Abstract
Background
Thailand’s hospitals may adopt different supervision approaches to improve tuberculosis (TB) treatment adherence.
Objective
The aim of this study was to compare out-of-pocket (OOP) expenditures, indirect costs, and health-related quality of life (HRQoL) among TB patients who received pharmaceutical care (pharmacist-led patient education and telephone consultation), home visit, and self-administered therapy (SAT) in Thailand.
Methods
We conducted a prospective study to collect OOP expenditures, indirect costs, and HRQoL from a subsample of 104 adult pulmonary TB patients who started treatment between January and May 2014 in three hospitals. The three sources of data included patient interviews, patient medical records, and the hospital billing database. Patients were followed from January 2014 to March 2015. Relevant OOP expenditures collected during the interviews included (1) healthcare costs and other medications costs (e.g. vitamins, antibiotics, anti-cough) occurring in private healthcare units; and (2) costs of transportation, food, and accommodation. Productivity loss was measured using the self-reported amount of time a patient was unable to work due to TB, travel time to and from the hospital, time spent at the hospital (waiting time, consultation time, and hospitalizations), and time spent accompanying family members on outpatient visits or during hospitalizations. Cost differences among treatment strategies were adjusted for baseline characteristics by generalized linear models (GLMs). All costs were converted to international dollars (I$).
Results
A total of 256 eligible patients who started pulmonary TB treatment during the specified period were approached, with 104 patients being included in the analysis (29, 38, and 37 patients receiving pharmaceutical care, home visit, and SAT, respectively). Mean OOP expenditures per patient receiving pharmaceutical care, home visit, and SAT were I$907.56 [confidence interval (CI) I$603.80–I$1269.41], I$148.47 (CI I$109.49–I$194.89), and I$95.35 (CI I$69.11–I$129.63), respectively. The GLM indicated statistically significantly lower OOP expenditures for patients receiving either home visit or SAT (ratio of mean costs 0.247, CI 0.142–0.427; and 0.318, CI 0.187–0.540, respectively) than those receiving pharmaceutical care. Patient’s indirect costs for receiving pharmaceutical care, home visit, and SAT were I$1925.68 (CI I$922.06–I$3284.94), I$2393.66 (CI I$1435.01–I$3501.98), and I$833.33 (CI I$453.87–I$1263.45), respectively. The GLM found no statistically significant differences in indirect costs for the home visit and SAT groups (ratio of mean costs 1.904, CI 0.754–4.802; and 0.792, CI 0.289–2.175, respectively) when pharmaceutical care was set as the reference. Mean utility scores [EuroQol five-dimensional three-level (EQ-5D-3L)] at baseline and treatment end were 0.679 and 0.830, 0.713 and 0.905, and 0.708 and 0.913 for patients receiving pharmaceutical care, home visit, and SAT, respectively.
Conclusion
Pharmaceutical care patients experienced the highest OOP expenditures, compared with home visit and SAT patients. Home-visit patients reached the highest indirect costs and utility score improvements. A large-scale prospective study is required in order to strengthen evidence to support policy making regarding the most efficient use of limited resources for the management of TB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Differences in patient characteristics [e.g. public insurance coverage, socioeconomic status, disease severity, and distance to tuberculosis (TB) services] among patients receiving different strategies had a high impact on a large variation in financial burden. |
The willingness of non-local patients to pay out of their own pockets when seeking TB care at a university hospital outside their district may be atypical in other settings. Caution should be exercised when generalizing the research findings to other populations. |
1 Introduction
Tuberculosis (TB) imposes a significant impact on not just the public healthcare system but also on patients and their families. Patients have to pay out of their own pockets for the cost of healthcare, food, and transportation when seeking care and receiving TB diagnosis and treatment at a health center or hospital [1].
Many TB patients are economically productive adults [2], and productivity loss due to the disease is the main driver of indirect costs [1]. A systematic literature review reported that the financial burden of TB borne by patients was composed of three parts: 20% direct medical costs [range in international dollars (I$) I$0–I$801.70], 20% direct non-medical costs (range I$0–I$1271.40), and 60% indirect costs (range I$29.80–I$2184.00) [3]. In addition to the economic impact, TB causes pain and psychological suffering, and consequently results in a reduction in the health-related quality of life (HRQoL) of patients [4, 5].
The economic burden of TB has a significant, multifaceted impact on low- and middle-income countries [6]. Patients may have poor compliance to medications due to financial burden, resulting in worsening health, potential spread of the disease, and/or death [6]. One of the World Health Organization (WHO) global targets for reducing economic impact is to push forward the provision of TB diagnosis and treatment, free of charge, through national universal health coverage [3, 7, 8].
Thailand established national universal coverage in 2002 [9]. All Thai citizens are covered under one of the three public insurance schemes, namely the Civil Servant Medical Benefits Scheme (CSMBS), the Social Security Scheme (SSS), and the Universal Coverage Scheme (UCS). Access to public TB diagnostic services and treatment is free of charge in Thailand. The national TB program adopts the daily dose regimen whereby most drug-susceptible patients take TB medication themselves at home and visit a health center or a district hospital at least once a month for a medication refill. Patients with multidrug-resistance TB (MDR-TB) are required to visit the nearest health center every day for treatment.
There are barriers to the implementation of directly observed therapy (DOT), a WHO recommended strategy to promote adherence for pulmonary TB patients [10], in resource-constrained countries [11, 12]. Other supportive strategies are therefore tailored to a country-specific context [13,14,15]. In Thailand, three alternative supervision approaches, pharmaceutical care (pharmacist-led health education), home visit, and self-administered therapy (SAT), are adopted by referral hospitals to improve adherence to TB treatment; however, evidence on the economic impact associated with these approaches is limited. To update the evidence of costs incurred by TB patients in Thailand, the objective of this longitudinal prospective study was to compare the out-of-pocket (OOP) expenditures, indirect costs, and HRQoL for TB patients who received pharmaceutical care compared with home visit and SAT in Thailand.
2 Methods
2.1 Data Source and Target Population
This was a prospective cohort study that followed patients for at least 6 months or until the treatment course was complete. Recruitment was between January and May 2014, while the data were collected from January 2014 to March 2015. OOP expenditures such as co-payment and healthcare costs not covered by public health insurance (i.e. non-essential drugs, seeking care outside their service area) were retrieved from the hospital billing database, while other OOP expenditures, productivity loss, and HRQoL were obtained through patient interviews.
The clinical data of patients were retrieved from patient medical records and TB registration records. Costs were separately estimated in two main phases: pre-TB treatment period (from illness onset to TB diagnosis) and TB treatment period (from the start to completion of TB treatment). All costs were calculated in Thai currency, adjusted using the cumulative inflation rate from the year of data collection (1.89% in 2014) to 2015 [16], and then converted to I$ using the 2015 purchasing power parity (PPP) conversion factor of private consumption [12.958 local currency unit (LCU) per I$] [17].
The study was conducted in three hospitals in Songkhla province, southern Thailand: Songklanagarind Hospital, Hatyai Hospital, and Songkhla Hospital, where pharmaceutical care, home visit, and SAT, respectively, have been adopted. Songklanagarind Hospital is an 850-bed teaching hospital affiliated to the Prince of Songkla University (PSU). Hatyai Hospital (700-bed, tertiary care hospital) and Songkhla Hospital (480-bed, secondary care hospital) are the main provincial referral hospitals under the Ministry of Public Health. This study was approved by the Research Ethics Board at each of the participating hospitals.
Patient eligibility criteria included the following: patients ≥18 years of age, confirmed pulmonary TB by a physician and classified bacteriologically as smear-positive or smear-negative, TB treatment started between January and May 2014, and a treatment period of ≥2 months in one of the study hospitals. Every patient who met the criteria was recruited in each of the study hospitals. Eligible patients were informed of the purpose of the study and assured that refusal to participate would not affect their TB care.
2.2 Tuberculosis Supervision Strategies
Pharmaceutical care provides pharmacist-led patient education at every outpatient visit, home visit provide regular home visit until treatment completion, and SAT is an approach where patients take medications by themselves at home without any additional supportive approach (see Online Appendix Table 1 for a description of these strategies). The treatment regimen was described in our previous retrospective cohort study [18, 19].
2.3 Data Collection
Patients were interviewed three times during clinic visits in the intensive phase (first 2 months), and were then interviewed once every 2 months during clinic visits in the continuous phase of treatment (the remaining 4 months). Most patients were interviewed while waiting in the clinic. Eligible patients who missed the interview at the clinic visit were contacted and interviewed by telephone within 14 days after the clinic visit. If patients could not be reached by telephone, they were interviewed at their next clinic visit. Patients were interviewed using a questionnaire developed by the authors (Online Appendix 1) and consisting of three parts: (1) sociodemographic information (first visit only); (2) OOP expenditures and productivity loss (the value of paid and unpaid production loss due to illness, time spent seeking treatment, disability [20]); and (3) HRQoL measured using the EuroQol five-dimensional three-level (EQ-5D-3L) Thai version [21, 22].
Since there is no validated TB-specific tool, the EQ-5D-3L was used in HRQoL measurement in this study. The EQ-5D-3L is the most preferred utility-based questionnaire in Thailand because it is short and easy to understand [23]. It includes five attributes: mobility, self-care, usual activity, pain/discomfort, and anxiety/depression [24]. Each attribute has three response levels representing no, some, and severe problems. Patients were asked to select the level that best described their current health status for each of these five attributes, and then assess their global health status by using the visual analog scale (VAS). The EQ-5D-3L health utility score was calculated using the Thai value set [25], in which the utility score can range between −0.45 and 1. A score of 1 and 0 represents perfect health and death, respectively, while negative values indicate states worse than death. For the VAS, patients were asked to mark the point on a vertical scale that best described their current health status where 100 and 0 represent best and worst imaginable health, respectively.
Relevant OOP expenditures collected during the interviews included (1) healthcare costs and other medications costs (e.g. vitamins, antibiotics, anti-cough) incurred in private healthcare units; and (2) costs of transportation, food, and accommodation. Productivity loss was measured, including time unable to work due to TB, travel time to and from the hospital, time spent at the hospital (waiting time, consultation time, and hospitalizations), and time spent accompanying family members on outpatient visits or during hospitalizations. If patients were hospitalized, we assumed 8 h of working time lost per day and one accompanying family member per patient. To estimate the indirect costs due to productivity loss, the amount of time lost due to TB illness was multiplied by the patient’s hourly wage rate. If the wage was not available, we used the daily minimum wage in Songkhla province (300 baht per day for no more than 8 h of work per day, in 2015) [26]. This was also applied to patients who were not in paid employment.
2.4 Statistical Analysis
Descriptive analyses were conducted to explore the distributions and categorize baseline characteristics, treatment outcome, and costs. Categorical variables (e.g. baseline characteristics) were analyzed using the Chi squared test, unless expected cell counts were low, in which case Fisher’s exact test was used. Means and standard deviations (SD) were calculated for continuous variables (e.g. patients’ income, treatment duration, OOP expenditures, and indirect costs). Differences in means were evaluated using analysis of variance (ANOVA). The EQ-5D-3L utility scores between the start of the intensive phase and the end of the continuous phase were compared using the paired t test.
We reported the cost data as arithmetic mean with SD, since other measures (e.g. median costs, log-transformed costs) did not provide sufficient information to estimate total costs at the population level, which is informative for healthcare policy decisions [27]. The Shapiro–Wilk test indicated that all cost data were positively skewed (p < 0.001), and the non-parametric bootstrap technique was thus used to compare arithmetic mean costs and to derive the 95% confidence intervals (CIs) [28].
Differences in the mean costs (e.g. OOP expenditures and indirect costs) among treatment strategies adjusted for baseline characteristics were investigated by generalized linear models (GLMs) [29, 30]. The model included seven baseline covariates, namely treatment strategy, sex, age, habitat, education, income, and health Insurance.
The performance of GLMs was assessed by comparing two different distributions (e.g. inverse Gaussian and gamma distribution) and two different link functions (e.g. identity and log link). Because the covariates act additively on the mean, the models with an identity link present the results as the difference in mean costs. On the contrary, those with a log link present the ratio of means due to the fact that the covariates act multiplicatively [29]. Model performance was assessed using Akaike Information Criterion (AIC) [29, 31] and graphical analyses: (1) scatter plots of deviance residuals versus fitted values; (2) normal probability plots (P–P plots) of the deviance residuals; and (3) normal quantile plots (Q–Q plots) of the deviance residuals [29, 32]. Smaller AIC values indicate a more appropriate model. Differences of 10 or more in AIC indicate the better-fitting model [29]. The more suitable models provided normally distributed deviance residuals (indicated by a random scatter of points from the scatter plots and the straight line on the normal plots) [29, 32, 33]. Data analyses were performed using SPSS software [34]. Significance for all statistical analysis was set at p < 0.05 whenever applicable.
3 Results
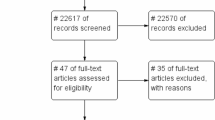
Between January and March 2014, 205 adult patients started pulmonary TB treatment in three hospitals in Songkhla province (Songklanagarind Hospital, Hatyai Hospital, and Songkhla Hospital). Given a change in policy at one study hospital, the recruitment period was extended until May 2014 and resulted in 13 additional patients being considered in the analysis (Fig. 1). In total, 104 patients from the three hospitals met our inclusion criteria and were included in the analysis (29, 38, and 37 patients receiving pharmaceutical care, home visit, and SAT, respectively). Baseline characteristics are described in Table 1 (see Online Appendix Table 2 for complications in TB treatment). Since the study hospital is an academic tertiary hospital, 38% of patients in the pharmaceutical care group came from neighboring provinces to seek TB care at the study hospital. A possible explanation may be that they had more confidence in the quality of care provided in this hospital. On the other hand, all patients in the home visit and SAT groups were from Songkhla province. Overall, 41.4, 78.9, and 67.6% of patients in the pharmaceutical care, home visit, and SAT groups, respectively, had an education level less than high school. Pharmaceutical care had the lowest percentage of patients (6.9%) who were unable to work due to TB, compared with the home visit (23.7%) and SAT groups (18.9%). Mean monthly income before TB and current income were, respectively, I$1381.97 and I$1184.59 in the pharmaceutical care group, I$738.67 and I$421.76 in the home visit group, and I$569.48 and I$417.79 in the SAT group. The pharmaceutical care group had the highest percentage of patients who accessed healthcare not covered by their public insurance (37.9, 7.9, and 0% for patients receiving pharmaceutical care, home visit, and SAT, respectively).
Selection of study participants. TB tuberculosis, SAT self-administered therapy, DOT directly observed therapy. 1Previously, Songkhla Hospital adopted the modified DOT (a combination of short-course DOT and home visit) as an adherence enhancement strategy. However, given the change in treatment policy in October 2013, most patients had SAT instead of receiving modified DOT; therefore, the recruitment period was extended until May 2014 in order to observe the performance of the hospital’s new policy and recruit more patients
Treatment success in 104 patients receiving pharmaceutical care, home visit, and SAT was 100, 97.37, and 83.78%, respectively (Table 2). However, when only focusing on the 72 new smear-positive patients, the success rates were 100, 95.80, and 80.95% for patients receiving pharmaceutical care, home visit, and SAT, respectively. The WHO sets the treatment success target for new smear-positive pulmonary TB patients as 85% [35]. One patient (2.6%) in the home visit group died, while in the SAT group, two patients (5.4%) died and four (10.8%) defaulted on treatment. The average durations of the intensive phase were 2.5, 2.7, and 2.2 months, and 4.3, 5.5, 4.6 months in the continuous phase, in patients receiving pharmaceutical care, home visit, and SAT, respectively.
3.1 Out-of-Pocket Expenditure
The mean total OOP expenditures per patient were I$907.56 (CI I$603.80–I$1,269.41), I$148.47 (CI I$109.49–I$194.89), and I$95.35 (CI I$69.11–I$129.63) for those receiving pharmaceutical care, home visit, and SAT, respectively (Table 3). The median results are reported in Online Appendix Table 3. The main expense across all treatment strategies was transportation costs (Fig. 2). The differences in mean total OOP expenditures between the treatment strategies when adjusted for baseline characteristics were investigated by the GLMs. The model performance assessment using the AICs (Online Appendix Table 4) and graphical analyses (Online Appendix Fig. 1) indicated a strong preference for the gamma model and the log link. After adjustment for baseline characteristics (Table 4), there were statistically significantly lower OOP expenditures for patients receiving either home visit or SAT (ratio of mean costs 0.247, CI 0.142–0.427; and 0.318, CI 0.187–0.540, respectively), when compared with patients receiving pharmaceutical care.
In the pre-TB treatment period, the pharmaceutical care group incurred higher OOP expenditures (I$169.48, CI I$120.04–I$233.31) compared with those receiving either home visit (I$42.09, CI I$19.81–I$72.17) or SAT (I$19.11, CI I$13.52–I$26.71) (Table 3). OOP expenditures for the pharmaceutical care group were mostly driven by outpatient visits that were not covered by the patients’ health insurance (40.94%) and transportation costs (28.51%). Patients receiving home visit had the main expenses from hospitalizations that were not covered by health insurance (32.36%) and food supplements (30.94%). The largest expenses in patients receiving SAT were transportation (44.22%) and healthcare costs that occurred in private healthcare units (23.13%).
During the TB treatment period, the main OOP cost driver across all treatment strategies was transportation (I$278.53, CI I$173.94–I$400.67; I$57.15, CI I$43.81–I$72.55; and I$47.19, CI I$36.75–I$60.48 for those receiving pharmaceutical care, home visit, and SAT, respectively) (Table 3). In addition, patients in the pharmaceutical care group also had high expenses due to outpatient visits (24.63%) and hospitalizations (20.43%) that occurred outside the service area and were not covered by public health insurance. Patients in the pharmaceutical care group had higher OOP expenditures (I$738.07, CI I$458.96–I$1,095.58) compared with those in either the home visit or SAT groups (I$106.38, CI I$79.24–I$136.36; and I$76.24, CI I$50.90–I$109.50, respectively).
3.2 Indirect Costs Incurred by Patients
In the pre-TB treatment period, ten patients in the home visit group had lost, on average, a total of 37 work days, while five patients each in the SAT and pharmaceutical care groups reported a loss of 24 and 12 work days, respectively. In the TB treatment period, patients had lost a total of 107, 76, and 54 work days among 18, 10, and 12 patients who received home visit, SAT, and pharmaceutical care, respectively (Table 5).
Indirect costs were I$1925.68 (CI I$922.06–I$3,284.94), I$2393.66 (CI I$1435.01–I$3501.98), and I$833.33 (CI I$453.87–I$1,263.45) for patients receiving pharmaceutical care, home visit, and SAT, respectively (Table 5). The median results are reported in Online Appendix Table 5.
GLMs with an identity link, regardless of distribution, did not converge. The AICs (Online Appendix Table 4) and graphical analyses (Online Appendix Fig. 2) clearly showed that the gamma distribution with a log link was a better-performing model. After adjustment for baseline characteristics (Table 4), the ratios of mean costs were 1.904 (CI 0.754–4.802) and 0.792 (CI 0.289–2.175) for the home visit and SAT groups, respectively, when pharmaceutical care was set as the reference.
On average, OOP expenditures accounted for 7.93, 2.00, and 1.29% of the patient’s reported annual income, while indirect costs accounted for 12.35, 27.05, and 11.35% of the patient’s reported annual income for patients receiving pharmaceutical care, home visit, and SAT, respectively (Fig. 3).
3.3 Health-Related Quality of Life
When patients were followed until March 2015, a total of 104 and 90 patients completed the questionnaire at the end of the intensive and continuous phases, respectively. The overall study dropout rate was 13.5%.
The percentage of patients who had no problem with mobility at baseline and at the end of treatment were 72.4 and 84.6, 78.9 and 88.9, and 64.9 and 100% in the pharmaceutical care, home visit, and SAT groups, respectively. Ninety percent and almost 100% of all patients had no problem with self-care at baseline and at the end of treatment, respectively. At baseline, 60% of all patients had no problem with usual activities, increasing to more than 80% at the end of treatment. Also at baseline, 10.3, 5.3, and 5.4% of patients receiving pharmaceutical care, home visit, and SAT, respectively, had extreme pain or discomfort. Four patients (10.5%) and one patient receiving home visit and SAT, respectively, also reported extreme anxiety or depression, whereas at the end of treatment, no patients reported either extreme pain/discomfort or anxiety/depression. The mean EQ-5D scores at baseline and at the end of treatment were 0.679 and 0.830, 0.713 and 0.905, and 0.708 and 0.913 (Online Appendix Table 6).
Table 6 shows the differences in paired mean scores between the beginning and end of treatment. All three treatment strategies had significant increases in utility scores at the end of treatment (0.169, CI 0.028–0.310; 0.185, CI 0.115–0.255; and 0.176, CI 0.093–0.258) for the pharmaceutical care, home visit and SAT groups, respectively). Home visit resulted in the highest difference in paired mean EQ-5D and VAS scores.
4 Discussion
Although patients’ access to TB care is free of charge in Thailand, this prospective study highlights the financial burden imposed by TB on patients and society. Mean OOP expenditure in pharmaceutical care was I$907.56, which was higher than the mean direct costs reported in the systematic literature review on the financial burden of TB borne by patients and their families in low- and middle-income countries (mean I$296.80 [SD I$376.00]) [3]. Productivity loss was a large contributor to the financial burden. Mean indirect costs incurred by patients receiving all three strategies (I$1925.68, I$2393.66, and I$833.33 for patients receiving pharmaceutical care, home visit, and SAT, respectively) exceeded mean indirect costs reported in the systematic literature review (mean I$450.80 (SD I$553.40), range I$29.80–I$2184.00) [3]. All three strategies were associated with a significant improvement in the HRQoL of patients. The financial burden varied markedly across different treatment strategies due to the influence of several factors, such as public insurance coverage, socioeconomic status, disease severity, and distance to TB services.
The pharmaceutical care group incurred the highest OOP expenditures, which is not surprising as many patients came from other provinces. In order to seek TB treatments, these patients incurred sixfold higher transportation costs compared with the other two strategies. In addition, 38% of these patients paid their medical care themselves because the healthcare at the study hospital was outside their service area. Consequently, costs due to outpatient visits and hospitalizations became the second largest contributor to OOP expenditures among these patients. This issue has been discussed in our previous retrospective cohort study [19].
People with TB-related symptoms may pay for seeking care in a private unit prior to a diagnosis of TB being made by a public healthcare provider. In all treatment strategies, average costs due to visiting private facilities prior to TB treatment were much higher than those incurred during the TB treatment period. Prior to a TB diagnosis, a larger proportion of patients receiving pharmaceutical care make a visit to private facilities, presumably due to the higher average income in this group. After a TB diagnosis, patient costs were much lower because the healthcare costs were mostly covered under patients’ health insurance.
Indirect costs affect both patients and society. Income loss due to TB affects patients directly, while production loss due to time seeking treatment, as well as illness and disability, also affect society. A large proportion of patients receiving home visit and SAT had no fixed income, and an absence from work due to seeking treatment or illness resulted in income loss. Productivity loss posed a substantial financial burden on patients receiving home visit due to the high proportion of patients who were too sick to work and those who were unemployed because of TB. The home visit group had the highest number of hospitalized patients, and one possible explanation for this may be that the home visit study hospital was the main provincial referral hospital, under the Ministry of Public Health, that generally has higher numbers of TB patients and more severe cases. The other reason may be the lower education level in patients receiving home visit compared with those receiving pharmaceutical care. A previous study in Thailand indicated that lower education levels were associated with poorer TB knowledge [36]. These patients may be more likely to delay visiting a hospital and choose other options instead (e.g. purchasing medications from drug stores, visiting community health centers). In such cases, disease is likely to be more advanced before hospital care begins.
The systematic literature review indicated that 50% of the total costs to patients were incurred prior to TB treatment [3]. The financial burden incurred between pre-diagnosis and the first 2 months of TB treatment may delay TB diagnosis, lead to spread of the disease in the community, and become a barrier to treatment adherence [3]; therefore, the changes in public healthcare services could be implemented. For example, providing overtime services could increase accessibility and reduce OOP expenditures; the use of effective referral procedures in community healthcare services could support early TB diagnosis and reduce possible productivity loss due to TB illness. In addition, the use of a fast and precise diagnostic test could control transportation costs and work time loss incurred by patients.
At baseline, TB impaired the HRQoL of patients in both physical and psychological domains across all five EQ-5D attributes. TB treatment, under all three strategies, was associated with significantly improved quality of life over time. Our findings demonstrated the longitudinal changes in the quality of life of TB patients over at least 6 months, which has been rarely reported. Most studies on the HRQoL of TB patients have been based on a cross-sectional design [4]. Of the seven studies with a prospective longitudinal design that assessed quality of life during the various stages of treatment, most had a follow-up of <6 months. Only two of the seven studies (one from China and the other from Malaysia) followed patients until treatment was completed, and reported improvement in patients’ quality of life over time; however, both used a different non-utility-based quality-of-life measure—the SF-36 [37, 38]. Furthermore, only one study used the SF-36 alongside the EQ-5D, but the change in health status was only investigated at the beginning of treatment and at 2 months [39].
Nevertheless, there were some limitations in our study. Given its nature, a prospective cohort study generally follows patients for a long time, which can lead to dropouts and consequently requires a large initial sample size. This prospective study had a relatively small sample size. Due to both the long treatment duration of TB and the time constraints, recruiting a large number of TB cases was very challenging. Thus, findings that indicate treatment success in patients receiving SAT below the WHO’s target of 85% [35] are highly preliminary and need to be confirmed by a future, large-scale, prospective study.
Financial burden varied markedly across different treatment strategies due to several factors, such as public insurance coverage, socioeconomic status, disease severity, and distance to TB services. In particular, the socioeconomic characteristics appear to differ substantially, which is not surprising since the three groups came from different areas of the province. In addition, the willingness of non-local patients to pay out of their own pockets to seek TB care at the university hospital outside their district may be atypical in other settings. Thus, caution should be exercised when generalizing the research findings to other populations.
Another limitation is related to the long treatment duration of TB. We initially planned to follow our participants for at least 6 months or until the treatment course was completed. Eventually, the interview was stopped in March 2015. In addition, although we followed the treatment outcomes for almost 2 years, two patients who had developed MDR-TB were still going through treatment at the end of December 2015. Lastly, a very small number of MDR-TB cases were included in the analysis. Research findings from this study were derived from mostly drug-susceptible pulmonary TB patients. Caution should be exercised when generalizing the research findings to other populations with a high proportion of MDR-TB.
5 Conclusions
Patients receiving pharmaceutical care experienced the highest OOP expenditures compared with those receiving home visit and SAT. Patients receiving home visit had the highest indirect costs, as well as the highest improvement in utility scores. Differences in patient characteristics among patients receiving different strategies had a high impact on the large variation in financial burden. A large-scale prospective study is required in order to strengthen evidence to support policy making regarding the most efficient use of limited resources for TB management.
References
World Health Organization (WHO). The Stop TB initiative: the economic impacts of tuberculosis. Amsterdam: Ministerial Conference; March 2000.
World Health Organization (WHO). Tuberculosis. 2016. http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed 3 Dec 2015.
Tanimura T, Jaramillo E, Weil D, Raviglione M, Lonnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J. 2014;43(6):1763–75.
Brown J, Capocci S, Smith C, Morris S, Abubakar I, Lipman M. Health status and quality of life in tuberculosis. Int J Infect Dis. 2015;32:68–75.
Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcomes. 2009;7:14-7525-7-14.
World Health Organization (WHO). Eliminating the financial hardship of TB via Universal Health Coverage and other Social Protection measures. 2013. http://www.who.int/tb/publications/UHC_SP_factsheet.pdf. Accessed June 2016.
World Health Organization (WHO). Global tuberculosis report 2015. 2015. http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf. Accessed 20 Dec 2015.
Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–801.
Tangcharoensathien V, Patcharanarumol W, Chitpranee,Prakongsai P, Jongudomsuk P, Srithamrongsawat S, et al. Thailand Health Financing Review 2010. 2010. http://ssrn.com/abstract=1623260. Accessed 10 June 2016.
World Health Organization (WHO). Treatment of tuberculosis: guidelines. 4th ed. 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf. Accessed 10 Sep 2013.
Pungrassami P, Johnsen SP, Chongsuvivatwong V, Olsen J, Sorensen HT. Practice of directly observed treatment (DOT) for tuberculosis in southern Thailand: comparison between different types of DOT observers. Int J Tuberc Lung Dis. 2002;6(5):389–95.
Khan MA, Walley JD, Witter SN, Imran A, Safdar N. Costs and cost-effectiveness of different DOT strategies for the treatment of tuberculosis in Pakistan. Directly Observed Treatment. Health Policy Plan. 2002;17(2):178–86.
Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev 2015;(5):CD003343. doi:10.1002/14651858.CD003343.pub4.
Grange JM, Zumla A. Making DOTS succeed. Directly Observed Treatment, Short Course. Lancet. 1997;350(9072):157.
Ormerod LP. Directly observed therapy (DOT) for tuberculosis: why, when, how and if? Thorax. 1999;54(Suppl 2):S42–5.
World Bank. Inflation, consumer prices (annual %) in the World Bank Database. 2015. http://data.worldbank.org/indicator/FP.CPI.TOTL.ZG?end=2014&locations=TH&start=2011. Accessed 11 June 2016.
World Bank. PPP conversion factor, private consumption (LCU per international $) in the World Bank Database. 2015. http://data.worldbank.org/indicator/PA.NUS.PRVT.PP?end=2015&locations=TH&start=2011. Accessed 11 June 2016.
Tanvejsilp P, Pullenayegum E, Loeb M, Dushoff J, Xie F. Role of pharmaceutical care for self-administered pulmonary tuberculosis treatment in Thailand. J Clin Pharm Ther. 2017;42(3):337–44.
Tanvejsilp P, Loeb M, Dushoff J, Xie F. Healthcare resource uses and out-of-pocket expenses associated with pulmonary TB treatment in Thailand. PharmacoEconomics Open. 2017. doi:10.1007/s41669-017-0053-0.
Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics. 2013;31(7):537–49.
EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
EuroQol Group. EQ-5D-3L self-complete version on paper. 2016. http://www.euroqol.org/eq-5d-products/eq-5d-3l/self-complete-version-on-paper.html. Accessed 26 Dec 2013.
Sakthong P. Measurement of clinical-effect: utility. J Med Assoc Thai. 2008;91(Suppl 2):S43–52.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72.
Tongsiri S, Cairns J. Estimating population-based values for EQ-5D health states in Thailand. Value Health. 2011;14(8):1142–5.
Ministry of Labour. Wage committee announcement on minimum wage rate (No.7) and Explanation and Summary of minimum wage rate 2013, entered into force 1 January 2013. 2015. http://www.mol.go.th/sites/default/files/downloads/pdf/Wage_2013_Eng.pdf. Accessed 11 June 2016.
Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320(7243):1197–200.
Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19(23):3219–36.
Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9(4):197–204.
Dodd S, Bassi A, Bodger K, Williamson P. A comparison of multivariable regression models to analyse cost data. J Eval Clin Pract. 2006;12(1):76–86.
Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59–68.
Moran JL, Solomon PJ, Peisach AR, Martin J. New models for old questions: generalized linear models for cost prediction. J Eval Clin Pract. 2007;13(3):381–9.
Gan FF, Koehler KJ, Thompson JC. Probability plots and distribution curves for assessing the fit of probability models. Am Stat. 1991;45:14–21.
IBM Corp. IBM SPSS Statistics for Windows, Version 23.0. 2015.
World Health Organization (WHO). Global tuberculosis report 2013. 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed 20 Dec 2015.
Chimbanrai B, Fungladda W, Kaewkungwal J, Silachamroom U. Treatment-seeking behaviors and improvement in adherence to treatment regimen of tuberculosis patients using intensive triad-model program, Thailand. South Asian J Trop Med Public Health. 2008;39:526–41.
Chamla D. The assessment of patients’ health-related quality of life during tuberculosis treatment in Wuhan, China. Int J Tuberc Lung Dis. 2004;8(9):1100–6.
Atif M, Sulaiman SA, Shafie AA, Asif M, Sarfraz MK, Low HC, et al. Impact of tuberculosis treatment on health-related quality of life of pulmonary tuberculosis patients: a follow-up study. Health Qual Life Outcomes. 2014;12:19-7525-12-19.
Kruijshaar ME, Lipman M, Essink-Bot ML, Lozewicz S, Creer D, Dart S, et al. Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis. 2010;14(3):296–302.
Acknowledgements
The authors thank Dr. Eleanor Pullenayegum for her guidance with the statistics used in this study. A very special gratitude goes out to Siwakarn Sukma, Udomrut Chauychana, Deeya Mungmee, Ammorn Datbamrung, and all other hospital staff at TB clinics in Songklanagarind, Hatyai, and Songkhla Hospitals, Thailand, for helping with and providing all support in the data collection process.
Availability of Data and Material
The datasets generated and analyzed during the current study are not publicly available due to protecting participant confidentiality, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors developed the concept and design of the study; PT performed the data collection, analyzed the data, and prepared the manuscript; FX, ML, and JD performed critical revision of the manuscript.
Corresponding author
Ethics declarations
Pimwara Tanvejsilp is supported through the Office of the Higher Education Commission, Thailand, under the Strategic Scholarships Fellowships Frontier Research Networks (Specific for Southern region) program.
Conflict of interest
Pimwara Tanvejsilp, Mark Loeb, Jonathan Dushoff, and Feng Xie have no conflicts of interest to declare that are directly relevant to the content of this article.
Ethical approval
This study was approved by the Research Ethics Board at each of the participating hospitals.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tanvejsilp, P., Loeb, M., Dushoff, J. et al. Out-of-Pocket Expenditures, Indirect Costs and Health-Related Quality of Life of Patients with Pulmonary Tuberculosis in Thailand. PharmacoEconomics Open 2, 281–296 (2018). https://doi.org/10.1007/s41669-017-0057-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-017-0057-9