Abstract
We have a limited understanding of the role of exercise on memory function among those with Parkinson’s disease (PD). This review discusses the mechanisms influencing PD, examines the extent and mechanisms through which exercise can reduce the risk of developing PD, details the extent and mechanisms through which exercise is associated with memory function among PD, and indicates the effects of exercise on various non-memory outcomes among PD patients. A narrative and systematic review approach was employed. We specifically highlight the role of dopamine in PD; indicate the protective effect of exercise in reducing PD risk, which may occur from exercise-induced alterations in dopamine levels, inflammation, oxidative stress, and neurotrophic factor levels; detail the literature (in human and animal models) demonstrating that exercise can enhance memory function among PD patients, which may occur from exercise-induced changes in dopamine and modulation of mechanisms associated with memory (e.g., AMPA and NMDA receptor expression); and demonstrate that regular exercise engagement (including various exercise modalities) among PD patients can improve motor function, psychological function, and prevent early mortality. Exercise may be a putative strategy to help prevent PD and treat the memory impairment associated with PD. Recommendations for future research are discussed.
Similar content being viewed by others
References
Aarsland, D., Andersen, K., Larsen, J. P., Lolk, A., & Kragh-Sorensen, P. (2003). Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Archives of Neurology, 60(3), 387–392.
Aarsland, D., Zaccai, J., & Brayne, C. (2005). A systematic review of prevalence studies of dementia in Parkinson’s disease. Movement Disorders, 20(10), 1255–1263. https://doi.org/10.1002/mds.20527.
Aguiar Jr., A. S., Araujo, A. L., da Cunha, T. R., Speck, A. E., Ignacio, Z. M., De-Mello, N., & Prediger, R. D. (2009). Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Research Bulletin, 79(6), 452–457. https://doi.org/10.1016/j.brainresbull.2009.05.005.
Aguiar Jr., A. S., Lopes, S. C., Tristao, F. S., Rial, D., de Oliveira, G., da Cunha, C., et al. (2016). Exercise improves cognitive impairment and dopamine metabolism in MPTP-treated mice. Neurotoxicity Research, 29(1), 118–125. https://doi.org/10.1007/s12640-015-9566-4.
Allen, N. E., Sherrington, C., Suriyarachchi, G. D., Paul, S. S., Song, J., & Canning, C. G. (2012). Exercise and motor training in people with Parkinson’s disease: a systematic review of participant characteristics, intervention delivery, retention rates, adherence, and adverse events in clinical trials. Parkinsons Dis, 2012, 854328. https://doi.org/10.1155/2012/854328.
Altmann, L. J., Stegemoller, E., Hazamy, A. A., Wilson, J. P., Bowers, D., Okun, M. S., & Hass, C. J. (2016). Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: results of a controlled study. Journal of the International Neuropsychological Society, 22(9), 878–889. https://doi.org/10.1017/S135561771600076X.
Beatty, W. W., Monson, N., & Goodkin, D. E. (1989). Access to semantic memory in Parkinson’s disease and multiple sclerosis. Journal of Geriatric Psychiatry and Neurology, 2(3), 153–162.
Beaulieu, J. M., & Gainetdinov, R. R. (2011). The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological Reviews, 63(1), 182–217. https://doi.org/10.1124/pr.110.002642.
Berzosa, C., Cebrian, I., Fuentes-Broto, L., Gomez-Trullen, E., Piedrafita, E., Martinez-Ballarin, E., et al. (2011). Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. Journal of Biomedicine & Biotechnology, 2011, 540458. https://doi.org/10.1155/2011/540458.
Bethus, I., Tse, D., & Morris, R. G. (2010). Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. The Journal of Neuroscience, 30(5), 1610–1618. https://doi.org/10.1523/JNEUROSCI.2721-09.2010.
Bell, P. T., Gilat, M., & Shine, J. M. (2017). Striatal dysfunction during dual-task performance in Parkinson’s disease. Brain, 140(5), 1174–1177. https://doi.org/10.1093/brain/awx063.
Bouchard, T. P., Malykhin, N., Martin, W. R., Hanstock, C. C., Emery, D. J., Fisher, N. J., & Camicioli, R. M. (2008). Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiology of Aging, 29(7), 1027–1039. https://doi.org/10.1016/j.neurobiolaging.2007.02.002.
Brienesse, L. A., & Emerson, M. N. (2013). Effects of resistance training for people with Parkinson’s disease: a systematic review. Journal of the American Medical Directors Association, 14(4), 236–241. https://doi.org/10.1016/j.jamda.2012.11.012.
Bruck, A., Kurki, T., Kaasinen, V., Vahlberg, T., & Rinne, J. O. (2004). Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. Journal of Neurology, Neurosurgery, and Psychiatry, 75(10), 1467–1469. https://doi.org/10.1136/jnnp.2003.031237.
Blesa, J., Trigo-Damas, I., Quiroga-Varela, A., & Jackson-Lewis, V. R. (2015). Oxidative stress and Parkinson’s disease. Frontiers in Neuroanatomy, 9, 91. https://doi.org/10.3389/fnana.2015.00091.
Cepeda, C., Andre, V. M., Jacoy, E. L., & Levine, M. S. (2009). NMDA and dopamine: diverse mechanisms applied to interacting receptor systems. In A. M. Van Dongen (Ed.), Biology of the NMDA receptor. Boca Raton, FL: CRC Press/Taylor & Francis.
Chen, H., Zhang, S. M., Schwarzschild, M. A., Hernan, M. A., & Ascherio, A. (2005). Physical activity and the risk of Parkinson disease. Neurology, 64(4), 664–669. https://doi.org/10.1212/01.WNL.0000151960.28687.93.
Chiaravalloti, N. D., Ibarretxe-Bilbao, N., DeLuca, J., Rusu, O., Pena, J., Garcia-Gorostiaga, I., & Ojeda, N. (2014). The source of the memory impairment in Parkinson’s disease: acquisition versus retrieval. Movement Disorders, 29(6), 765–771. https://doi.org/10.1002/mds.25842.
Cho, H. S., Shin, M. S., Song, W., Jun, T. W., Lim, B. V., Kim, Y. P., & Kim, C. J. (2013). Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil, 9(3), 354–361. https://doi.org/10.12965/jer.130048.
Calne, D. B. (1993). Treatment of Parkinson’s disease. The New England Journal of Medicine, 329(14), 1021–1027. https://doi.org/10.1056/NEJM199309303291408.
Coffeen, U., Ortega-Legaspi, J. M., de Gortari, P., Simon-Arceo, K., Jaimes, O., Amaya, M. I., & Pellicer, F. (2010). Inflammatory nociception diminishes dopamine release and increases dopamine D2 receptor mRNA in the rat’s insular cortex. Molecular Pain, 6, 75. https://doi.org/10.1186/1744-8069-6-75.
Conradsson, D., Lofgren, N., Nero, H., Hagstromer, M., Stahle, A., Lokk, J., & Franzen, E. (2015). The effects of highly challenging balance training in elderly with Parkinson’s disease: a randomized controlled trial. Neurorehabilitation and Neural Repair, 29(9), 827–836. https://doi.org/10.1177/1545968314567150.
David, F. J., Robichaud, J. A., Leurgans, S. E., Poon, C., Kohrt, W. M., Goldman, J. G., et al. (2015). Exercise improves cognition in Parkinson’s disease: the PRET-PD randomized, clinical trial. Movement Disorders, 30(12), 1657–1663. https://doi.org/10.1002/mds.26291.
Delghandi, M. P., Johannessen, M., & Moens, U. (2005). The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cellular Signalling, 17(11), 1343–1351. https://doi.org/10.1016/j.cellsig.2005.02.003.
Dias, V., Junn, E., & Mouradian, M. M. (2013). The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis, 3(4), 461–491. https://doi.org/10.3233/JPD-130230.
Fama, R., Sullivan, E. V., Shear, P. K., Stein, M., Yesavage, J. A., Tinklenberg, J. R., & Pfefferbaum, A. (2000). Extent, pattern, and correlates of remote memory impairment in Alzheimer’s disease and Parkinson’s disease. Neuropsychology, 14(2), 265–276.
Felger, J. C. (2017). The role of dopamine in inflammation-associated depression: mechanisms and therapeutic implications. Current Topics in Behavioral Neurosciences, 31, 199–219. https://doi.org/10.1007/7854_2016_13.
Fisher, B. E., Petzinger, G. M., Nixon, K., Hogg, E., Bremmer, S., Meshul, C. K., & Jakowec, M. W. (2004). Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. Journal of Neuroscience Research, 77(3), 378–390. https://doi.org/10.1002/jnr.20162.
Fisher, G., Schwartz, D. D., Quindry, J., Barberio, M. D., Foster, E. B., Jones, K. W., & Pascoe, D. D. (2011). Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J Appl Physiol (1985), 110(3), 730–737. https://doi.org/10.1152/japplphysiol.00575.2010.
Fisher, B. E., Li, Q., Nacca, A., Salem, G. J., Song, J., Yip, J., et al. (2013). Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport, 24(10), 509–514. https://doi.org/10.1097/WNR.0b013e328361dc13.
Galvan, A., & Wichmann, T. (2008). Pathophysiology of parkinsonism. Clinical Neurophysiology, 119(7), 1459–1474. https://doi.org/10.1016/j.clinph.2008.03.017.
Gao, C., & Wolf, M. E. (2007). Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. The Journal of Neuroscience, 27(52), 14275–14285. https://doi.org/10.1523/JNEUROSCI.2925-07.2007.
Gao, L., Zhang, J., Hou, Y., Hallett, M., Chan, P., & Wu, T. (2017). The cerebellum in dual-task performance in Parkinson’s disease. Scientific Reports, 7, 45662. https://doi.org/10.1038/srep45662.
Garraux, G. (2008). Preserve brain function...through physical exercice? Revue Médicale de Liège, 63(5–6), 293–298.
Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., & Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews. Immunology, 11(9), 607–615. https://doi.org/10.1038/nri3041.
Goes, A. T., Souza, L. C., Filho, C. B., Del Fabbro, L., De Gomes, M. G., Boeira, S. P., & Jesse, C. R. (2014). Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience, 256, 61–71. https://doi.org/10.1016/j.neuroscience.2013.09.042.
Gomez-Cabrera, M. C., Borras, C., Pallardo, F. V., Sastre, J., Ji, L. L., & Vina, J. (2005). Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. The Journal of Physiology, 567(Pt 1), 113–120. https://doi.org/10.1113/jphysiol.2004.080564.
Gonzalez-Burgos, I., & Feria-Velasco, A. (2008). Serotonin/dopamine interaction in memory formation. Progress in Brain Research, 172, 603–623. https://doi.org/10.1016/S0079-6123(08)00928-X.
Hanagasi, H. A., Tufekcioglu, Z., & Emre, M. (2017). Dementia in Parkinson’s disease. Journal of the Neurological Sciences, 374, 26–31. https://doi.org/10.1016/j.jns.2017.01.012.
Hazamy, A. A., Altmann, L. J., Stegemoller, E., Bowers, D., Lee, H. K., Wilson, J., et al. (2017). Improved cognition while cycling in Parkinson’s disease patients and healthy adults. Brain and Cognition, 113, 23–31. https://doi.org/10.1016/j.bandc.2017.01.002.
Higuchi, M., Cartier, L. J., Chen, M., & Holloszy, J. O. (1985). Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. Journal of Gerontology, 40(3), 281–286.
Huber, S. J., Shuttleworth, E. C., & Paulson, G. W. (1986). Dementia in Parkinson’s disease. Archives of Neurology, 43(10), 987–990.
Hurwitz, A. (1989). The benefit of a home exercise regimen for ambulatory Parkinson’s disease patients. The Journal of Neuroscience Nursing, 21(3), 180–184.
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., & Morris, J. G. (2008). The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement Disorders, 23(6), 837–844. https://doi.org/10.1002/mds.21956.
Inskip, M., Mavros, Y., Sachdev, P. S., & Fiatarone Singh, M. A. (2016). Exercise for individuals with Lewy body dementia: a systematic review. PLoS One, 11(6), e0156520. https://doi.org/10.1371/journal.pone.0156520.
Jokinen, P., Bruck, A., Aalto, S., Forsback, S., Parkkola, R., & Rinne, J. O. (2009). Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism & Related Disorders, 15(2), 88–93. https://doi.org/10.1016/j.parkreldis.2008.03.005.
Klein, C., Rasinska, J., Empl, L., Sparenberg, M., Poshtiban, A., Hain, E. G., et al. (2016). Physical exercise counteracts MPTP-induced changes in neural precursor cell proliferation in the hippocampus and restores spatial learning but not memory performance in the water maze. Behavioural Brain Research, 307, 227–238. https://doi.org/10.1016/j.bbr.2016.02.040.
Kramberger, M. G., Stukovnik, V., Cus, A., Repovs, G., Tomse, P., Meglic, N. P., et al. (2010). Parkinson’s disease dementia: clinical correlates of brain spect perfusion and treatment. Psychiatria Danubina, 22(3), 446–449.
Kuroda, K., Tatara, K., Takatorige, T., & Shinsho, F. (1992). Effect of physical exercise on mortality in patients with Parkinson’s disease. Acta Neurologica Scandinavica, 86(1), 55–59.
Lanier, W. L. (1997). The afferentation theory of cerebral arousal. Neuroanesthesia. (pp. 27-38).
Lanier, W. L., Iaizzo, P. A., & Milde, J. H. (1989). Cerebral function and muscle afferent activity following intravenous succinylcholine in dogs anesthetized with halothane: the effects of pretreatment with a defasciculating dose of pancuronium. Anesthesiology, 71(1), 87–95.
Lanier, W. L., Iaizzo, P. A., Milde, J. H., & Sharbrough, F. W. (1994). The cerebral and systemic effects of movement in response to a noxious stimulus in lightly anesthetized dogs. Possible modulation of cerebral function by muscle afferents. Anesthesiology, 80(2), 392–401.
Logroscino, G., Sesso, H. D., Paffenbarger Jr., R. S., & Lee, I. M. (2006). Physical activity and risk of Parkinson’s disease: a prospective cohort study. Journal of Neurology, Neurosurgery, and Psychiatry, 77(12), 1318–1322. https://doi.org/10.1136/jnnp.2006.097170.
Loprinzi, P. D., Herod, S. M., Cardinal, B. J., & Noakes, T. D. (2013). Physical activity and the brain: a review of this dynamic, bi-directional relationship. Brain Research, 1539, 95–104. https://doi.org/10.1016/j.brainres.2013.10.004.
Loprinzi, P. D., Danzl, M. M., Ulanowski, E., & Paydo, C. (2017a). A pilot study evaluating the association between physical activity and cognition among individuals with Parkinson’s disease. Disability and Health Journal. https://doi.org/10.1016/j.dhjo.2017.05.004.
Loprinzi, P. D., Edwards, M. K., & Frith, E. (2017b). Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. The European Journal of Neuroscience, 46(5), 2067–2077. https://doi.org/10.1111/ejn.13644.
Loprinzi, P. D., Sng, E., & Frith, E. (2017c). “Memorcise”: implications for patient compliance and medication adherence. The Physician and Sportsmedicine. https://doi.org/10.1080/00913847.2018.1402664.
Loprinzi, P. D., Frith, E., & Ponce, P. (2018). Memorcise and Alzheimer’s disease. The Physician and Sportsmedicine, 1–10. https://doi.org/10.1080/00913847.2018.1445932.
Luscher, C., & Malenka, R. C. (2012). NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harbor Perspectives in Biology, 4(6). https://doi.org/10.1101/cshperspect.a005710.
Mahmmoud, R. R., Sase, S., Aher, Y. D., Sase, A., Groger, M., Mokhtar, M., et al. (2015). Spatial and working memory is linked to spine density and mushroom spines. PLoS One, 10(10), e0139739. https://doi.org/10.1371/journal.pone.0139739.
Marchant, D. W. (2016). Dancing with disease: a dancer’s reflections on moving with people with Parkinson’s and memory loss. Frontiers in Neurology, 7, 137. https://doi.org/10.3389/fneur.2016.00137.
Martin, W. R., Wieler, M., Gee, M., & Camicioli, R. (2009). Temporal lobe changes in early, untreated Parkinson’s disease. Movement Disorders, 24(13), 1949–1954. https://doi.org/10.1002/mds.22680.
Mattson, M. P. (2015). Late-onset dementia: a mosaic of prototypical pathologies modifiable by diet and lifestyle. NPJ Aging Mech Dis, 1. https://doi.org/10.1038/npjamd.2015.3.
Methajarunon, P., Eitivipart, C., Diver, C. J., Phys, G. D., & Foongchomcheay, A. (2016). Systematic review of published studies on aquatic exercise for balance in patients with multiple sclerosis, Parkinson’s disease, and hemiplegia. Hong Kong Physiotherapy Journal, 35, 12–20.
Mohr, E., Fabbrini, G., Williams, J., Schlegel, J., Cox, C., Fedio, P., & Chase, T. N. (1989). Dopamine and memory function in Parkinson’s disease. Movement Disorders, 4(2), 113–120. https://doi.org/10.1002/mds.870040202.
Morrin, H., Fang, T., Servant, D., Aarsland, D., & Rajkumar, A. P. (2017). Systematic review of the efficacy of non-pharmacological interventions in people with Lewy body dementia. International Psychogeriatrics, 1–13. https://doi.org/10.1017/S1041610217002010.
Murray, D. K., Sacheli, M. A., Eng, J. J., & Stoessl, A. J. (2014). The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener, 3(1), 5. https://doi.org/10.1186/2047-9158-3-5.
Muslimovic, D., Post, B., Speelman, J. D., & Schmand, B. (2007). Motor procedural learning in Parkinson’s disease. Brain, 130(Pt 11), 2887–2897. https://doi.org/10.1093/brain/awm211.
Naismith, S. L., Mowszowski, L., Diamond, K., & Lewis, S. J. (2013). Improving memory in Parkinson’s disease: a healthy brain ageing cognitive training program. Movement Disorders, 28(8), 1097–1103. https://doi.org/10.1002/mds.25457.
Narayanan, N. S., Rodnitzky, R. L., & Uc, E. Y. (2013). Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Reviews in the Neurosciences, 24(3), 267–278. https://doi.org/10.1515/revneuro-2013-0004.
Neal, M., Cunningham, J., Lever, I., Pezet, S., & Malcangio, M. (2003). Mechanism by which brain-derived neurotrophic factor increases dopamine release from the rabbit retina. Investigative Ophthalmology & Visual Science, 44(2), 791–798.
Neurauter, G., Schrocksnadel, K., Scholl-Burgi, S., Sperner-Unterweger, B., Schubert, C., Ledochowski, M., & Fuchs, D. (2008). Chronic immune stimulation correlates with reduced phenylalanine turnover. Current Drug Metabolism, 9(7), 622–627.
Nieuwhof, F., Bloem, B. R., Reelick, M. F., Aarts, E., Maidan, I., Mirelman, A., et al. (2017). Impaired dual tasking in Parkinson’s disease is associated with reduced focusing of cortico-striatal activity. Brain, 140(5), 1384–1398. https://doi.org/10.1093/brain/awx042.
Nocera, J. R., Amano, S., Vallabhajosula, S., & Hass, C. J. (2013). Tai Chi exercise to improve non-motor symptoms of Parkinson’s disease. J Yoga Phys Ther, 3. https://doi.org/10.4172/2157-7595.1000137.
Paillard, T., Rolland, Y., & de Souto Barreto, P. (2015). Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol, 11(3), 212–219. https://doi.org/10.3988/jcn.2015.11.3.212.
Pereira, J. B., Junque, C., Marti, M. J., Ramirez-Ruiz, B., Bargallo, N., & Tolosa, E. (2009). Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Movement Disorders, 24(8), 1193–1199. https://doi.org/10.1002/mds.22560.
Perez, C. A., & Cancela, J. M. (2014). Effectiveness of water-based exercise in people living with Parkinson’s disease: a systematic review. European Review of Aging and Physical Activity, 11, 107–118.
Petzinger, G. M., Holschneider, D. P., Fisher, B. E., McEwen, S., Kintz, N., Halliday, M., et al. (2015). The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast, 1(1), 29–39.
Poo, M. M., Pignatelli, M., Ryan, T. J., Tonegawa, S., Bonhoeffer, T., Martin, K. C., et al. (2016). What is memory? The present state of the engram. BMC Biology, 14, 40. https://doi.org/10.1186/s12915-016-0261-6.
Pothakos, K., Kurz, M. J., & Lau, Y. S. (2009). Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neuroscience, 10, 6. https://doi.org/10.1186/1471-2202-10-6.
Powers, S. K., Criswell, D., Lawler, J., Ji, L. L., Martin, D., Herb, R. A., & Dudley, G. (1994). Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. The American Journal of Physiology, 266(2 Pt 2), R375–R380.
Powers, S. K., Radak, Z., & Ji, L. L. (2016). Exercise-induced oxidative stress: past, present and future. The Journal of Physiology, 594(18), 5081–5092. https://doi.org/10.1113/JP270646.
Ramazzina, I., Bernazzoli, B., & Costantino, C. (2017). Systematic review on strength training in Parkinson’s disease: an unsolved question. Clinical Interventions in Aging, 12, 619–628. https://doi.org/10.2147/CIA.S131903.
Rosenthal, L. S., & Dorsey, E. R. (2013). The benefits of exercise in Parkinson disease. JAMA Neurology, 70(2), 156–157. https://doi.org/10.1001/jamaneurol.2013.772.
Sampaio, T. B., Savall, A. S., Gutierrez, M. E. Z., & Pinton, S. (2017). Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural Regeneration Research, 12(4), 549–557. https://doi.org/10.4103/1673-5374.205084.
Salazar, R. D., Ren, X., Ellis, T. D., Toraif, N., Barthelemy, O. J., Neargarder, S., & Cronin-Golomb, A. (2017). Dual tasking in Parkinson’s disease: cognitive consequences while walking. Neuropsychology, 31(6), 613–623. https://doi.org/10.1037/neu0000331.
Steenland, K., MacNeil, J., Seals, R., & Levey, A. (2010). Factors affecting survival of patients with neurodegenerative disease. Neuroepidemiology, 35(1), 28–35. https://doi.org/10.1159/000306055.
Shu, H. F., Yang, T., Yu, S. X., Huang, H. D., Jiang, L. L., Gu, J. W., & Kuang, Y. Q. (2014). Aerobic exercise for Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. PLoS One, 9(7), e100503. https://doi.org/10.1371/journal.pone.0100503.
da Silva, F. C., Arancibia, B. A., Ferreira, E. G., Hernandez, S. S., & da Silva, R. (2016). Effects of Nordic walking on Parkinson’s disease: a systematic review of randomized clinical trials. Fisioter Pesqui, 23(4).
Sleiman, S. F., Henry, J., Al-Haddad, R., El Hayek, L., Abou Haidar, E., Stringer, T., . . . Chao, M. V. (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife, 5. doi: https://doi.org/10.7554/eLife.15092.
Salmazo-Silva, H., Parente, M. A., Rocha, M. S., Baradel, R. R., Cravo, A. M., Sato, J. R., et al. (2017). Lexical-retrieval and semantic memory in Parkinson’s disease: the question of noun and verb dissociation. Brain and Language, 165, 10–20. https://doi.org/10.1016/j.bandl.2016.10.006.
Snyder, G. L., Fienberg, A. A., Huganir, R. L., & Greengard, P. (1998). A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. The Journal of Neuroscience, 18(24), 10297–10303.
de Souza Fortaleza, A. C., Mancini, M., Carlson-Kuhta, P., King, L. A., Nutt, J. G., Chagas, E. F., et al. (2017). Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait & Posture, 56, 76–81. https://doi.org/10.1016/j.gaitpost.2017.05.006.
Strouwen, C., Molenaar, E., Munks, L., Keus, S. H. J., Zijlmans, J. C. M., Vandenberghe, W., et al. (2017). Training dual tasks together or apart in Parkinson’s disease: results from the DUALITY trial. Movement Disorders, 32(8), 1201–1210. https://doi.org/10.1002/mds.27014.
Saltychev, M., Barlund, E., Paltamaa, J., Katajapuu, N., & Laimi, K. (2016). Progressive resistance training in Parkinson’s disease: a systematic review and meta-analysis. BMJ Open, 6(1), e008756. https://doi.org/10.1136/bmjopen-2015-008756.
Sun, X., Zhao, Y., & Wolf, M. E. (2005). Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. The Journal of Neuroscience, 25(32), 7342–7351. https://doi.org/10.1523/JNEUROSCI.4603-04.2005.
Sung, Y. H. (2015). Effects of treadmill exercise on hippocampal neurogenesis in an MPTP/probenecid-induced Parkinson’s disease mouse model. Journal of Physical Therapy Science, 27(10), 3203–3206. https://doi.org/10.1589/jpts.27.3203.
Tam, C. W., Burton, E. J., McKeith, I. G., Burn, D. J., & O'Brien, J. T. (2005). Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology, 64(5), 861–865. https://doi.org/10.1212/01.WNL.0000153070.82309.D4.
Tamura, I., Kikuchi, S., Otsuki, M., Kitagawa, M., & Tashiro, K. (2003). Deficits of working memory during mental calculation in patients with Parkinson’s disease. Journal of the Neurological Sciences, 209(1–2), 19–23.
Thacker, E. L., Chen, H., Patel, A. V., McCullough, M. L., Calle, E. E., Thun, M. J., et al. (2008). Recreational physical activity and risk of Parkinson’s disease. Movement Disorders, 23(1), 69–74. https://doi.org/10.1002/mds.21772.
Tufekci, K. U., Meuwissen, R., Genc, S., & Genc, K. (2012). Inflammation in Parkinson’s disease. Advances in Protein Chemistry and Structural Biology, 88, 69–132. https://doi.org/10.1016/B978-0-12-398314-5.00004-0.
Uhrbrand, A., Stenager, E., Pedersen, M. S., & Dalgas, U. (2015). Parkinson’s disease and intensive exercise therapy—a systematic review and meta-analysis of randomized controlled trials. Journal of the Neurological Sciences, 353(1–2), 9–19. https://doi.org/10.1016/j.jns.2015.04.004.
Villalba, R. M., Lee, H., & Smith, Y. (2009). Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Experimental Neurology, 215(2), 220–227. https://doi.org/10.1016/j.expneurol.2008.09.025.
Whitfield, J. A., & Goberman, A. M. (2017). Speech motor sequence learning: effect of Parkinson disease and normal aging on dual-task performance. Journal of Speech, Language, and Hearing Research, 60(6S), 1752–1765. https://doi.org/10.1044/2017_JSLHR-S-16-0246.
Wosiski-Kuhn, M., & Stranahan, A. M. (2012). Transient increases in dendritic spine density contribute to dentate gyrus long-term potentiation. Synapse, 66(7), 661–664. https://doi.org/10.1002/syn.21545.
Xu, Q., Park, Y., Huang, X., Hollenbeck, A., Blair, A., Schatzkin, A., & Chen, H. (2010). Physical activities and future risk of Parkinson disease. Neurology, 75(4), 341–348. https://doi.org/10.1212/WNL.0b013e3181ea1597.
Ying, S. W., Futter, M., Rosenblum, K., Webber, M. J., Hunt, S. P., Bliss, T. V., & Bramham, C. R. (2002). Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. The Journal of Neuroscience, 22(5), 1532–1540.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Appendix
Appendix
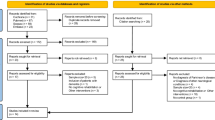
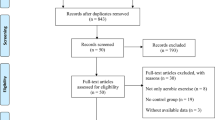
To be eligible for inclusion for aim 3, studies had to be published in English, indexed in PubMed, and employ a cross-sectional, prospective, or experimental design. To be as inclusive as possible, both animal and human studies were included. Outside these criteria, no specific exclusionary criteria were applied.
We employed the following PubMed computerized search on November 18, 2017:
(“exercise”[MeSH Terms] OR “exercise”[All Fields] OR (“physical”[All Fields] AND “activity”[All Fields]) OR “physical activity”[All Fields]) AND (“memory”[MeSH Terms] OR “memory”[All Fields]) AND (“parkinson disease”[MeSH Terms] OR (“parkinson”[All Fields] AND “disease”[All Fields]) OR “parkinson disease”[All Fields] OR (“parkinson’s”[All Fields] AND “disease”[All Fields]) OR “parkinson’s disease”[All Fields])
Following the aforementioned search, 86 studies were identified. We identified 10 papers that met the inclusion criteria for review. The full article of these 10 papers were read, and among these, 10 were retained for inclusion. An additional 2 articles were included after referencing the bibliographies of these retrieved articles. This resulted in 12 articles evaluated for this aim.
Rights and permissions
About this article
Cite this article
Loprinzi, P.D., Frith, E. Memorcise in the Context of Parkinson’s Disease. J Cogn Enhanc 2, 208–216 (2018). https://doi.org/10.1007/s41465-018-0075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-018-0075-2