Abstract
Acute bouts of physical activity of at least moderate intensity have shown to enhance cognition in young as well as older adults. This effect has been observed for different kinds of activities such as aerobic or strength and coordination training. However, only few studies have directly compared these activities regarding their effectiveness. Further, most previous studies have mainly focused on inhibition and have not examined other important core executive functions (i.e., updating, switching) which are essential for our behavior in daily life (e.g., staying focused, resisting temptations, thinking before acting), as well. Therefore, this study aimed to directly compare two kinds of activities, aerobic and coordinative, and examine how they might affect executive functions (i.e., inhibition, updating, and switching) in a test-retest protocol. It is interesting for practical implications, as coordinative exercises, for example, require little space and would be preferable in settings such as an office or a classroom. Furthermore, we designed our experiment in such a way that learning effects were controlled. Then, we tested the influence of acute bouts of physical activity on the executive functioning in both young and older adults (young 16–22 years, old 65–80 years). Overall, we found no differences between aerobic and coordinative activities and, in fact, benefits from physical activities occurred only in the updating tasks in young adults. Additionally, we also showed some learning effects that might influence the results. Thus, it is important to control cognitive tests for learning effects in test-retest studies as well as to analyze effects from physical activity on a construct level of executive functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, many studies have focused on acute bouts of moderate physical activities to improve cognitive performance. A main aspect of cognitive processes is executive functions, which are important in various daily activities (i.e., driving a car, walking along a crowded street), sports, and academic achievement (Diamond 2013; Diamond and Ling 2016; Tomporowski et al. 2014). Previous studies have examined executive functions following acute bouts of physical activities in children and young adults (Chang et al. 2014a; Chen et al. 2014; Hillman et al. 2009; Hung et al. 2013) as well as middle-aged and older adults (Barella et al. 2010; Chang and Etnier 2009; Chang et al. 2014b). Overall, they have found small effects which differed depending on age and type of activity.
Especially age-related differences are interesting, since research indicates life span adaptations in cognition (Salthouse and Davis 2006) and since fitness, which is also reduced at an older age, moderates the interaction between activity and cognition (Pesce 2009). Interestingly, only few studies directly compared younger and older adults. For instance, one study examined working memory following 15 min of moderate cycling in three different age groups (i.e., 19–39 years, 40–64 years, and 65 + years) and reported cognitive benefits independently of age (Hogan et al. 2013). Another work examined inhibition following light and moderate cycling intensities in younger and older adults; again, both age groups improved their cognitive results following moderate physical activity (Kamijo et al. 2009). These and other studies used one specific exercise (e.g., cycling exercises) and examined only one executive function each. Thus, there is still a lack of research that compares effects of different physical activities on multiple executive functions in different age groups. A better understanding of such age- and fitness-related effects is essential for practical implications: to improve physical activity regimes regarding cognitive development in youth as well as regarding cognitive maintenance at an older age.
One recent meta-analysis examined how age and fitness level affect the relationship between cognition and physical activity (Ludyga et al. 2016). The authors reported that the highest effects on executive functions occurred in preadolescents and older adults, and the effects were independent of fitness level. Their review also yielded that most involved studies looked at the effect of physical activity on a particular executive function (i.e., inhibition) without considering potential effects on other core executive functions such as updating and shifting. This is in line with another meta-analysis that reported significant results for an inhibition task (i.e., Stroop) and mixed results for memory tasks (i.e., short-term memory, updating), but not for shifting tasks (Chang et al. 2012).
The meta-analysis also showed that different types of physical activities vary in their effects on cognition (Chang et al. 2012). Aerobic activities showed a small positive effect, anaerobic or strengthening activities showed a negative effect, and the highest effects on cognition were provided when both single bouts of aerobic and strengthening activities were applied (Chang et al. 2012). In other studies, it was assumed that differences can also be found between physical fitness and motor fitness tasks (Marchetti et al. 2015; Pesce 2009). This might be due to acute physical fitness tasks (i.e., running, cycling), which can benefit cognition by metabolic changes (e.g., catecholamine expression, see McMorris and Hale (2015)), whereas motor fitness tasks (i.e., movement coordination) are related to changes in information processing (Voelcker-Rehage and Niemann 2013). Since most studies only applied one type of physical activity, there is need for further comparative studies. Concerning this matter, there are a few studies on children (aerobic vs coordinative vs strengthening activities, see Ludyga et al. (2017); van den Berg et al. (2016)) and middle-aged adults (aerobic vs strengthening activities, see Alves et al. (2012)). However, we are not aware of comparative studies on older adults.
There are also other important moderators for the relationship between physical activities and cognition, namely activity durations and intensities. Previous research has shown that short bouts of activity (i.e., 20 min) of moderate intensities (e.g., 40–79% Wmax or equivalentFootnote 1) were most promising for positive effects on cognition (Brisswalter et al. 2002; Chang et al. 2012; McMorris and Hale 2015). Light intensities showed rather small effects and vigorous intensities needed a longer recovery phase (> 15 min.) to induce cognitive benefits (Chang et al. 2012). Chang et al. (2012) also recommended that physical activities should be longer than 11 min.
Besides all of the aspects mentioned above, a lack of validity and reliability in tasks of executive functions is named as a serious issue of cognitive testing (Chung et al. 2014). Specifically, the test-retest reliability of tasks of executive functioning was found to be low (Lowe and Rabbitt 1998; Miyake et al. 2000). However, tests for internal reliability (i.e., split-half method adjusted by Spearman-Brown) showed better results (Fournier-Vicente et al. 2008; Hull et al. 2008). Regarding test-retest designs, there are additional participant-related factors (i.e., task learning, motivation, mood, daily fitness) which decrease test stability. Therefore, results from studies concerning the effects of physical activities on cognitive performance without measuring the test-retest reliability should be interpreted carefully.
A very topical review (Pontifex et al. 2019) reported most of the named as well as additional aspects in the framework of acute effects from physical activity on cognition. Regarding our initial considerations and other research gaps described by Pontifex et al. (2019), the current study aimed to contribute to this field of research by comparing the effects of different acute physical activities (i.e., coordinative and aerobic) on a diverse (i.e. inhibition, updating, task shifting as single functions) and unitary approach of executive functions in two age groups. A unitary approach is particularly interesting since a participant’s performance might differ depending on the tasks and participant. A recent study (Bock et al. 2019) did not find indications of distinct executive functions. However, other studies reported bi-factorial models (Adrover-Roig et al. 2012; Hull et al. 2008) or discussed both the unity and the diversity of executive functions (Miyake and Friedman 2012), which reveals the discrepancies in the literature.
To address all these factors, two test sessions were conducted in our study, i.e., one contained physical activity and cognitive tests, and the other contained only cognitive tests (in balanced order, to reduce test-learning effects). Additionally, a control group performed two sessions without physical activity to also measure the test-retest reliability. We hypothesized (I) that there would be activity-related benefits on three core executive functions in young as well as older adults, (II) that these effects would also be pronounced in the unitary approach on both age groups, (III) that there would be a difference between both activity modalities with slightly higher effects in aerobic activities, (IV) that older adults would show higher improvements compared with younger ones, and (V) that our tests would show good test-retest reliability with negligible learning effects.
Methods
Participants
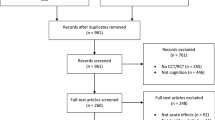
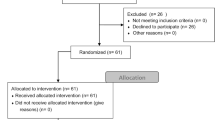
This was a controlled, non-blinded, parallel group study conducted in Germany. Overall, 120 participants from two age groups (young 16–22 years and old 65–80 years) were recruited via an internal participant pool and media articles. A total of 19 participants were excluded from the statistical analysis because they had missed the second test session or because they did not meet the inclusion criteria. In terms of our heart rate measurements, another 11 participants could not be analyzed due to technical problems during data recording. The inclusion criteria were recorded based on self-reporting. Participants had to have no health restrictions to perform physical activity for 20 min at a moderate to high intensity. They were also asked not to engage in any additional sports activity on the same day before our test sessions.
This study was approved by the Ethics Committee of the German Sport University Cologne (148/2016) in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Participants were informed verbally as well as in writing about our study and signed an informed consent form.
First, participants were informed about the physical activity tasks (aerobic and coordinative) and were then randomly assigned to one of the three groups (in blocks, 4:4:4, from a computer-generated list) by the principal investigator (one control group (CG) and two activity groups (AG)). We calculated the required sample size using G-Power® 3.1 as follows: reviews reported small (f = 0.10) to medium (f = 0.25) effect sizes from physical activity (Chang et al. 2012; Ludyga et al. 2016), therefore we expected a mean effect size of f = 0.175. For the interaction term of a 2 (age) × 3 (group) × 2 (time) analysis of variance with repeated measures on the latter factor, including f = 0.175, α = 0.05, β = 0.80, and a correlation among repeated measures of 0.6, G-Power yielded a required sample size of 90. In total, 101 participants were included in the analysis, of which 70 (39 young adults, 16 female, Mage = 18.23 ± 1.33 years; 31 older adults, 10 female, Mage = 71.45 ± 4.58 years) were in our AGs and 31 (15 young adults, 5 female, Mage = 17.87 ± 0.74 years; 16 older adults, 5 female, Mage = 72.31 ± 3.66 years) made up the CG. The primary outcome was the differences in activity-related effects on cognition between age and activity groups. The participants’ assignment for our study is illustrated in Fig. 1.
Procedure
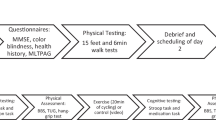
All three groups were tested during two test sessions with a break of four to five weeks in between. This period was chosen for two reasons: Firstly, test-learning effects seem to be negligible only after four weeks (Falleti et al. 2006), and secondly, longer periods increase the risk of a training-induced bias (i.e., improved physical fitness). Each test session consisted of six computerized tasks for executive functions that were examined sequentially in a randomized order. These tasks represented three core executive functions (i.e., inhibition, task shifting, and updating; Miyake et al. 2000) and were adapted from tasks described in the literature (see corresponding sections below). Each session took place in small groups (i.e., up to four participants) and lasted about 50 to 60 min (without physical activity). Everyone had a single user workstation, and all participants started each of the six tasks at the same time.
The CG, which did not perform any physical activity during the period, was examined to control for the first reason (task learning effects and task reliability). The AGs either performed an aerobic activity (i.e., running) or a sequence of coordinative activities for 20 min, before they performed the computerized tasks. Performing activities were counter-balanced between the first and second session, active (with physical activity) sessions lasted about 1.5 hours on average. After the physical activity and before starting the computerized tasks, participants needed an average of 7 min to switch from the gym to the computer. To ensure that physical exercise was synchronized with participants’ individual threshold regarding the moderate intensity, all of them wore a heart rate monitor (Polar RS 400®) during the activities and following tasks. Additionally, during the aerobic activity, participants were asked to control their heart rate and maintain moderate to high intensities. The coordinative activity group was not asked to maintain its heart rate since our planned coordinative tasks had changing intensities and short resting periods in between.
The timing of physical activity in our AGs and the sequence of cognitive tests were also determined using a computer-generated randomized list. The instruction of the activities and tests was carried out by students after they had been trained by the study supervisor. All participants who did not perform the activities as instructed or who did not match the inclusion criteria (i.e., had performed additional physical activity the same day) were logged by the instructors and subsequently excluded from the analysis (see Fig. 1).
Physical Activity
The aerobic activity consisted of a short warm-up (i.e., mobilization and walking) and 20 min of continuous movement (i.e., running). We asked participants to run at a moderate to high intensity, about 80% of their maximum heart rate. This value was slightly higher than the recommendations of the American College of Sports Medicine (moderate intensities 64–76% HRmax). Thus, participants who performed below this level were definitely in the range of moderate intensity, and those who performed above the threshold were not negatively affectedFootnote 2 as there was the transition time from the gym to the computer. Our HR calculation was based on mean age in our AG and the formula [208 − (0.7 x age)] for maximum heart rate (Tanaka et al. 2001). We created physical activity intervals calculated on this basis which resulted in about 150–160 beats per minute (bpm) for young and about 120–130 bpm for older adults.
Our coordinative activities consisted of five tasks separated by individual stations and were explained by one student. Additionally, each station had a short, written instruction to remind the participants of the task. Participants performed the activity at each station for 3 min and then had 1 min to switch to the next station, reread the task instructions if necessary, and try out the task before the next 3-min phase started. The chosen tasks concerned coordinative skills including hand-eye coordination, postural control, bilateral movements, and step/jump coordination (including different variations):
-
Task 1, jumping sequence over two ropes arranged in a square (backward, forward, double-footed, alternating left/right)
-
Task 2, throwing balls at a target 4 m away (balls had different sizes, one eye closed, using non-dominant hand)
-
Task 3, postural control on an unstable surface for 10 s (one eye or both closed, rotating movements with free upper/lower limbs)
-
Task 4, stepping coordination on an agility ladder (three different movement patterns, from slow to fast step speed)
-
Task 5, balancing on a rope on the floor while dribbling one ball (using two balls, walking back and forth, one ball dribbling and the other one throwing and catching, one eye closed)
Cognitive Tasks
We used two cognitive tasks for each of the three core executive functions. We were able to control effects from physical exercises on a construct level of cognitive abilities (i.e., as diverse constructs) and not solely on a task level. Task order was randomized across each AG to avoid negative sequence effects (i.e., tiredness) on individual tasks.
Inhibition Tasks
We used the Stroop task (Stroop 1935) as one part of our inhibition testing. This computerized version of the task was adapted from existing tests in the literature (Alvarez-Alamilla et al. 2016; Bugg and Hutchison 2012). We randomly presented (in the same quantity) the words yellow, blue, red, and green one by one, either in a color that matched the words’ meaning (congruent trials) or in a different color (incongruent trials). Participants had to press a response button as fast as possible to indicate the color of each word, irrespective of its meaning. In each trial, two answer options, one matching the color and the other not matching, were presented on the screen and could be selected with one of the two buttons. We recorded accuracy in the form of correct answers and used the reaction time to calculate inhibition costs (i.e., incongruent trials minus congruent trials) across all correct trials. Our version of the Stroop task consisted of six blocks, each including 32 trials.
The second task was the Simon task, which was also modified from a previous research (Bialystok et al. 2008). A right or left pointing arrow appeared randomly on the right or left side of the central screen, either matching the side that it was pointing to (congruent trial) or not (incongruent trial). Participants were told to indicate as fast as possible the direction the arrow was pointing using one of the two buttons (i.e., left or right keyboard buttons). As in the Stroop task, this task consisted of six blocks, each including 32 trials, and we recorded accuracy as well as reaction times and calculated inhibition costs the same way.
Task Shifting
In the literature, “task shifting” or “switching” describes the process of switching between two alternating tasks. We used a paradigm that has previously been described in the literature (Karbach and Kray 2009; Kray and Lindenberger 2000). Our two tasks used the same mixed block format (alternating form: AABBAABB …) where participants had to switch intra-individual tasks (“A” and “B”) after every other trial. Thus, we had two types of trials, i.e., repetition trials (i.e., every second A or B task) and switching trials (i.e., every first A or B task). One task contained two visuospatial stimuli, and participants were required to differentiate between shapes (circles or square) or between sizes (i.e., small or large) of the presented stimulus. The other task contained a semantic component, and participants had to differentiate between animate and inanimate words or between one- or multiple-syllable words. Participants had to keep the alternating order in mind and had to respond to each task by pressing one of the two buttons. Both tasks consisted of six blocks, each including 17 trials, and participants were told to answer as fast and correct as possible. We measured accuracy in terms of correct answers as well as response times and calculated switching costs by subtracting repetition tasks from switching tasks in all correct trials.
Updating Tasks
Updating tasks are described by tracking or monitoring working memory representations, and therefore are related to the notion of working memory (Miyake et al. 2000). We used a modified computerized version of the n-back task (Schmiedek et al. 2009a; Schmiedek et al. 2009b). We presented black dots sequentially on a 4 × 4 grid, and participants had to indicate whether a dot appeared in the same field of the grid as the occurrence of the dot before the last. There were two answer buttons (i.e., one for new field, the other for the same field as the dot before the last), and we registered accuracy in terms of correct answers as well as reaction times. However, we only used accuracy values for further calculations to be comparable with the results of the second task (see below). Our n-back task consisted of six blocks, each including 19 trials.
The second updating task was a modified keep track task (Miyake et al. 2000; Yntema 1963). The participants were presented with a table with six categories, each of which included three words. Afterwards, participants were required to remember six blocks, including a different number of categories (the number increased every two blocks, 2 × 3, 2 × 4, and 2 × 5 categories). In each block, we presented a sequence of 15 randomized words from the table, and participants had to track the last word of each given category. At the end of the block, they wrote down the last word, and we registered accuracy in terms of correct answers.
We calculated an inhibition score (i.e., mean value of inhibition costs from reaction times in both tasks), a task shifting score (i.e., mean value of switching costs from reaction times in both tasks), and an updating score (i.e., mean value of accuracy in both tasks) for the subsequent analyses. The three core functions were used for our diverse approach. In a further step, we transformed them to z-scores:
This formula includes zi = individual z-score, MVi as individual mean value, MV as mean value, and SD as standard deviation (note MV and SD were calculated across both age groups and all conditions to preserve differences). Subsequently, we calculated individual mean values from all three core functions to examine the unitary approach.
Statistics
We analyzed the heart rate measurements to verify physical activity intensities. Therefore, we calculated mean values from the entire 20 min of physical activity in each condition as well as for the entire test session. Then, we conducted a 2 (condition, aerobic, coordination) × 2 (time, during activity, during tests) analysis of variance (ANOVA) with repeated measurements on the latter factor for each age group.
Furthermore, we conducted a 2 (age, young, old) × 3 (condition, aerobic, coordination, control) × 2 (activity, with or without previous activity) ANOVA with repeated measurements for each core function of our diverse approach and the MV from our unitary approach. In the CG, we additionally examined reliability by the intraclass correlation coefficient (ICC) for both age groups (i.e., young and old) and further conducted a 2 (age, young, old) × 2 (time, first, second session) ANOVA. The latter tested whether participants improved their task performance between tests (i.e., learning effects).
Results
Results from heart rate measurements showed that young (F(1,31) = 11.36, p < 0.01, ɳ2 = 0.268) as well as older (F(1,24) = 14.99, p < 0.01, ɳ2 = 0.385) participants differed in their heart rate between both activity conditions and mean heart rate during the cognitive tests. The post hoc analysis indicated that mean heart rate values were higher in aerobic than in coordinative activity groups (young, mean difference (MD) = 17 bpm; old, MD = 21 bpm). Regarding intensities in our aerobic AGs, only mean heart rate in older participants (MV = 126 ± 19.3 bpm) met our instructions. Heart rates in young participants were slightly lower (MV = 148 ± 12.8 bpm) than instructed, however, it equaled 76% HRmax and therefore still met the range for moderate intensity.
Regarding our diverse approach, we found significant age effects for inhibition (F(1,95) = 22.40, p < 0.01, ɳ2 = 0.167, Fig. 2), updating (F(1,95) = 69.82, p < 0.01, ɳ2 = 0.388, Fig. 3), and task shifting (F(1,95) = 28.09, p < 0.01, ɳ2 = 0.220, Fig. 4). Older participants showed a decreased performance for inhibition (MD = − 39.14 ms) and updating (MD = − 21.7%) and better results for task shifting (MD = 48.59 ms) compared with younger participants. There was also a significant interaction of age × condition for inhibition (F(2,95) = 5.99, p < 0.01, ɳ2 = 0.090, Fig. 2) and updating (F(2,95) = 5.29, p < 0.01, ɳ2 = 0.059, Fig. 3). Regarding physical activity, results indicated a significant interaction of activity × condition for inhibition (F(2,95) = 3.43, p = 0.04, ɳ2 = 0.009, Fig. 2). Moreover, updating showed a main effect for activity (F(1,95) = 9.36, p < 0.01, ɳ2 = 0.008, Fig. 3) and a significant interaction for activity × age (F(1,95) = 5.76, p = 0.02, ɳ2 = 0.005, Fig. 3).
In view of the activity × condition interaction of inhibition, the post hoc comparisons (Bonferroni-corrected) no longer showed significant differences. For updating, post hoc comparisons (Bonferroni-corrected) of activity × age indicated that only young participants showed significant activity-related differences (MD = 5.93%, p < 0.01).
Regarding our unitary approach, we found only a significant age effect (F(1,95) = 22.32, p < 0.01, ɳ2 = 0.177), indicating a slightly better performance in young compared with older participants (MD = 0.41, p < 0.01).
Our analysis for the test-retest reliability showed that three of the six tasks in young and four of the six tasks in older participants revealed a significant moderate to good reliability (see Table 1). Nearly all tasks revealed significant differences between both age groups. Independently of age, the post hoc analysis of the semantic switch task showed a significantly decreased performance (MD = − 34.506, ɳ2 = 0.153, CI (95%) = − 65.304 to − 3.709) across time, indicated by higher switching costs. The Stroop task (MD = 27.037, ɳ2 = 0.172, CI (95%) = 4.469 to 49.606) as well as the Simon task (MD = 10.398, ɳ2 = 0.147, CI (95%) = 0.874 to 19.921) showed a significant increase across time, indicating lower inhibition costs in both age groups. Post hoc analysis of the time × age interaction in the n-back task showed significant learning effects for younger adults (see Table 1).
Discussion
The aim of this study was to test the effects of different acute bouts of physical activity with a diverse as well as a unitary approach of executive functions (EF) in young and older adults. In a control group, we additionally assessed our cognitive tasks for reliability and test-retest effects (i.e., learning). We found overall age effects in each approach. Physical activity appeared to only improve updating in young adults, however, this effect seemed to be stable across all conditions, and therefore we cannot exclude any potential learning effects. Additionally, our results also indicated a lack of test-retest reliability and varied regarding testing periods, which we attribute to the mentioned learning effects.
We are not aware of any other study that has used different tests of core EF to analyze the effects of physical activity on a construct level (i.e., using latent variable approach). Interestingly, previous studies that have used single tests have found many benefits of exercise on cognition (Chang et al. 2012; Ludyga et al. 2016). This contradicts the results of our study and could be due to a variety of factors such as age, exercise intensities, length of cognitive tasks battery (between one and three tasks vs six tasks), and insufficient or unverified test-retest reliability (for a review see Pontifex et al. (2019)). Therefore, the main aspects are discussed in the following sections.
Regarding the age factor, it should be mentioned that EF change during the developmental phases from children to young adults (Best and Miller 2010). Regarding aerobic exercises, a previous study in adolescents showed effects on inhibition and working memory during but not after moderate physical activity (i.e., 60% and 70% HRmax) (Soga et al. 2015). In contrast, other research in preadolescents revealed benefits only on inhibition following 60% HRmax treadmill running (Hillman et al. 2009); or on task shifting, updating, and inhibition following running at 60–70% HRmax on a playing field (Chen et al. 2014). We used comparable intensities (our intensities were slightly lower than 80% HRmax); however, our results are only consistent in terms of updating. Importantly, most of the named studies recruited younger participants (e.g., children), which highlights the abovementioned factor of age. A further aspect is the task modality; our keep track task was quite similar to a free recall memory task. In this regard, Coles and Tomporowski (2008) suggested that an activity-induced revival might be beneficial for long-term memory processes. Besides those methodological issues, there could also be a physiological explanation. Previous studies have shown that exercise results in biochemical adaptations (i.e., catecholamine and hormone levels), which affects cognition (McMorris et al. 2015; Piepmeier and Etnier 2015). More precisely, increased levels of brain-derived neurotrophic factor (BDNF) might be responsible for positive post-exercise effects on memory functions. This assumption is based on the fact that both memory functions and BDNF expression rely on the hippocampus (Piepmeier and Etnier 2015). Additionally, the authors showed that such an increase in the BDNF level can last for 10 to 60 min (Piepmeier and Etnier 2015), whereby a previous study indicated that this time is primarily influenced by the exercise duration (Schmolesky et al. 2013). Thus, a potential explanation for our results could be that the increased BDNF level only affected the updating task performance. However, this interpretation should be considered carefully as we did not analyze BDNF.
To the best of our knowledge, this is the first study that used simple coordinative activities to test benefits on cognition in older adults. Other similar studies have examined more complex activities such as dancing (Kimura and Hozumi 2012), yoga (Gothe and McAuley 2015), or exergames (Monteiro-Junior et al. 2017) that included multiple different coordinative components at the same time. Regarding those studies, effects on EF were limited but promising. Authors reported reduced switching costs following a combination style dancing program, overall improved EF following yoga, and no effects on EF from exergames. However, regarding yoga, the results should be interpreted cautiously due to a lot of inconsistencies between yoga programs in the reviewed studies (Gothe and McAuley 2015). Thus, research on how acute bouts of coordinative activities affect executive functioning in healthy older adults is limited. Regarding other physical activities, recent studies have shown benefits on inhibition after acute bouts of aerobic (Johnson et al. 2016) or resistance (Chang, Tsai, et al., 2014; Johnson et al. 2016) activities. In contrast, earlier studies reported no benefits of acute bouts of aerobic activity on the inhibition part of a Stroop task in older adults (Barella et al. 2010) or acute bouts of resistance activity on a trail making test in middle-aged adults (Chang and Etnier 2009). In summary, there is mixed evidence concerning benefits of acute bouts of physical activity on inhibition with increasing age.
Our current research showed no effects of different activities in this age group. Additionally, most cognitive tasks showed acceptable test-retest reliability, while three tasks also indicated variable performances. Thus, in older adults, we showed relatively stable results indicating that 20 min of physical activity on the threshold to high intensity did not improve EF. In contrast to our results, another study found activity-related benefits on reaction times but not on accuracy in a working memory task (n-back task) independently of age (Hogan et al. 2013). These differences might occur because of some methodological issues. Hogan et al. (2013) used two independent groups and one testing session, whereas our study was based on a test-retest design with counter-balanced performance of physical activity. We discussed above the influence of BDNF on cognitive performance in young adults, but in older adults, this relationship might be influenced by structural changes of the brain during the (healthy) aging process (Fjell and Walhovd 2010; Voelcker-Rehage and Niemann 2013). More precisely, another study showed a reduced hippocampal volume as well as decreased working memory performance at an older age (Erickson et al. 2010). Generally, there could also be exercise-induced BDNF expression at an older age; however, regarding our results, 20 min of physical activity might be insufficient. This is in line with another study with older adults which showed increased BDNF levels as well as better working memory performance following 35 min of moderate intensity exercise (Håkansson et al. 2017).
Furthermore, we anticipated similar results between different conditions of physical activity (aerobic and coordinative activities), but our results did not indicate any differences in both age groups. The overall lack of findings, however, is partly in line with previous studies. They did not find differences between exercise modalities such as aerobic, coordination, and strength (van den Berg et al. 2016); aerobic and coordination (Ludyga et al. 2017); or aerobic and strength, either (Alves et al. 2012). However, it should be mentioned that these studies only included children or adult females. Furthermore, those and other studies also differed in their characteristics (e.g., type, duration, intensities) and settings (e.g., urban areas, nature) of physical activity (Pontifex et al. 2019), which makes a direct comparisons more difficult. As a first step towards a solution, another review described standardized guidelines to improve comparability between studies (Basso and Suzuki 2017).
We further conducted test-retest comparisons using intraclass correlations and analysis of variance for each test in both age groups to prove reliability of our cognitive tasks. In both age groups, we discovered mixed results which indicate that test-retest reliability should be interpreted with caution. Our results are partly in line with another study that examined the test-retest reliability for attentional and executive tasks in middle-aged and older adults (Lemay et al. 2004). They showed acceptable results for most of their tasks; however, for error-based scores (i.e., reaction time tasks, Stroop task), they reported a lack of reliability. Similar results of reduced test-retest reliability were shown for more complex tasks (i.e., Tower of Hanoi, Wisconsin Card Sorting Test) of EF (Lowe and Rabbitt 1998; Miyake et al. 2000). Miyake et al. (2000) therefore suggested that people used a strategy adaptation when performing these tasks. Regarding such adaptations, it would be advantageous to learn each task extensively prior to the testing. In another study, the authors showed moderate scores for reliability of a neurocognitive test battery (Gualtieri and Johnson 2006). Despite the authors’ findings, these researchers also pointed out that neurocognitive test batteries can be less reliable since memory, attention, and reaction times can be affected by external (i.e., time of day, drugs, disease) as well as internal (i.e., motivation, fatigue, mood) factors. In summary, reliability of computerized tasks of EF might be influenced by internal/external factors, task complexity, and task learning effects. Specifically, increasing task complexity allows more than one solution strategy, and therefore participants’ performances could vary. Thus, in test-retest study designs, a reliability test seems to be important to prove the aptitude of the cognitive tasks. Our results support this view, and additional testing concerning the reliability would be helpful regarding the interpretation of further results.
It is also possible that our lack of findings is based on some limitations. First, the mixed results from reliability testing indicate a necessity of learning sessions for some cognitive tests. A further limitation concerns our overall quite long cognitive task duration, which could negatively change internal factors (e.g., mood, fatigue, motivation) that affect task performance and therefore reduce possible benefits. Furthermore, the additional transition time from the gym to the laboratory might have influenced the participants’ BDNF level which could have been higher for 10 to 60 min and also depends entirely on the duration of the exercise. Thus, the transition time plus our time-consuming cognitive tasks might have been too long to show effects on cognition. Furthermore, we used a percentage of the groups’ means HRmax and defined upper/lower intensity limits. However, instead of using these means, it might have been more effective and precise to calculate the individual intensity range. A further limitation is the time difference of about 30 min between our exercise and no exercise sessions. Thus, there might be a confounding effect in the AG between both sessions from the shorter duration of our “control” session.
Overall, we found improvements for updating in young adults following acute bouts of physical activity, which should however, be interpreted with caution due to our methodological limitations. Additionally, there were no differences between aerobic and coordinative activities in the young or in the older adults. In future studies, it would be important to control cognitive tasks for test-retest reliability. Furthermore, the activity prescriptions should be written in such a way that studies can be compared with others, and participants’ physical performance should be measured intensively to differentiate between effects from various types of physical activities. As an additional aspect, it would be interesting to extend the research on a construct level (i.e., using latent variables) of EF.
Notes
Equivalent includes VO2 and heart rate. However, it should be mentioned that there are also other definitions for exercise physiology or endocrinology in literature (e.g., 64–76 % HRmax, from the American College of Sports Medicine (2010) guidelines).
Cognitive performance seems to be impaired immediately after high intensity exercise (Chang et al. 2012), thus a short delay was necessary.
References
Adrover-Roig, D., Sesé, A., Barceló, F., & Palmer, A. (2012). A latent variable approach to executive control in healthy ageing. Brain and Cognition, 78(3), 284–299. https://doi.org/10.1016/j.bandc.2012.01.005.
Alvarez-Alamilla, J., Velasco, A., & Río-Portilla, Y. (2016). Conflict processing and response inhibition in patients with temporal lobe epilepsy fMRI Study. Epilepsy Journal, 2(3). https://doi.org/10.4172/2472-0895.1000113.
Alves, C. R. R., Gualano, B., Takao, P. P., Avakian, P., Fernandes, R. M., Morine, D., & Takito, M. Y. (2012). Effects of acute physical exercise on executive functions: a comparison between aerobic and strength exercise. Journal of Sport and Exercise Psychology, 34(4), 539–549. https://doi.org/10.1123/jsep.34.4.539.
Barella, L. A., Etnier, J. L., & Chang, Y.-K. (2010). The immediate and delayed effects of an acute bout of exercise on cognitive performance of healthy older adults. Journal of Aging and Physical Activity, 18, 87–98 Retrieved from http://journals.humankinetics.com/doi/pdf/10.1123/japa.18.1.87.
Basso, J. C., & Suzuki, W. A. (2017). The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plasticity, 2, 127–152. https://doi.org/10.3233/BPL-160040.
Best, J. R., & Miller, P. H. (2010). A developmental perspective on executive function. Child Development, 81(6), 1641–1660. https://doi.org/10.1111/j.1467-8624.2010.01499.x.
Bialystok, E., Craik, F., & Luk, G. (2008). Cognitive control and lexical access in younger and older bilinguals. Journal of Experimental Psychology-Learning Memory and Cognition, 34(4), 859–873. https://doi.org/10.1037/0278-7393.34.4.859.
Bock, O., Haeger, M., & Voelcker-Rehage, C. (2019). Structure of executive functions in young and in older persons. PLOS One, 14(5), e0216149. https://doi.org/10.1371/journal.pone.0216149.
Brisswalter, J., Callardeau, M., & René, A. (2002). Effects of acute physical exercise on cognitive performance. Sports Medicine (Auckland, N.Z.), 32(9), 555–566. https://doi.org/10.1097/00005768-199701000-00009.
Bugg, J. M., & Hutchison, K. a. (2012). Converging evidence for control of color–word stroop interference at the item level. Journal of Experimental Psychology: Human Perception and Performance, 39(2), 433–449. https://doi.org/10.1037/a0029145.
Chang, Y.-K., & Etnier, J. L. (2009). Effects of an acute bout of localized resistance exercise on cognitive performance in middle-aged adults: a randomized controlled trial study. Psychology of Sport & Exercise, 10, 19–24. https://doi.org/10.1016/j.psychsport.2008.05.004.
Chang, Y.-K., Labban, J. D., Gapin, J. I., & Etnier, J. L. (2012). The effects of acute exercise on cognitive performance: a meta-analysis. Brain Research, 1453(March), 87–101. https://doi.org/10.1016/j.brainres.2012.02.068.
Chang, Y.-K., Chi, L., Etnier, J. L., Wang, C.-C., Chu, C.-H., & Zhou, C. (2014a). Effect of acute aerobic exercise on cognitive performance: role of cardiovascular fitness. Psychology of Sport and Exercise, 15(5), 464–470. https://doi.org/10.1016/j.psychsport.2014.04.007.
Chang, Y.-K., Tsai, C.-L., Huang, C.-C., Wang, C.-C., & Chu, I.-H. (2014b). Effects of acute resistance exercise on cognition in late middle-aged adults: general or specific cognitive improvement? Journal of Science and Medicine in Sport, 17, 51–55. https://doi.org/10.1016/j.jsams.2013.02.007.
Chen, A.-G., Yan, J., Yin, H.-C., Pan, C.-Y., & Chang, Y.-K. (2014). Effects of acute aerobic exercise on multiple aspects of executive function in preadolescent children. Psychology of Sport and Exercise, 15(6), 627–636. https://doi.org/10.1016/j.psychsport.2014.06.004.
Chung, H. J., Weyandt, L. L., & Swentosky, A. (2014). Handbook of executive functioning. In S. Goldstein & J. A. Naglieri (Eds.), Handbook of Executive Functioning (pp. 1–567). New York: Springer Science+Business Media. https://doi.org/10.1007/978-1-4614-8106-5.
Coles, K., Tomporowski, P. D. (2008) Effects of acute exercise on executive processing, short-term and long-term memory. Journal of Sports Sciences, 26(3), 333–344. https://doi.org/10.1080/02640410701591417.
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750.Executive.
Diamond, A., & Ling, D. S. (2016). Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Developmental Cognitive Neuroscience, 18, 34–48. https://doi.org/10.1016/j.dcn.2015.11.005.
Erickson, K. I., Prakash, R. S., Voss, M. W., Chaddock, L., Heo, S., Mclaren, M., et al. (2010). BDNF is associated with age-related decline in hippocampal volume. Journal of Neuroscience, 30(15), 5368–5375. https://doi.org/10.1523/JNEUROSCI.6251-09.2010.
Falleti, M., Maruff, P., Collie, A., & Darby, D. (2006). Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. Journal of Clinical and Experimental Neuropsychology, 28(7), 1095–1112. https://doi.org/10.1080/13803390500205718.
Fjell, A. M., & Walhovd, K. B. (2010). Structural brain changes in aging: courses, causes and cognitive consequences. Reviews in the Neurosciences, 21(3), 187–221. https://doi.org/10.1515/REVNEURO.2010.21.3.187.
Fournier-Vicente, S., Larigauderie, P., & Gaonac’h, D. (2008). More dissociations and interactions within central executive functioning: a comprehensive latent-variable analysis. Acta Psychologica, 129(1), 32–48. https://doi.org/10.1016/j.actpsy.2008.04.004.
Gothe, N., & McAuley, E. (2015). Yoga and cognition: a meta-analysis of chronic and acute effects. Kinesiology, Health and Sport Studies, 48 Retrieved from http://digitalcommons.wayne.edu/coe_khs/48.
Gualtieri, C. T., & Johnson, L. G. (2006). Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Archives of Clinical Neuropsychology, 21, 623–643. https://doi.org/10.1016/j.acn.2006.05.007.
Håkansson, K., Ledreux, A., Daffner, K., Terjestam, Y., Bergman, P., Carlsson, R., et al. (2017). BDNF responses in healthy older persons to 35 minutes of physical exercise , cognitive training and mindfulness: associations with working memory function. Journal of Alzheimer’s Disease, 55(2), 645–657. https://doi.org/10.3233/JAD-160593.
Hillman, C. H., Pontifex, M. B., Raine, L. B., Castelli, D. M., Hall, E. E., & Kramer, A. F. (2009). The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience, 159(3), 1044–1054. https://doi.org/10.1016/j.neuroscience.2009.01.057.
Hogan, C. L., Mata, J., & Carstensen, L. L. (2013). Exercise holds immediate benefits for affect and cognition in younger and older adults. Psychology and Aging, 28(2), 587–594. https://doi.org/10.1037/a0032634.
Hull, R., Martin, R. C., Beier, M. E., Lane, D., & Hamilton, A. C. (2008). Executive function in older adults: a structural equation modeling approach. Neuropsychology, 22(4), 508–522. https://doi.org/10.1037/0894-4105.22.4.508.
Hung, T. M., Tsai, C. L., Chen, F. T., Wang, C. C., & Chang, Y. K. (2013). The immediate and sustained effects of acute exercise on planning aspect of executive function. Psychology of Sport and Exercise, 14(5), 728–736. https://doi.org/10.1016/j.psychsport.2013.05.004.
Johnson, L., Addamo, P. K., Selva Raj, I., Borkoles, E., Wyckelsma, V., Cyarto, E., & Polman, R. C. (2016). An acute bout of exercise improves the cognitive performance of older adults. Journal of Aging and Physical Activity, 24, 591–598. https://doi.org/10.1123/japa.2015-0097.
Kamijo, K., Hayashi, Y., Sakai, T., Yahiro, T., Tanaka, K., & Nishihira, Y. (2009). Acute effects of aerobic exercise on cognitive function in older adults. Journal of Gerontology: Psychological Sciences, 64(3), 356–363. https://doi.org/10.1093/geronb/gbp030.
Karbach, J., & Kray, J. (2009). How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science, 12(6), 978–990. https://doi.org/10.1111/j.1467-7687.2009.00846.x.
Kimura, K., & Hozumi, N. (2012). Investigating the acute effect of an aerobic dance exercise program on neuro-cognitive function in the elderly. Psychology of Sport and Exercise, 13(5), 623–629. https://doi.org/10.1016/J.PSYCHSPORT.2012.04.001.
Kray, J., & Lindenberger, U. (2000). Adult age differences in task switching. Psychology and Aging, 15(1), 126–147.
Lemay, S., Bédard, M.-A., Rouleau, I., & Tremblay, P.-L. G. (2004). Practice effect and test-retest reliability of attentional and executive tests in middle-aged to elderly subjects*. The Clinical Neuropsychologist, 18(2), 1–19 Retrieved from https://s3.amazonaws.com/academia.edu.documents/39805706/Practice_effect_and_test-retest_reliabil20151108-24242-16fm228.pdf?AWSAccessKeyId = AKIAIWOWYYGZ2Y53UL3A&Expires=1508514416&Signature=%2Bai5%2FdKitP%2FsLC32QiCtpFeNO3A%3D&response-content-disposition=.
Lowe, C., & Rabbitt, P. (1998). Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Neuropsychologia, 36(9), 915–923 Retrieved from https://ac.els-cdn.com/S0028393298000360/1-s2.0-S0028393298000360-main.pdf?_tid=26f5f396-cdf2-11e7-ab00-00000aacb35e&acdnat=1511182738_7a6a5122136129c6218bc64a598e6ba8.
Ludyga, S., Gerber, M., Brand, S., Holsboer-Trachsler, E., & Pühse, U. (2016). Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology, 53(11), 1611–1626. https://doi.org/10.1111/psyp.12736.
Ludyga, S., Brand, S., Gerber, M., Weber, P., Brotzmann, M., Habibifar, F., & Pühse, U. (2017). An event-related potential investigation of the acute effects of aerobic and coordinative exercise on inhibitory control in children with ADHD. Developmental Cognitive Neuroscience, 28, 21–28. https://doi.org/10.1016/J.DCN.2017.10.007.
Marchetti, R., Forte, R., Borzacchini, M., Vazou, S., Tomporowski, P. D., & Pesce, C. (2015). Physical and Motor Fitness, Sport Skills and Executive Function in Adolescents: A Moderated Prediction Model. Psychology, 6(6), 1915–1929. https://doi.org/10.4236/psych.2015.614189.
McMorris, T., & Hale, B. J. (2015). Is there an acute exercise-induced physiological/biochemical threshold which triggers increased speed of cognitive functioning? A meta-analytic investigation. Journal of Sport and Health Science, 4(1), 4–13. https://doi.org/10.1016/j.jshs.2014.08.003.
McMorris, T., Turner, A., Hale, B. J., & Sproule, J. (2015). Beyond the Catecholamines Hypothesis for an Acute Exercise-Cognition Interaction: A Neurochemical Perspective. In T. McMorris (Ed.), Exercise-Cognition Interaction: Neuroscience Perspectives (1st ed., pp. 65–103). Elsevier Inc.. https://doi.org/10.1016/B978-0-12-800778-5.00004-9.
Miyake, A., & Friedman, N. P. (2012). The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Current Directions in Psychological Science, 21(1), 8–14. https://doi.org/10.1177/0963721411429458.
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734.
Monteiro-Junior, R. S., da Silva Figueiredo, L. F., de Tarso Maciel-Pinheiro, P., Abud, L. E. R., Braga, A. M. E. M., Barca, M. L., et al. (2017). Acute effects of exergames on cognitive function of institutionalized older persons: a single-blinded, randomized and controlled pilot study. Aging Clinical and Experimental Research, 29, 387–394. https://doi.org/10.1007/s40520-016-0595-5.
Pesce, C. (2009). An integrated approach to the effect of acute and chronic exercise on cognition: the linked role of individual and task constraints. In T. McMorris, P. D. Tomporowski, & M. Audiffren (Eds.), Exercise and Cognitive Function (pp. 212–226). Chichester: Wiley-Heinrich.
Piepmeier, A. T., & Etnier, J. L. (2015). Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. Journal of Sport and Health Science, 4(1), 14–23. https://doi.org/10.1016/j.jshs.2014.11.001.
Pontifex, M. B., McGowan, A. L., Chandler, M. C., Gwizdala, K. L., Parks, A. C., Fenn, K., & Kamijo, K. (2019). A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychology of Sport and Exercise, 40(August 2018), 1–22. https://doi.org/10.1016/j.psychsport.2018.08.015.
Salthouse, T. A., & Davis, H. P. (2006). Organization of cognitive abilities and neuropsychological variables across the lifespan. Developmental Review, 26(1), 31–54. https://doi.org/10.1016/j.dr.2005.09.001.
Schmiedek, F., Hildebrandt, A., Lövdén, M., Lindenberger, U., & Wilhelm, O. (2009a). Complex span versus updating tasks of working memory: the gap is not that deep. Journal of Experimental Psychology. Learning, Memory, and Cognition, 35(4), 1089–1096. https://doi.org/10.1037/a0015730.
Schmiedek, F., Li, S.-C., & Lindenberger, U. (2009b). Interference and facilitation in spatial working memory: age-associated differences in lure effects in the n-back paradigm. Psychology and Aging, 24(1), 203–210. https://doi.org/10.1037/a0014685.
Schmolesky, M. T., Webb, D. L., & Hansen, R. A. (2013). The effects of aerobic exercise intensity and duration on levels of brain- derived neurotrophic factor in healthy men. Journal of Sports Science and Medicine, 12, 502–511 Retrieved from http://www.jssm.org.
Soga, K., Shishido, T., & Nagatomi, R. (2015). Executive function during and after acute moderate aerobic exercise in adolescents. Psychology of Sport & Exercise, 16, 7–17. https://doi.org/10.1016/j.psychsport.2014.08.010.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662 Retrieved from https://psych.hanover.edu/classes/Cognition/papers/stroop1933.pdf.
Tanaka, H., Monahan, K. D., & Seals, D. R. (2001). Age-predicted maximal heart rate revisited. https://doi.org/10.1016/S0735-1097(00)01054-8.
Tomporowski, P. D., McCullick, B., Pendleton, D. M., & Pesce, C. (2014). Exercise and children’s cognition: the role of exercise characteristics and a place for metacognition. Journal of Sport and Health Science, 4(1), 47–55. https://doi.org/10.1016/j.jshs.2014.09.003.
van den Berg, V., Saliasi, E., de Groot, R. H. M., Jolles, J., Chinapaw, M. J. M., & Singh, A. S. (2016). Physical activity in the school setting: cognitive performance is not affected by three different types of acute exercise. Frontiers in Psychology, 7, 723. https://doi.org/10.3389/fpsyg.2016.00723.
Voelcker-Rehage, C., & Niemann, C. (2013). Structural and functional brain changes related to different types of physical activity across the life span. Neuroscience & Biobehavioral Reviews, 37(9 Pt B), 2268–2295. https://doi.org/10.1016/j.neubiorev.2013.01.028.
Yntema, D. B. (1963). Keeping track of several things at once. Human Factors, 8–17.
Funding
Open Access funding provided by Projekt DEAL. This work was supported by a stipend “Impaired Mobility in older Age” (number 820002) sponsored by the German Sport University Cologne and awarded to M. Haeger.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of the German Sport University Cologne (148/2016) in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Participants were informed verbally as well as in writing about our study and signed an informed consent form.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haeger, M., Bury, N., Endres, C. et al. Are There Extended Cognitive Improvements from Different Kinds of Acute Bouts of Physical Activity?. J Cogn Enhanc 4, 401–411 (2020). https://doi.org/10.1007/s41465-020-00177-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-020-00177-1