Abstract
Background
Evidence Review Groups (ERGs) critically appraise company submissions as part of the National Institute for Health and Care Excellence (NICE) Single Technology Appraisal (STA) process. As part of their critique of the evidence submitted by companies, the ERGs undertake exploratory analyses to explore uncertainties in the company’s model. The aim of this study was to explore pre-defined factors that might influence or predict the extent of ERG exploratory analyses.
Objective
The aim of this study was to explore predefined factors that might influence or predict the extent of ERG exploratory analyses.
Methods
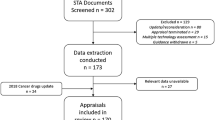
We undertook content analysis of over 400 documents, including ERG reports and related documentation for the 100 most recent STAs (2009–2014) for which guidance has been published. Relevant data were extracted from the documents and narrative synthesis was used to summarise the extracted data. All data were extracted and checked by two researchers.
Results
Forty different companies submitted documents as part of the NICE STA process. The most common disease area covered by the STAs was cancer (44%), and most ERG reports (n = 93) contained at least one exploratory analysis. The incidence and frequency of ERG exploratory analyses does not appear to be related to any developments in the appraisal process, the disease area covered by the STA, or the company’s base-case incremental cost-effectiveness ratio (ICER). However, there does appear to be a pattern in the mean number of analyses conducted by particular ERGs, but the reasons for this are unclear and potentially complex.
Conclusions
No clear patterns were identified regarding the presence or frequency of exploratory analyses, apart from the mean number conducted by individual ERGs. More research is needed to understand this relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As part of their critique of the evidence submitted by companies in the National Institute for Health and Care Excellence Single Technology Appraisal (STA) process, the Evidence Review Groups (ERGs) undertake exploratory analyses to explore uncertainties. |
Of the 100 STAs included in this analysis, 93 had exploratory analyses undertaken by the ERG. |
There is no clear pattern to the presence or frequency of exploratory analyses; these cannot be obviously explained by the disease area covered by the STA, the time the STA took place, or the company’s base-case incremental cost-effectiveness ratio. |
There may be a pattern in the mean number of analyses conducted by individual ERGs. |
1 Introduction
The UK National Institute for Health and Care Excellence (NICE) Single Technology Appraisal (STA) process is usually undertaken for a technology for a single indication and includes the production of a submission by the manufacturer or sponsor of the technology. NICE commissions independent Evidence Review Groups (ERGs) to critically appraise company submissions as part of the STA process. In their critique of the evidence submitted by companies, the ERGs often undertake exploratory analyses to explore uncertainties around the company’s model and their implications for decision making. The number and type of exploratory analyses undertaken varies between appraisals. The ERG reports are a central component of the evidence considered by the NICE Technology Appraisal Committees (AC) in forming their recommendations. The findings of the committee are used to produce the Appraisal Consultation Document (ACD) and, after further considerations and a consultation period, a Final Appraisal Determination (FAD) is produced, which results in NICE guidance. The STA process is outlined in detail in NICE’s Guide to the Process of Technology Appraisal [1]. The company is expected to follow the decision-analytic approaches as described in the Guide to the Methods of Technology Appraisal [2], and the submission is expected to contain an evaluation of the clinical effectiveness and cost effectiveness of the technology. It is the responsibility of the ERG to determine what additional analyses are required and to undertake them, although they may be constrained by the availability of data, the model structure, or time. The ERG may also identify and correct technical or programming errors. This critical appraisal of the company submission, and any additional work as a consequence of this critical appraisal, forms the basis of the ERG’s report. NICE’s remit to the ERGs is not overly prescriptive, allowing ERGs to use their acknowledged expertise and judgement in the methods used to critically appraise company submissions. This is appropriate due to the wide variation in complexity and quality of the company submissions received.
There are currently nine ERGs:
-
BMJ Technology Assessment Group (BMJ-TAG), BMJ Group.
-
Centre for Reviews and Dissemination (CRD)/Centre for Health Economics (CHE), University of York.
-
Health Economics Research Unit and Health Services Research Unit, University of Aberdeen.
-
Kleijnen Systematic Reviews Ltd.
-
Liverpool Reviews and Implementation Group (LRiG), University of Liverpool.
-
Peninsula Technology Assessment Group (PenTAG), Peninsula Medical School, University of Exeter.
-
School of Health and Related Research (ScHARR), University of Sheffield.
-
Southampton Health Technology Assessment Centre (SHTAC), University of Southampton.
-
Warwick Evidence, University of Warwick.
An additional ERG, the West Midlands Health Technology Assessment Collaboration, undertook STAs during the period 2005–2010. ERGs are assigned to work on STAs based on availability and lack of conflicts for specific topics.
Some assessment of ERG report production process has been undertaken. For example, Wong et al. [3] assessed approaches used by ERGs to critically appraise search strategies within company submissions. Previous research has highlighted issues with company submissions that are particularly challenging to the ERGs [4]. Carroll et al. [5] suggested that company STA submissions could be improved if attention were paid to transparency in the reporting, conduct and justification of the review, and modelling processes and analyses, as well as greater robustness in the choice of data in the model and closer adherence to the scope or decision problem. Kaltenthaler et al. [6] also recommended the need for clear and transparent reporting of company submissions, and for a clear and concise rationale for the synthesis of clinical data, the development of economic models and the assumptions used to develop models.
The aim of this study was to note the number of exploratory analyses undertaken by the ERGs within the NICE STA process and to assess whether their frequency might be explained by variables such as disease area or a company’s base-case incremental cost-effectiveness ratio (ICER). For the purpose of this research, an exploratory analysis was defined as any additional analysis that generated an ICER and was included in the ERG report section titled ‘Exploratory and sensitivity analyses undertaken by the ERG’ (most commonly reported in Sect. 6 of the suggested ERG report template). This is an underresearched area and this study was commissioned by NICE to develop understanding of this aspect of the STA process. This research is of interest to all key stakeholders in the STA process, including the ERGs, pharmaceutical companies, AC members and NICE. The full research report forms part of the National Institute for Health Research Health Technology Assessment (NIHR HTA) monograph series [7]. The objectives of the study presented here were twofold.
-
1.
To identify the extent of ERG exploratory analyses, as defined above.
-
2.
To identify factors that influence or predict the extent of ERG exploratory analyses.
2 Methods
This was a joint project undertaken by researchers from the University of Sheffield and the University of York, all with considerable experience of the NICE STA process. The 100 most recently completed STAs (2009–2014) for which guidance has been published were selected for inclusion in the analysis. A list of the STAs included in this analysis is provided in Appendix 1. Data were extracted from over 400 separate documents associated with these STAs, including ERG reports (unredacted versions, including confidential information used by the ACs), the first ACD issued and the final FAD issued.
A data extraction tool to extract relevant data to address the project objectives was developed and piloted to ensure usability and to standardise extraction [7]. The following items were included in the data extraction form:
-
basic characteristics including company, disease area and ERG;
-
company’s base-case ICER(s);
-
number and type of exploratory analyses conducted by the ERG.
All data extractions were double-checked by a second researcher, and a narrative synthesis [8] of the extracted data was performed, summarising key data through text and tables to highlight any potentially important patterns or relationships in the data. The mean and median numbers of exploratory analyses per ERG report were calculated. The mean number of analyses was used to provide a simple binary variable with which to test some assumptions about relationships between the number of exploratory analyses and variables such as disease area and ICER. It was considered a priori that the disease area and an estimated cost-effectiveness ratio of £20,000 per quality-adjusted life-year (QALY) were the key variables potentially most likely to predict the incidence and frequency of exploratory analyses. This was due to the known impact of disease area on other elements of STAs [9] and the perceived importance of the £20,000 per QALY ICER for NICE decision making [10]. An assessment was also made to identify any changes in the number of exploratory analyses undertaken over time, and whether the number and type of exploratory analyses differed by the ERG undertaking the critical appraisal. A more in-depth investigation of the types of exploratory analyses undertaken, and their role and impact on NICE decision making, is provided elsewhere [7].
The key data used in the synthesis were then reduced to whether an STA conducted more or less than the overall mean number of exploratory analyses, and whether just ‘one or more’ exploratory analyses were explicitly cited as having an influence on a recommendation (defined as being mentioned in the ACD or FAD). These arbitrary selections were made as a means of making the most of the data to address the objectives of the project and are explained more fully elsewhere [7, 11].
3 Results
3.1 Overview of the 100 Single Technology Appraisals
Forty different companies made submissions as part of the NICE STA process. The companies with the largest number of submissions were Roche (n = 16), Novartis (n = 9), Glaxo Smith Kline (n = 7), Bristol-Meyers Squibb (n = 7) and Bayer (n = 6). Other companies involved had five or fewer submissions and the majority of companies made only one or two submissions: Alimera Sciences, Alimta, Allergan, Amgen, Astellas Pharma, Astra Zeneca, Biogen, Boehringer-Ingelheim, Celgene, Cell Therapeutics Inc., Eli Lilly, Eliquis, Eisai, Genzyme, InterMune, Janssen, Laboratoires Servier, Movetis, MSD, Napp, Novo Nordisk, Otsuka Pharmaceuticals, Pfizer, PharmaMar, Pharmaxis, Pierre Fabre, Sanofi Aventis, Savient Pharmaceuticals, Schering-Plough, Stelara, Sucampo Pharma Europe, Takeda UK Ltd, The Medicines Company, Thrombogenics, and UCB.
The principal disease areas covered by the STAs were cancer (44%), blood and the immune system conditions (11%), cardiovascular conditions (10%) and musculoskeletal conditions (8%). Figure 1 shows a breakdown of disease area by company. Cancer is clearly covered by many companies, although Roche, GlaxoSmithKline and Novartis have contributed the most STAs of this nature. Roche and GlaxoSmithKline have both also contributed the most blood and immune system STAs.
Most of the ERG reports contained at least one exploratory analysis (93%), and a total of 40 (43%) included eight or more such analyses. The mean number of exploratory analyses per report containing exploratory analyses was 8.5, with a median of 7 and a range of 1–29. For this reason, a cut-off point of 8 exploratory analyses was chosen for these analyses. Figure 2 shows the distribution of exploratory analyses. With regard to the seven ERG reports that did not include any exploratory analyses, the majority (five ERG reports, 71%) stated that no exploratory analyses were undertaken by the ERG as the company models were considered to have serious flaws. One ERG report stated that no exploratory analyses were undertaken due to no ICERs being presented by the company (TA 191) [12], and one (TA 267) [13] because the ERG was satisfied with the company model and the sensitivity analyses presented by the company.
3.2 Factors That May Have Influenced the Number of Exploratory Analyses Undertaken by the Evidence Review Group
Four variables that may influence the incidence and frequency of exploratory analyses were considered:
-
disease area;
-
ICER;
-
changes over time;
-
the ERG undertaking the critical appraisal.
The incidence and frequency of ERG exploratory analyses do not appear to be related to the disease area covered by the STA (see Table 1). STAs in the blood/immune system category did have a slightly higher proportion of ERG reports, with more than eight exploratory analyses (7/11, 64%) than the other disease areas. However, as most disease area categories had small numbers of STAs, it is difficult to draw any firm conclusions from this.
With regard to the estimated cost-effectiveness ratio, there does not appear to be a relationship between the company’s base-case ICER and the incidence or number of exploratory analyses (see Table 2). Of the 93 ERG reports presenting one or more exploratory analyses, the proportion of ERG reports in which the company’s base-case ICER was below £20,000 per QALY gained was similar to the proportion at or above this ICER (47 and 53%), and the proportion of ERG reports below or above £30,000 per QALY gained shifts slightly but was also similar (54 and 46%). The likelihood of an ERG performing eight or more exploratory analyses did not appear to be affected by the company’s base-case ICER. The proportion of ERG reports with eight or more exploratory analyses was very similar in the STAs in which the company base-case ICER was below £20,000 per QALY gained (41%) or above this ICER (45%).
Developments in the STA process between 2009 and 2014 do not appear to have influenced the frequency of exploratory analyses undertaken, as shown in Fig. 3. In this sample, the total number of STAs undertaken has varied from three in 2009 to 23 in 2011; however, the number of ERG reports with eight or more exploratory analyses appears to be largely consistent over time. For example, between 2011 and 2014, the percentage was consistent in each year and was always between 38 and 45%.
However, there does appear to be a possible pattern in the mean number of analyses conducted by particular ERGs, as shown in Table 3.
The University of York CRD/CHE produced the highest number of reports (n = 18), while Warwick Evidence produced the fewest (n = 5). The number of reports varies between the ERGs depending on how long they have been undertaking STAs and the size of their agreed contract. The ScHARR had the highest mean number of exploratory analyses per report (11.4), while West Midlands had the fewest (2.3). Of the ERGs currently involved in the STA process, the teams with the lowest mean number of exploratory analyses per report were Kleijnen Systematic Reviews (5.3) and Liverpool Reviews (5.6). As stated above, the number of exploratory analyses appears to be largely consistent over time for the whole sample. We also looked at the number of exploratory analyses by ERGs over time and present data here for the ScHARR as an example. The other ERGs showed similar results. The number of exploratory analyses undertaken by the ScHARR appears to be the highest and is quite stable over time. In 2011, the ScHARR produced five ERG reports, and the number of exploratory analyses ranged from 3 to 19; in 2012, the ScHARR produced three ERG reports, with the number of analyses ranging from 2 to 22; and in 2013 two ERG reports were produced, with 5 and 19 exploratory analyses. It therefore appears that the number of exploratory analyses is likely to be more dependent on the individual requirements of each STA or other possible factors, rather than due to the ERG becoming more rigorous in its critique (such that the number of exploratory analyses might increase year-on-year) or simply more focused (such that numbers of exploratory analyses might decrease year-on-year).
4 Discussion
In this analysis, the vast majority (93%) of ERG reports reported one or more exploratory analyses, with a mean of 8.5 exploratory analyses per report for the 93 reports where exploratory analyses were undertaken. Of the 93 ERG reports with at least one exploratory analysis, a total of 40 (43%) included eight or more such analyses. The likelihood of an ERG performing eight or more exploratory analyses does not appear to be affected by the disease area covered by the STA or the company’s base-case ICER. In previous research, Barham [9] analysed data from the first 18 STAs undertaken by NICE and found 56% of these to cover cancer topics. In an analysis of the first 4 years of the NICE STA process, 48% of STAs were undertaken in cancer topics [14], slightly higher than reported in this study. This shows that the percentage of STAs that are cancer topics may be decreasing over time.
The proportion of ERG reports with eight or more analyses appears to be relatively stable over time and not related to any developments in the process between 2009 and 2014. Although there have been changes to the NICE technology appraisal process and methods guides during this time period, these appear to have had little effect on the number of exploratory analyses undertaken by the ERGs. As shown in the example of the analysis of ScHARR data, there does not appear to be a time-dependent trend as the number of analyses undertaken by the ERG appears to be stable with respect to time. Additional analyses of the data, both from other ERGs and from pharmaceutical companies, show similar results (data not shown).
The number of exploratory analyses varied by ERG. For the 10 ERGs undertaking STAs, the mean number of exploratory analyses per report ranged from 2.3 (West Midlands) to 11.4 (ScHARR). The reasons for this variation are unclear and potentially complex, and will include such factors as how thoroughly the company has explored the uncertainties and plausible alternative scenarios. It should be noted that no regression analyses were performed to explore the relationship between the mean number of analyses per report and variables such as ERG, disease area or year due to the limitations of the data because there was only one independent variable in any category (cancer in disease area) that exceeded 20 in number, with the majority being very small numbers, and with the result being that any such analysis would be underpowered. Other potentially influential factors, such as the complexity and perceived quality of company submissions, were also not explored or analysed. Therefore, this finding should be interpreted with caution and more research is needed to determine the relationship between variables such as ERG and number of exploratory analyses undertaken, which may also vary according to the skills, experience and judgements of the ERGs and that of individuals within the ERGs. This issue was not explored within this study.
Only seven of the STAs included in this analysis had no exploratory analyses undertaken by the ERGs, principally due to serious flaws identified by the ERG in the company model, which would have rendered analyses irrelevant. In only one of the STAs were no exploratory analyses undertaken by the ERG because the ERG was satisfied with the model and analyses presented by the company. The exploratory analyses undertaken by the ERGs serve various functions: to correct errors and violations within the company’s model; to address uncertainties in the evidence base; and explore a range of plausible scenarios, and therefore support NICE AC decision making. The exploratory analyses undertaken by ERGs frequently influence both ACD and FAD recommendations [11].
This analysis is a good reflection of current practice as the most recent 100 STAs were included. The data extraction tool was extensively piloted and the double-checking of all key data across the 100 STAs by at least two experienced cost-effectiveness modellers reduced the likelihood of inconsistency and inaccuracy in the data. The use of narrative synthesis was principally descriptive, and reduced the likelihood of overstating relationships in the data. A reductive approach was taken to managing data that might be affected by interpretation or by poor reporting in the original documents. However, there are some limitations to this research. The descriptions of analyses undertaken were often highly specific to a particular STA and could be inconsistent across ERG reports, and thus difficult to interpret and categorise. In addition, small inconsistencies might have affected the data extraction, which may be due to several people being involved. A number of other factors not considered in this study may have influenced the number of exploratory analyses undertaken by the ERGs but this was beyond the remit of this research.
This analysis of over 400 documents provides an overview of some of the principal factors potentially affecting the number of exploratory analyses undertaken by the ERGs. The wider study reported that four types of exploratory analysis were conducted in relation to companies’ models: fixing errors; addressing violations; addressing matters of judgement; and the provision of a new, ERG-preferred base-case. Ninety-three of the 100 ERG reports contained at least one of these analyses, and the most frequently reported type of analysis related to the category ‘matters of judgement’. The results of the wider study also suggest that these additional analyses undertaken by ERGs were highly influential in the policy and decision-making process [7, 11]. More in-depth analysis is needed to understand how ERGs make decisions regarding the exploratory analyses to be undertaken. More research is also needed to fully understand the types of exploratory analyses most useful to ACs in their decision making.
5 Conclusions
There is no clear pattern to the presence or frequency of exploratory analyses; they do not appear to be predicted by the disease area covered by the STA, the time the STA took place, or the company’s base-case ICER. In addition, there does appear to be a pattern in the mean number of analyses conducted by individual ERGs, but more research is needed to understand this relationship.
References
National Institute for Health and Care Excellence. Guide to the processes of technology appraisal. NICE. 2014. https://www.nice.org.uk/article/pmg19/chapter/Acknowledgements. Accessed 25 March 2015.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. NICE. 2013. https://www.nice.org.uk/article/pmg9/chapter/Foreword. Acessed 4 Aug 2015.
Wong R, Paisley S, Carroll C. Assessing searches in NICE single technology appraisals: practice and checklist. Int J Technol Assess Health Care. 2013;29(3):315–22.
Kaltenthaler E, Boland A, Carroll C, Dickson R, Fitzgerald P, Papaioannou D. Evidence Review Group approaches to the critical appraisal of manufacturer submissions for the NICE STA process: a mapping study and thematic analysis. Health Technol Assess 2011;15(22):1–iv.
Carroll C, Kaltenthaler E, Fitzgerald P, Boland A, Dickson R. A thematic analysis of the strengths and weaknesses of manufacturers’ submissions to the NICE Single Technology Assessment (STA) process. Health Policy. 2011;102(2–3):136–44.
Kaltenthaler EC, Dickson R, Boland A, Carroll C, Fitzgerald P, Papaioannou D, Akehurst R. A qualitative study of manufacturers’ submissions to the UK NICE single technology appraisal process. BMJ Open. 2012;2:e000562.
Kaltenthaler E, Carroll C, Hill-McManus D, Scope A, Holmes M, Rice S, et al. The use of exploratory analyses within the NICE Single Technology Appraisal process. Health Technol Assess. 2016;20(26):1–46.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduce of narrative synthesis in systematic reviews. Swindon: ESRC Methods Programme; 2006. http://www.lancaster.ac.uk/shm/research/nssr/research/dissemination/publications/NS_Synthesis_Guidance_v1.pdf. Accessed 11 April 16.
Barham L. Single technology appraisals by NICE: are they delivering faster guidance to the NHS? Pharmacoeconomics. 2008;26:1037–43.
McCabe C, Claxton KF, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733–44.
Carroll C, Kaltenthaler E, Tappenden P, Hill-McManus D, Scope A, Holmes M, et al. The type and impact of Evidence Review Group exploratory analyses in the NICE Single Technology (STA) process. Value Health (in press).
Capecitabine for the treatment of advanced gastric cancer. NICE technology appraisal guidance TA191. 2010. https://www.nice.org.uk/guidance/ta191. Accessed 27 Apr 2015.
Ivabradine for treating chronic heart failure. NICE technology appraisal guidance TA267. 2012. https://www.nice.org.uk/guidance/ta267. Accessed 2 May 2015.
Kaltenthaler E, Papaioannou D, Boland A, Dickson R. The National Institute for Health and Clinical Excellence Single Technology Appraisal process: lessons from the first 4 years. Value Health. 2011;14(8):1158–65.
Acknowledgements
Authors’ contributions
Eva Kaltenthaler contributed to the design of the study, undertook data extraction and developed the first draft of the manuscript. Christopher Carroll contributed to the design of the study and undertook data extraction. Daniel Hill-McManus undertook data extraction and data checking. Alison Scope undertook data extraction. Michael Holmes undertook data extraction. Stephen Rice undertook data extraction and data checking. Micah Rose undertook data extraction and data checking. Paul Tappenden contributed to the design of the study and undertook data checking. Nerys Woolacott contributed to the design of the study. All authors contributed to the development of the manuscript and agreed the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Eva Kaltenthaler, Christopher Carroll, Daniel Hill-McManus, Alison Scope, Michael Holmes,, Stephen Rice, Micah Rose, Paul Tappenden, and Nerys Woolacott have undertaken work for NICE and declare no conflicts of interest. This work was conducted at the request of NICE.
Financial support
This project was funded by the NIHR HTA Programme. Visit the HTA Programme website for more details http://www.nets.nihr.ac.uk/projects/hta/1415104. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA Programme, NICE, NIHR, National Health Service or the Department of Health.
Appendix 1: 100 included STAs
Appendix 1: 100 included STAs
NICE TA number | Full appraisal title |
|---|---|
TA181 | Pemetrexed for the first-line treatment of non-small-cell lung cancer |
TA182 | Prasugrel for the treatment of acute coronary syndromes with percutaneous coronary intervention |
TA183 | Topotecan for the treatment of recurrent and stage IVB cervical cancer |
TA185 | Trabectedin for the treatment of advanced soft tissue sarcoma |
TA186 | Certolizumab pegol for the treatment of rheumatoid arthritis |
TA189 | Sorafenib for the treatment of advanced hepatocellular carcinoma |
TA190 | Pemetrexed for the maintenance treatment of non-small-cell lung cancer |
TA191 | Capecitabine for the treatment of advanced gastric cancer |
TA192 | Gefitinib for the first-line treatment of locally advanced or metastatic non-small-cell lung cancer |
TA193 | Rituximab for the treatment of relapsed or refractory chronic lymphocytic leukaemia |
TA196 | Imatinib for the adjuvant treatment of gastrointestinal stromal tumours |
TA197 | Dronedarone for the treatment of non-permanent atrial fibrillation |
TA198 | Tocilizumab for the treatment of rheumatoid arthritis |
TA201 | Omalizumab for the treatment of severe persistent allergic asthma in children aged 6–11 years |
TA202 | Ofatumumab for the treatment of chronic lymphocytic leukaemia refractory to fludarabine and alemtuzumab |
TA203 | Liraglutide for the treatment of type 2 diabetes mellitus |
TA204 | Denosumab for the prevention of osteoporotic fractures in postmenopausal women |
TA205 | Eltrombopag for the treatment of chronic immune (idiopathic) thrombocytopenic purpura |
TA208 | Trastuzumab for the treatment of HER2-positive metastatic gastric cancer |
TA211 | Prucalopride for the treatment of chronic constipation in women |
TA212 | Bevacizumab in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer |
TA213 | Aripiprazole for the treatment of schizophrenia in people aged 15 to 17 years |
TA214 | Bevacizumab in combination with a taxane for the first-line treatment of metastatic breast cancer |
TA215 | Pazopanib for the first-line treatment of advanced renal cell carcinoma |
TA216 | Bendamustine for the first-line treatment of chronic lymphocytic leukaemia |
TA218 | Azacitidine for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia |
TA219 | Everolimus for the second-line treatment of advanced renal cell carcinoma |
TA220 | Golimumab for the treatment of psoriatic arthritis |
TA221 | Romiplostim for the treatment of chronic immune (idiopathic) thrombocytopenic purpura |
TA222 | Trabectedin for the treatment of relapsed ovarian cancer |
TA225 | Golimumab for the treatment of rheumatoid arthritis after the failure of previous disease-modifying anti-rheumatic drugs |
TA226 | Golimumab for the treatment of rheumatoid arthritis after the failure of previous disease-modifying anti-rheumatic drugs |
TA227 | Erlotinib monotherapy for maintenance treatment of non-small-cell lung cancer |
TA229 | Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion |
TA230 | Bivalirudin for the treatment of ST-segment-elevation myocardial infarction |
TA232 | Retigabine for the adjunctive treatment of partial onset seizures in epilepsy |
TA233 | Golimumab for the treatment of ankylosing spondylitis |
TA234 | Abatacept for the treatment of rheumatoid arthritis after the failure of conventional disease-modifying anti-rheumatic drugs |
TA235 | Mifamurtide for the treatment of osteosarcoma |
TA236 | Ticagrelor for the treatment of acute coronary syndromes |
TA237 | Ranibizumab for the treatment of diabetic macular oedema |
TA238 | Tocilizumab for the treatment of systemic juvenile idiopathic arthritis |
TA239 | Fulvestrant for the treatment of locally advanced or metastatic breast cancer |
TA244 | Roflumilast for the management of severe chronic obstructive pulmonary disease |
TA245 | Apixaban for the prevention of venous thromboembolism after total hip or knee replacement in adults |
TA248 | Exenatide prolonged-release suspension for injection in combination with oral antidiabetic therapy for the treatment of type 2 diabetes |
TA249 | Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation |
TA250 | Eribulin for the treatment of locally advanced or metastatic breast cancer |
TA252 | Telaprevir for the treatment of genotype 1 chronic hepatitis C |
TA253 | Boceprevir for the treatment of genotype 1 chronic hepatitis C |
TA254 | Fingolimod for the treatment of highly active relapsing–remitting multiple sclerosis |
TA255 | Cabazitaxel for hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen |
TA256 | Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation |
TA258 | Erlotinib for the first-line treatment of locally advanced or metastatic EGFR-TK mutation-positive non-small-cell lung cancer |
TA259 | Abiraterone for castration-resistant metastatic prostate cancer previously treated with a docetaxel-containing regimen |
TA260 | Botulinum toxin type A for the prevention of headaches in adults with chronic migraine |
TA261 | Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism |
TA263 | Bevacizumab in combination with capecitabine for the first-line treatment of metastatic breast cancer |
TA264 | Alteplase for treating acute ischaemic stroke |
TA266 | Mannitol dry powder for inhalation for treating cystic fibrosis |
TA267 | Ivabradine for treating chronic heart failure |
TA268 | Ipilimumab for previously treated advanced (unresectable or metastatic) melanoma |
TA269 | Vemurafenib for treating locally advanced or metastatic BRAF V600 mutation-positive malignant melanoma |
TA271 | Fluocinolone acetonide intravitreal implant for the treatment of chronic diabetic macular oedema after an inadequate response to prior therapy |
TA272 | Vinflunine for the treatment of advanced or metastatic transitional cell carcinoma of the urothelial tract |
TA275 | Apixaban for preventing stroke and systemic embolism in people with nonvalvular atrial fibrillation |
TA282 | Pirfenidone for treating idiopathic pulmonary fibrosis |
TA283 | Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion |
TA284 | Bevacizumab in combination with paclitaxel and carboplatin for first-line treatment of advanced ovarian cancer |
TA285 | Bevacizumab in combination with gemcitabine and carboplatin for treating the first recurrence of platinum-sensitive advanced ovarian cancer |
TA287 | Bevacizumab in combination with gemcitabine and carboplatin for treating the first recurrence of platinum-sensitive advanced ovarian cancer |
TA288 | Dapagliflozin in combination therapy for treating type 2 diabetes |
TA289 | Ruxolitinib for disease-related splenomegaly or symptoms in adults with myelofibrosis |
TA290 | Mirabegron for treating symptoms of overactive bladder |
TA291 | Pegloticase for treating severe debilitating chronic tophaceous gout |
TA292 | Aripiprazole for treating moderate to severe manic episodes in adolescents with bipolar I disorder |
TA293 | Eltrombopag for treating chronic immune (idiopathic) thrombocytopenic purpura |
TA294 | Aflibercept solution for injection for treating wet age-related macular degeneration |
TA295 | Everolimus in combination with exemestane for treating advanced HER2-negative hormone-receptor-positive breast cancer after endocrine therapy |
TA296 | Crizotinib for previously treated non-small-cell lung cancer associated with an anaplastic lymphoma kinase fusion gene |
TA297 | Ocriplasmin for treating vitreomacular traction |
TA298 | Ranibizumab for treating choroidal neovascularisation associated with pathological myopia |
TA299 | Bosutinib for previously treated chronic myeloid leukaemia |
TA303 | Teriflunomide for treating relapsing–remitting multiple sclerosis |
TA305 | Aflibercept for treating visual impairment caused by macular oedema secondary to central retinal vein occlusion |
TA306 | Pixantrone monotherapy for treating multiply relapsed or refractory aggressive non-Hodgkin’s B-cell lymphoma |
TA307 | Aflibercept in combination with irinotecan and fluorouracil-based therapy for treating metastatic colorectal cancer that has progressed following prior oxaliplatin-based chemotherapy |
TA308 | Rituximab in combination with glucocorticoids for treating anti-neutrophil cytoplasmic antibody-associated vasculitis |
TA309 | Pemetrexed maintenance treatment following induction therapy with pemetrexed and cisplatin for non-squamous non-small-cell lung cancer |
TA310 | Afatinib for treating epidermal growth factor receptor mutation-positive locally advanced or metastatic non-small-cell lung cancer |
TA311 | Bortezomib for induction therapy in multiple myeloma before high-dose chemotherapy and autologous stem cell transplantation |
TA312 | Alemtuzumab for treating relapsing-remitting multiple sclerosis |
TA313 | Ustekinumab for treating active psoriatic arthritis |
TA315 | Canagliflozin in combination therapy for treating type 2 diabetes |
TA316 | Enzalutamide for metastatic hormone-relapsed prostate cancer previously treated with a docetaxel-containing regimen |
TA318 | Lubiprostone for treating chronic idiopathic constipation |
TA319 | Ipilimumab for previously untreated advanced (unresectable or metastatic) melanoma |
TA320 | Dimethyl fumarate for treating relapsing-remitting multiple sclerosis |
TA321 | Dabrafenib for treating unresectable or metastatic BRAF V600 mutation-positive melanoma |
TA322 | Lenalidomide for treating myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality |
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kaltenthaler, E., Carroll, C., Hill-McManus, D. et al. Issues Related to the Frequency of Exploratory Analyses by Evidence Review Groups in the NICE Single Technology Appraisal Process. PharmacoEconomics Open 1, 99–108 (2017). https://doi.org/10.1007/s41669-016-0001-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-016-0001-4