Before randomisation, each patient gave their informed consent to participate, and patients were informed of the possibility to withdraw from the study at any time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pirhonen, L., Bolin, K., Olofsson, E.H. et al. Person-Centred Care in Patients with Acute Coronary Syndrome: Cost-Effectiveness Analysis Alongside a Randomised Controlled Trial. PharmacoEconomics Open 3, 495–504 (2019). https://doi.org/10.1007/s41669-019-0126-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-019-0126-3