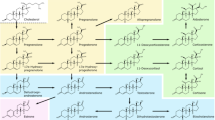
Abstract
An improved method is described for the fast and sensitive determination of multiple endogenous steroid hormones in steroidogenic pathway by liquid chromatography–tandem mass spectrometry (LC–MS/MS), in which serum samples, after a simple protein precipitation and a derivatization by 2-hydrazinopyridine (HP), were subjected to LC–MS analysis. The protein precipitation of serum samples is achieved via adding acetonitrile, while the derivatization of endogenous steroid hormones in serum samples after protein precipitation is performed using 2-hydrazinopyridine as a derivatization reagent. The derivatives of the steroid hormones exhibit good detection sensitivity in LC–MS analysis. Linearity range of the improved method for the target steroid hormones is in two orders of magnitude and the correlation coefficients (r) are in the range from 0.9885 to 0.9998. The limits of detection (signal/noise = 3) range from 0.07 to 65.26 ng/mL. The recoveries are between 80.2 and 116.4% with acceptable intra- and inter-day relative standard deviations (RSDs) ranging from 0.3 to 18.9%. The improved method can be applied to explore the differences of steroid hormones between healthy controls and polycystic ovary syndrome patients.
Graphic Abstract








Similar content being viewed by others
References
Ceglarek U, et al. Rapid quantification of steroid patterns in human serum by on-line solid phase extraction combined with liquid chromatography-triple quadrupole linear ion trap mass spectrometry. Clin Chim Acta. 2009;401(1–2):114–8.
Deng YY, et al. Steroid hormone profiling in obese and nonobese women with polycystic ovary syndrome. Sci Rep. 2017;7(1):14156.
Li AD, et al. Follicular hyperandrogenism and insulin resistance in polycystic ovary syndrome patients with normal circulating testosterone levels. J Biomed Res. 2018;32(3):208–14.
Kamboj MK, Bonny AE. Polycystic ovary syndrome in adolescence: diagnostic and therapeutic strategies. Transl Pediatr. 2017;6(4):248–55.
Lee YH, et al. Welsh onion root (Allium fistulosum) restores ovarian functions from letrozole induced-polycystic ovary syndrome. Nutrients. 2018;10(10):1430.
Shaaban Z, et al. Pathophysiological mechanisms of gonadotropins- and steroid hormones-related genes in etiology of polycystic ovary syndrome. Iran J Basic Med Sci. 2019;22(1):3–16.
Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010;121(3–5):491–5.
Krone N, et al. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010;121(3–5):496–504.
Blue SW, et al. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception. 2018;97(4):363–9.
Khedr A, Alandal AM. Liquid chromatography-tandem mass spectrometric analysis of ten estrogen metabolites at sub-picogram levels in breast cancer women. J Chromatogr B. 2016;1031:181–8.
Peitzsch M, et al. An LC-MS/MS method for steroid profiling during adrenal venous sampling for investigation of primary aldosteronism. J Steroid Biochem Mol Biol. 2015;145:75–84.
Caron P, Turcotte V, Guillemette C. A chromatography/tandem mass spectrometry method for the simultaneous profiling of ten endogenous steroids, including progesterone, adrenal precursors, androgens and estrogens, using low serum volume. Steroids. 2015;104:16–24.
Koal T, et al. Standardized LC-MS/MS based steroid hormone profile-analysis. J Steroid Biochem Mol Biol. 2012;129(3–5):129–38.
Licea-Perez H, et al. Development of a highly sensitive and selective UPLC/MS/MS method for the simultaneous determination of testosterone and 5 alpha-dihydrotestosterone in human serum to support testosterone replacement therapy for hypogonadism. Steroids. 2008;73(6):601–10.
Nishio T, et al. Development and application of electrospray-active derivatization reagents for hydroxysteroids. J Pharm Biomed Anal. 2007;44(3):786–95.
Leinonen A, Kuuranne T, Kostiainen R. Liquid chromatography/mass spectrometry in anabolic steroid analysis–optimization and comparison of three ionization techniques: electrospray ionization, atmospheric pressure chemical ionization and atmospheric pressure photoionization. J Mass Spectrom. 2002;37(7):693–8.
Yun-Qing H, et al. Use of isotope mass probes for metabolic analysis of the jasmonate biosynthetic pathway. Analyst. 2011;136(7):1515–22.
Ye T, et al. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J Pineal Res. 2019;66:e12531.
Zhu QF, et al. Comprehensive screening and identification of fatty acid esters of hydroxy fatty acids in plant tissues by chemical isotope labeling-assisted liquid chromatography-mass spectrometry. Anal Chem. 2018;90(16):10056–63.
Higashi T, et al. Salivary chenodeoxycholic acid and its glycine-conjugate: Their determination method using LC–MS/MS and variation of their concentrations with increased saliva flow rate. Steroids. 2010;75(4):338–45.
Im E, et al. Simultaneous determination of androgens and prostaglandins in human urine using ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2019;1109:45–53.
Hala D, et al. Quantification of 2-hydrazinopyridine derivatized steroid hormones in fathead minnow ( Pimephales promelas ) blood plasma using LC-ESI+/MS/MS. J Chromatogr B. 2011;879(9–10):591–8.
Shibayama Y, et al. Liquid chromatography-tandem mass spectrometric method for determination of salivary 17alpha-hydroxyprogesterone: a noninvasive tool for evaluating efficacy of hormone replacement therapy in congenital adrenal hyperplasia. J Chromatogr B. 2008;867(1):49–56.
Higashi TT, Nishio N, Shimada K. Alternative procedure for charged derivatization to enhance detection responses of steroids in electrospray ionization-MS. Chem Pharm Bull. 2007;55(4):662–5.
Lionetto L, et al. LC-MS/MS simultaneous analysis of allopregnanolone, epiallopregnanolone, pregnanolone, dehydroepiandrosterone and dehydroepiandrosterone 3-sulfate in human plasma. Bioanalysis. 2017;9(6):527–39.
Guo N, et al. Stable isotope labeling—liquid chromatography/mass spectrometry for quantitative analysis of androgenic and progestagenic steroids. Anal Chim Acta. 2016;905:106–14.
Rossi C, et al. Serum steroid profiling for congenital adrenal hyperplasia using liquid chromatography-tandem mass spectrometry. Clin Chim Acta. 2010;411(4):222–8.
Acknowledgements
The work is supported by the National Key R&D Program of China (2018YFA0900400), the National Natural Science Foundation of China (21635006, 31670373, 21721005, 21904099), and the Postdoctoral Science Foundation of China (Grant 2018M642893).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Bai, YL., Hong, ZD., Zhang, TY. et al. A Method for Simultaneous Determination of 14 Carbonyl-Steroid Hormones in Human Serum by Ultra High Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Anal. Test. 4, 1–12 (2020). https://doi.org/10.1007/s41664-020-00120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-020-00120-5