Abstract
Background
Ovarian cancer is a leading cause of cancer-related mortality. Although the disease is relatively rare, it carries a disproportionately large morbidity burden.
Objective
We conducted a cost-utility analysis from a Canadian public payer perspective to determine the cost effectiveness of bevacizumab, a newly available treatment option for recurrent ovarian cancer.
Methods
Using a 7-year time horizon, a three health-state cohort-based partitioned survival model was developed to assess the cost utility of bevacizumab plus chemotherapy (BEV) versus chemotherapy alone. We reconstructed individual patient data from published Kaplan–Meier curves. Clinical parameters, including progression-free survival and overall survival, were derived from the AURELIA phase III randomized controlled trial. Costs, resource utilization and utility values from recent Canadian sources were used to populate the model. Results were presented using incremental cost-utility ratios (ICURs). Uncertainty was examined through univariate and probabilistic sensitivity analyses.
Results
The reconstructed individual patient data matched the AURELIA trial results. Total costs for the BEV and chemotherapy treatment arms were $Can79,086 and $Can54,982, respectively. Total estimated quality-adjusted life-years (QALYs) were 1.1055 and 0.9926 for the BEV and chemotherapy arms, respectively. The ICUR was estimated to be $Can213,424 per QALY gained. At a willingness-to-pay threshold of $Can100,000 per QALY gained, the probability of BEV being cost effective was 0.
Conclusions
The results of our analysis suggest that the addition of bevacizumab to single-agent chemotherapy treatment, while improving patient outcomes, is unlikely to be cost effective in this Canadian patient population. The results also provide some preliminary validation for use of individual patient data-reconstruction techniques in pharmacoeconomic evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Very few therapeutic options are available for women with recurrent ovarian cancer, particularly in the platinum-resistant setting. |
This economic evaluation, which incorporated the results of the AURELIA clinical trial, found that bevacizumab plus chemotherapy may not be a cost-effective option for treating patients with platinum-resistant recurrent ovarian cancer in Canada. |
In addition, this study provides some initial validation of recently developed individual patient data digitization and reconstruction techniques for use in economic evaluation. |
1 Introduction
Ovarian cancer is a devastating disease that carries a substantial economic and patient burden. The prognosis for ovarian cancer is poor, typically around 12 months, and the risk of recurrence following primary therapy is between 60 and 70% [1]. Each year around the world, an estimated 230,000 cases of ovarian cancer are diagnosed [2]. Ovarian cancer results in approximately 150,000 deaths per year; more than any other gynaecological cancer [2]. In Canada, ovarian cancer has been estimated to affect approximately one in every 71 women [3]. Approximately 2800 women were estimated to develop ovarian cancer in 2015, and an estimated 1750 Canadian women died from ovarian cancer in 2015 [3]. The platinum-free interval is a strong predictor of treatment success in recurrent ovarian cancer, as nearly all patients with recurrent disease eventually develop platinum resistance [1]. Platinum-resistant disease is that which has relapsed within 6 months after receiving platinum-containing therapy. Approximately 85% of patients who achieve full remission after completion of first-line therapy will develop recurrent disease [4].
Bevacizumab is a recombinant humanized monoclonal antibody that selectively binds to and neutralises the biologic activity of vascular endothelial growth factor, reducing the vascularisation of tumours, thereby inhibiting tumour growth [5]. Bevacizumab has been approved for indications including, among others, advanced stages of colorectal cancer, non-small-cell lung cancer, kidney cancer and platinum-resistant ovarian cancer in the USA, Europe and Japan [5, 6]. In Canada, bevacizumab has received marketing authorization for the treatment of metastatic colorectal cancer, locally advanced metastatic or recurrent non-small-cell lung cancer, platinum-sensitive recurrent ovarian cancer and platinum-resistant recurrent ovarian cancer and has received a notice of compliance with conditions for malignant glioma [5]. Although an economic evaluation of bevacizumab as a front-line treatment for patients with stage III suboptimal de-bulking, stage III unresectable or stage IV epithelial ovarian cancer in Canada was previously conducted based on the results of the ICON7 (International Collaboration on Ovarian Neoplasms 7) clinical trial [7], the cost effectiveness of bevacizumab for treatment of advanced recurrent ovarian cancer in Canada is unknown. This paper presents a cost-effectiveness analysis conducted to compare, from the Canadian public payer perspective, bevacizumab plus chemotherapy (BEV) versus chemotherapy alone in patients with platinum-resistant recurrent ovarian cancer based on clinical data from the AURELIA trial.
2 Methods
The purpose of this economic analysis was to analyse the expected costs, effects and cost effectiveness of currently available pharmacotherapeutic options for managing patients with platinum-resistant recurrent ovarian cancer from a Canadian healthcare system perspective based on the results of the AURELIA trial. An economic evaluation was conducted to determine the cost effectiveness of bevacizumab plus pegylated liposomal doxorubicin, paclitaxel or topotecan versus these single-agent chemotherapy regimens alone.
2.1 Model Overview
A 7-year partitioned survival model using 1-month cycle lengths was developed in Microsoft® Excel with three health states: alive with no progression (pre-progression health state), alive with disease progression (post-progression health state) and death (Fig. 1). The model was populated using clinical estimates derived from reconstructed Kaplan–Meier (KM) curve data from the AURELIA trial. All patients entered the model through the pre-progression health state and could stay in this health state or transition either to the post-progression health state or to death according to transition probabilities calculated from reconstructed KM curves from the AURELIA trial. The progression-free survival (PFS) and overall survival (OS) curves were used to determine the distribution of patients in the ‘pre-progression’ health state over time and the proportion of patients that transition to the ‘death’ health state for each treatment arm, respectively. The difference between the OS curve and the PFS curve provided the proportion of patients experiencing progressive disease. The primary outcome of the analysis was calculated as the incremental cost per quality-adjusted life-year (QALY) gained. Univariate and probability sensitivity analyses were conducted to examine uncertainty in the data.
As recommended by the current Canadian reimbursement guidelines for oncology products, the analysis was undertaken from a public payer perspective [8]. Pharmacoeconomic modelling guidelines recommend running models until 99% of the simulated patients have died. In the AURELIA clinical trial, most patients had died after 4 years. To account for the unknown prognosis of patients who were alive at the time of data cut-off for the final OS analysis in AURELIA, and to be reasonably sure that no simulated patients remained alive in the cohort, a 7-year time horizon was assumed in the base-case analysis. Costs and outcomes were discounted at 5% annually as per Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines [8].
2.2 Clinical Inputs
The modelled population was based on the population described in the AURELIA trial, which included patients with platinum-resistant recurrent ovarian cancer.
The AURELIA trial was an open-label phase III clinical trial evaluating bevacizumab-containing therapy versus chemotherapy alone in the platinum-resistant setting. The primary endpoint was investigator-assessed PFS, defined as the interval between random assignment and first radiologically documented disease progression or death, whichever occurred first [1]. Eligible patients had histologically confirmed epithelial ovarian, fallopian tube or primary peritoneal cancer that had progressed within 6 months of completing four or more cycles of platinum-based therapy [1]. The vast majority of patients—157/182 (86%) and 167/179 (93%) patients in the chemotherapy alone and BEV arms, respectively—had cancer originating in the ovaries [1]. The results of the AURELIA trial have been described in detail elsewhere [1]. Briefly, 361 patients were enrolled in AURELIA, 182 in the chemotherapy-only arm and 179 in the BEV arm. Median patient age was 61 years in the chemotherapy-only arm and 62 in the BEV arm. A large majority of patients in both arms had serious/adenocarcinoma tumour types. The primary endpoint of the trial was PFS, and results indicated a median PFS of 6.7 months in the BEV arm compared with 3.4 months in the chemotherapy-only arm (two-sided stratified log-rank test P < 0.001). Median OS for the BEV and chemotherapy arms was reported as 16.6 and 13.3 months, respectively, but the difference was not statistically significant (unstratified log-rank test P < 0.174).
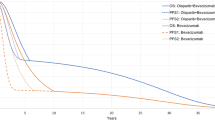
Transition probabilities were derived from digitizing and reconstructing individual-level patient data from OS and PFS curves in AURELIA [1] (Table 1). Transition probabilities were derived using a method described by Diaby et al. [9] and analysed using the statistical package R. A detailed description of the method is presented in Appendix A in the Electronic Supplementary Material (ESM). For both the chemotherapy-only and the BEV treatment arms in the OS analysis, the log-logistic distribution was found to best fit the reconstructed data based on statistical tests and confirmed through visual inspection (Fig. 2). For both the chemotherapy only and the BEV treatment arms in the PFS analysis, the lognormal distribution was found to best fit the reconstructed data (Fig. 2). Alternative distributions considered in the analysis are presented in Appendices C, D, E and F in the ESM. For the chemotherapy arm, the proportion of patients receiving each of the three single agents, respectively, was assigned according to the patient proportions reported in the AURELIA trial: 34.9% received pegylated liposomal doxorubicin, 31.9% received paclitaxel, and 33.2% received topotecan.
Fitted parametric distributions for chemotherapy and bevacizumab plus chemotherapy treatment arms: overall survival (log-logistic) and progression-free survival (lognormal). Left-hand column presents fitted curves for chemotherapy treatment arm, right-hand column presents fitted curves for bevacizumab plus chemotherapy arm. Black lines denote reconstructed trial data, black dotted lines indicate 95% confidence intervals. Red lines indicate fitted curves using two standard parametric distributions, 95% confidence intervals are represented by red dotted lines
Adverse events were modelled according to the adverse event rates reported in the AURELIA trial for each treatment arm. Only those adverse events of grade 3 or higher were modelled. In the chemotherapy-only arm, neutropenia, fatigue, leukopenia, abdominal pain and vomiting were present in ≥5% of patients, whereas, in the BEV arm, only neutropenia was observed in ≥5% patients.
2.3 Healthcare Resource Use and Costs
Unit costs of medications, healthcare resource utilization and costs relating to management of adverse events were taken from publically available sources, including the Ontario Schedule of Physician Benefits and the published Canadian oncology literature [9,10,11,12,13,14] (Table 1). The weekly cost of supportive care for the pre-progression and post-progression health state was taken from a recent Canadian cost-effectiveness analysis of bevacizumab [15]. The cost of adverse events used in model calculations was estimated by multiplying the proportion of patients with adverse events in each of the two treatment arms as reported in the AURELIA trial by the unit cost of each adverse event. These were then summed across each of the two treatment arms to give an average cost of management for each reported adverse event (Table 1). Patients in the BEV arm of the model received chemotherapy plus BEV treatment for six cycles, and patients in the chemotherapy arm received chemotherapy treatment for three cycles in accordance with the median treatment duration reported in AURELIA [1]. All unit costs were inflated, as necessary, to 2016 Canadian dollars ($Can) using the health and personal care component of the Canadian consumer price index [16]. Total costs for each treatment arm were estimated over the time horizon of the model by aggregating the cycle-specific costs associated with treatment, healthcare resource utilization and management of adverse events according to the number of patients in each health state.
2.4 Health State Utilities
A targeted literature search was conducted to identify health state utility values associated with ovarian cancer. Canadian-specific estimates were derived from a recent study that estimated health state utilities based on the time trade-off method according to the stage of ovarian cancer [17]. The utility decrement associated with disease progression was assumed to be 0.04, the difference between stage 2 (0.81) and stage 3 (0.77) ovarian cancer utility estimates. For each model cycle, the proportion of patients in each health state were multiplied by the health state-specific utility value to estimate the cycle-specific number of QALYs. Total QALYs for each treatment arm were estimated by summing QALYs across each cycle over the time horizon of the model. No utility decrements were associated with adverse events in the model.
2.5 Sensitivity Analyses
Uncertainty surrounding input parameter values was addressed by conducting deterministic sensitivity analyses in which one input parameter value was varied at a time. Base-case values were varied by ±20% and deterministic incremental cost-effectiveness ratios (ICERs) re-calculated. Probabilistic sensitivity analyses were undertaken to account for uncertainty in model assumptions by varying multiple parameter values simultaneously and running a Monte Carlo simulation on these values (1000 iterations were run). For utility and treatment efficacy parameter values, a beta distribution was assumed, whereas cost parameters were assumed to follow a gamma distribution. For transition probability parameter values, the Cholesky decomposition technique was used (Appendix B in the ESM).
2.6 Value of Information
Parameter uncertainty was also quantified using the population expected value of perfect information (EVPI) for future research. To estimate the per-patient EVPI, first the mean net benefit was calculated over all of the Monte Carlo simulations in the probabilistic sensitivity analysis. Next, the net benefit for each individual Monte Carlo simulation in each of the treatment arms was calculated, and the maximum net benefit across the treatment arms for each simulation was identified. The mean of these maximized net benefit values was then taken. Finally, the difference between the mean of the maximized net benefits and the maximum of the mean values was calculated. The EVPI was estimated to explore the value of conducting future research given a willingness-to-pay ceiling ratio of $Can100,000 per QALY gained and was calculated by multiplying per-patient EVPI by the effective population. The estimated 2015 incidence of ovarian cancer [3] multiplied by the proportion of patients with recurrent ovarian cancer [4] yielded an effective per annum population of 2380. In addition, expected value of perfect partial information (EVPPI) analyses were conducted to identify specific parameters for which additional data collection may be worthwhile.
3 Results
3.1 Base Case
The results of the KM curve digitization, individual patient-level data reconstruction and curve-fitting procedures were compared with the original results from the AURELIA clinical trial in terms of median OS, median PFS, number of events and hazard ratio. The results from the reconstructed curves were broadly consistent with those from AURELIA (Table 2).
Over the 7-year time horizon, total costs were estimated to be $Can79,086 for the BEV treatment arm and $Can54,982 for the chemotherapy-only treatment arm. The BEV and chemotherapy-only treatment arms generated 1.1055 and 0.9926 QALYs, respectively. The incremental cost per QALY gained when using BEV compared with chemotherapy alone was estimated to be $Can213,424 in the base-case analysis. To obtain an ICER of $Can100,000 per QALY gained, a commonly cited threshold in oncology, the unit price of bevacizumab per milligram would have to be reduced by 39%.
3.2 Sensitivity Analyses
Results from the deterministic sensitivity analysis are presented in the form of a tornado diagram (Fig. 3). The centre line in the diagram represents the base case. The ICER was most sensitive to the unit cost of bevacizumab, the discount factor for both costs and outcomes, and the time horizon.
Deterministic sensitivity analysis results (tornado diagram). Results of the one-way sensitivity analysis in which several model input parameters were varied to determine their effect on output. Blue bars represent the base-case input parameter values minus 20%, the red bars represent base case input parameter values plus 20%. The horizontal axis represents the incremental cost-effectiveness ratio value. QALY quality-adjusted life-year
Results of the probabilistic sensitivity analysis are presented in the form of cost-effectiveness acceptability curves in Fig. 4. The results indicated that BEV is not cost effective at either $Can50,000 or $Can100,000 per QALY gained, two commonly cited threshold values. At a probability of 0.5, the ICER was $Can213,424 per QALY gained.
3.3 Value of Information
The population EVPI is presented in Fig. 5. The EVPI was estimated to be $Can804,818 at a willingness-to-pay threshold of $Can100,000 per QALY gained. Further research may be worth conducting up to a maximum expected cost of $Can804,818. Results of the EVPPI analyses showed EVPPI to be the highest for the OS parameter values, suggesting that additional data collection could be worthwhile for these parameters.
Expected value of perfect information analysis (population). Line graph presenting the returns to future research estimated through the value-of-information analysis. The figure depicts the expected value of perfect information at various willingness-to-pay thresholds. EVPI expected value of perfect information, QALY quality-adjusted life-year
4 Discussion
Given the need for new treatment options among patients with recurrent ovarian cancer who have developed platinum resistance and the rapid increases in the cost of cancer therapies, an economic evaluation is a practical means of quantifying the value and benefits of bevacizumab use in this setting.
AURELIA was an open-label phase III clinical trial. While open-label trials may introduce bias that might not be encountered in a double-blinded trial design, there are compelling ethical reasons for using an open-label design in the area of oncology, particularly among terminally ill patients. The results of the present study, based on the open-label AURELIA trial data, should be considered valid for this patient population.
The base-case results of the present cost-effectiveness analysis suggested that BEV is cost effective only if payers are willing to pay more than $Can200,000 per QALY gained. While there is no explicit cost-effectiveness threshold in Canada, a willingness-to-pay threshold of approximately $Can100,000 per QALY gained has been suggested to be weak evidence for adoption or appropriate utilization [18]. In addition, oncology products with baseline ICERs above $Can100,000 per QALY gained tend to receive a negative recommendation from the pan-Canadian Oncology Drug Review (pCODR) or receive a positive recommendation only on condition of improved cost effectiveness [19]. On the basis of this apparent de facto cost-effectiveness threshold of approximately $Can100,000 per QALY gained, BEV may not be considered cost effective for treatment of patients with platinum-resistant recurrent ovarian cancer in Canada. The model was sensitive to the unit cost of bevacizumab, the discount factors for cost and outcomes and the time horizon. The model was relatively insensitive to other input parameters.
One of the primary factors contributing to the baseline ICER value in this study is the unit cost of the study drug. Bevacizumab commands a much higher price than other chemotherapy agents used for treatment of advanced recurrent ovarian cancer in Canada. At the same time, the total gain in QALYs from using BEV is only moderately higher than the QALYs gained from treating patients with chemotherapy alone. This relatively small incremental-effectiveness benefit yields a proportionately small denominator of the ICER, which in turn results in a relatively large ICER value.
A recent cost-effectiveness analysis by Ghatage et al. [15] focusing on ovarian cancer in Canada estimated the ICER of BEV versus chemotherapy alone to be $Can95,942 in the base case. While this is considerably less than the estimate reported in the present study, the population from this publication was taken from the ICON7 phase III clinical trial, which examined BEV versus chemotherapy alone as first-line treatment among patients with high-risk or advanced-stage ovarian cancer (stage III suboptimal de-bulking, stage III unresectable or stage IV). As the patients in ICON7 were receiving first-line therapy, the baseline characteristics of the population were also quite different in AURELIA. The specific chemotherapy treatment therapies examined also differed and are associated with a different place in therapy than those investigated in AURELIA. Patients with stage III and stage IV disease are also associated with different utility values, which can dramatically affect ICER estimates. In addition, the utility decrement in the present analysis due to disease progression was assumed to equal the difference (0.04) between the reported utility of stage II disease (0.81) and stage III disease (0.77), whereas Ghatage et al. [15] reported the utility of progression at 0.64. As a result, the reported PFS and OS estimates from ICON7 were different from estimates from the AURELIA trial; therefore, similar ICERs would not be expected.
Using the results of the AURELIA trial, a conference abstract was recently published in which a decision-tree-based cost-effectiveness analysis was undertaken from a US public payer perspective [20]. This study reported an ICER of $US285,624 per QALY gained over a 15-month time horizon. Applying the same time horizon in the present model resulted in an ICER of $Can516,652 per QALY gained, or approximately $US400,000 per QALY gained at current exchange rates. Differences in the results of the present study compared with the abstract in question could be at least partially accounted for by differences in modelled treatment patterns, possible inclusion of different utility values and different costs of managing adverse events between the USA and Canada. In addition, the specific cost components included in the US study were not identifiable from the published abstract alone.
4.1 Limitations
While the number of events and hazard ratios estimated from the reconstructed individual patient-level data in this study closely matched those reported in the AURELIA trial, median OS and median PFS estimates in the BEV arm were less precise, varying by approximately 0.9 and 0.3 months, respectively, compared with the trial. Though relatively small, these discrepancies could lead to inaccurate results. The analysis also assumed the per episode costs of adverse event management for leukopenia were equal to those for neutropenia. This assumption may have biased the results, though the impact of this assumption is expected to be minimal.
Patient-reported outcomes, the secondary endpoint of the AURELIA trial, were assessed in a separate publication using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Ovarian Cancer Module 28 (EORTC QLQ-OV28) [21]. The authors of this separate study concluded that the addition of bevacizumab to single-agent chemotherapy increased the proportion of patients achieving a 15% improvement in patient-reported abdominal and gastrointestinal symptoms. However, since patient-reported outcomes are not standardized, and because the outcomes reported in this separate study have not been mapped to a generic utility instrument, the results of the study were not amenable to inclusion in a cost-effectiveness analysis.
Although this analysis used Canadian-specific utility values, the decrement due to progressive disease was assumed to be the difference between stage 2 and stage 3 ovarian cancer as reported in Howel et al. [17]. The calculated decrement was therefore small (0.04) and may not reflect the true disutility associated with progressive disease. Uncertainty modelled in the sensitivity analyses was also introduced at ±20% of base-case values. This approach was used because the availability of uncertainty data was limited and access to experts was lacking; as such, it does not reflect the true uncertainty associated with the input values. In addition, the assumption that no utility decrements were associated with adverse events is, while clinically implausible, a conservative assumption since BEV was shown to be associated with higher adverse event rates than chemotherapy alone in the safety profile from the AURELIA trial. However, the expected impact of these adverse events on the results of this economic evaluation would be minimal, as the adverse events are predominantly short lived and patients typically recover quickly [22].
Finally, generalizability may be limited for several reasons. First, the highly controlled environment of randomized controlled trials such as AURELIA may limit generalizability to the Canadian and other patient contexts. Second, this analysis was conducted from a Canadian healthcare system perspective, and the clinical data were derived from a single multinational randomized controlled trial. There may therefore be important differences in terms of demography, epidemiology, specific characteristics of the Canadian healthcare system, and healthcare resource availability that could limit generalizability of the results of the present study to other jurisdictions.
5 Conclusion
Based on the results of this economic analysis, BEV is not considered cost effective at willingness-to-pay thresholds below approximately $Can200,000 per QALY gained and therefore may not be a cost-effective treatment option for women with advanced recurrent ovarian cancer in Canada.
References
Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8.
World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence in Europe (EU 27) in 2012. Geneva: WHO; 2014.
Canadian cancer society's advisory committee on cancer statistics. Canadian cancer statistics 2015. Toronto: Canadian Cancer Society; 2015.
Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park). 2013;27(4):288–94, 298.
Health Canada. AVASTIN® [bevacizumab] product monograph. 2015.
Group Media Relations. Media Release. Hoffmann-La Roche: 15 August 2014. http://www.roche.com/dam/jcr:fe9d7850-6e1b-4b89-9c4a-84f975d000bc/en/med-cor-2014-08-15-e.pdf.
Chan JK, Herzog TJ, Hu L, et al. Bevacizumab in treatment of high-risk ovarian cancer—a cost-effectiveness analysis. Oncologist. 2014;19(5):523–7.
Canadian Agency for Drugs and Technologies in Health. Addendum to CADTH’s guidelines for the economic evaluation of health technologies: specific guidance for oncology products. Ottawa: CADTH; 2009.
Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32(2):101–8.
Ontario Ministry of Health and Long-Term Care (MOHLTC). Schedule of benefits for physician services under the health insurance act. Toronto, ON: MOHLTC; 2015.
Ontario Ministry of Health and Long-Term Care (MOHLTC). Schedule of benefits for laboratory services. Toronto, ON: MOHLTC; 1999.
Bernard LM, Verma S, Thompson MF, et al. A canadian economic analysis of US oncology adjuvant trial 9735. Curr Oncol. 2011;18(2):67–75.
Lathia N, Mittmann N, DeAngelis C, et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116(3):742–8.
Tam VC, Ko YJ, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20(2):e90–106.
Ghatage P, Wright E, Martin Nunez I, et al. Bevacizumab for front-line treatment of epithelial ovarian, fallopian tube or primary peritoneal cancer patients with high risk of relapse: a cost effective option for Canadian patients. ISPOR 20th Annual International Meeting, Philadelphia, PA, May 2015.
Statistics Canada. Canadian consumer price index. ON: Ottawa; 2016.
Howel H, Naik D, Su J, et al. Stage specific health utility index scores of Canadian cancer patients. J Clin Oncol. 2015;33(15 Suppl 1):abstr 6614.
Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146(4):473–81.
Samjoo I, Grima D. Insights into the pan-Canadian oncology drug review recommendations—three years after its inception. Value Health. 2014;17(3):A100.
Schaffer EM, Coles TM, Wysham WZ, et al. Adding bevacizumab to single-agent chemotherapy for the treatment of platinum-resistant recurrent ovarian cancer: a cost-effectiveness analysis of the aurelia trial. Value Health. 2015;18(7):A461.
Stockler MR, Hilpert F, Friedlander M, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32(13):1309–16.
Moore DC. Drug-induced neutropenia. Pharm Ther. 2012;41(12):765–8.
pan-Canadian Oncology Drug Review. pan-Canadian oncology drug review final economic guidance report. Bevacizumab for ovarian cancer. pCODR; 2015.
Attard CL, Maroun JA, Alloul K, et al. Cost-effectiveness of oxaliplatin in the adjuvant treatment of colon cancer in Canada. Curr Oncol. 2010;17(1):17–24.
Attard CL, Brown S, Alloul K, Moore MJ. Cost-effectiveness of folfirinox for first-line treatment of metastatic pancreatic cancer. Curr Oncol. 2014;21(1):e41–51.
Hannouf MB, Sehgal C, Cao JQ, et al. Cost-effectiveness of adding cetuximab to platinum-based chemotherapy for first-line treatment of recurrent or metastatic head and neck cancer. PLoS One. 2012;7(6):e38557.
Newton N, Goetghebeur M, Ouagari K. Costs related to adverse events in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors in Canada. Value Health. 2008;11(3):A62–3.
Ontario Ministry of Health and Long-Term Care. Ontario drug benefit program: dispensing fees. http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx. 2015.
Author information
Authors and Affiliations
Contributions
GB, FX and J-ET designed the study. GB and J-ET built the model and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Graeme Ball, Feng Xie and Jean-Eric Tarride have no conflicts of interest.
Funding
No funding was provided for this work.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ball, G., Xie, F. & Tarride, JE. Economic Evaluation of Bevacizumab for Treatment of Platinum-Resistant Recurrent Ovarian Cancer in Canada. PharmacoEconomics Open 2, 19–29 (2018). https://doi.org/10.1007/s41669-017-0030-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-017-0030-7