Abstract
Sandvik 3R60™ is an AISI 316/316L type of stainless steel. In this paper, the structural stability of the material under long-term ageing or creep test has been studied. The material had been creep tested with a stress of 45 MPa at 700 °C. The predicted rupture time for the creep specimen was about 100,000 h; however, the specimen broke first after 240,131 h. The oxidation behavior and structural stability in both aged and creep-tested samples were studied using SEM/EDS, EBSD and ECCI techniques. Thin oxide layers near the sample surface are mainly spinel oxides and eskolaite (Cr2O3). Sigma phase, χ-phase, Eta phase, M23C6 and Cr2N have been observed in the matrix of the samples. In the crept sample, the amount of sigma phase has increased, so has Eta phase and χ-phase as well. Thermo-Calc evaluation can reasonably predict precipitation of sigma phase, Eta phase and M23C6, but not χ-phase and Cr2N phases. Creep crack initiation behavior has been studied. It is mainly noticed to start at surface oxide layer or coarse sigma particles at grain boundary or triple point. Additionally, it is also observed that the presence of a thin Cr2O3 layer between the oxide and matrix along with discontinuous sigma phase distribution at grain boundary that will reduce the risk for creep crack initiation. Further, the crack propagation behavior has also been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-temperature materials are usually defined as materials that are able to withstand temperatures above 540 °C (Meetham 2000). Austenitic stainless steels are a very important group of structural materials that have been widely used for elevated temperature applications such as boilers, nuclear reactors, super-heater tubes and re-heater tubes in ultra-supercritical power plants (Gianfrancesco 2017). This is mainly due to its good combination of excellent mechanical properties and corrosion resistance at high temperatures (Zinkle et al. 2009).
The requirements of the materials for high-temperature applications are not only their physical, thermal, corrosion, and mechanical properties, but also the cost, which includes the material cost and service life. The latter is more important. For fossil fuel power stations, the design life is more than 25 years (Gianfrancesco 2017). For nuclear power plant, the design lifetime is even longer (Horak et al. 1983; Féron 2012). This requires the material to have certain service life. A prolonged service life of a material is more desirable from an economic point of view by decreasing the total cost of the system (AZO Materials 2019). It is a very hard challenge to design a material for high-temperature application with long service life since the material can be degraded during the service due to the material structural instability. Another challenge is the long-term mechanical and corrosion tests to get data for the applications.
Austenitic stainless steels are a group of Fe–Cr–Ni alloys with an austenitic or FCC structure at room temperature. Mn, Si, Mo, W, Ti, Nb, Cu and N are sometime added to improve the material properties or with the purposes for metallurgy and production process. The microstructures of austenitic heat-resistant steels after long-term service at high temperature generally consist of austenitic phase and different precipitates. They are mainly M23C6, MX, Z-phase, M6C, sigma phase, Laves phase, χ-phase, ETA phase, G-phase, nitride, and others, and have been well reviewed by Sourmail (2001) and Zhou (2017). Small fine precipitates such as nano MX particles can increase both tensile and creep strength at high temperatures. MX carbonitrides are the main precipitates for the strengthening of austenitic stainless steels (Rainforth et al. 2002). Sigma phase is a brittle phase. Precipitation of large size sigma phase at grain boundary is reported to deteriorate creep strength, toughness, and corrosion resistance (Babakr et al. 2008). M23C6 is a commonly observed precipitate in the matrix and at grain boundary. Fine discontinuous M23C6 carbides are effective in strengthening boundaries by acting as obstacles for migrating boundaries, leading to improvement of creep strength, the agglomeration and coarsening of M23C6 carbides severely deteriorate long-term creep properties (Pilloni et al. 2000).
316L type of stainless steels is well-developed material for different applications at elevated temperatures. Since it contains 2%–3% Mo, they exhibit excellent corrosion resistance and high mechanical properties, and have been used in nuclear power plants currently (Féron 2012). They can become a candidate material for the components in advanced nuclear power reactors (Was et al. 2017). The purpose of this paper will provide a discussion on the structural stability behavior in a Sandvik 3R60™ material, an AISI 316/316L type alloy, crept and aged at 700 °C for 240 131 h (about 27.4 years). The mechanisms for creep crack initiation and propagation behavior are also discussed.
Materials and Experimental Work
Sandvik 3R60™ is an austenitic stainless steel with low C content and alloyed with Mo, fulfilling the standards of AISI type 316/316L grades. The chemical composition is given in Table 1. Sandvik 3R60™ has good corrosion resistance and performs better than regular AISI type 316/316L and AISI type 304. Fields of applications can be as heat exchangers, pipelines, or cooling/heating coils. Other notable properties of Sandvik 3R60™ are its good weldability and good cryogenic properties (Sandvik 2019).
The material used was a tube material with an outside diameter of 162 mm and a thickness of 11 mm. It was in the solution annealed condition with a grain size of 59 µm or ASTM number of 5. The sample for the creep test was taken from the longitudinal direction of the tube wall. The creep test was performed in Escher Wyss (EW) creep testing machine at 700 °C. In this machine, multiple samples can be tested simultaneously. Figure 1a shows the geometry of the sample for the creep testing. The sample was mounted in the EW boxes. The load from the compressed spring was applied after the samples had been heated in the furnace for one hour until it was broken. In this study, Sandvik 3R60™ material had been creep tested with a stress of 45 MPa at 700 °C for 240,131 h until it failed.
To evaluate and analyze the microstructural evolution and stability of the aged and creep-tested specimens, two microstructural samples were selected in the ruptured creep-tested sample as shown in Fig. 1b. One is the crept or deformed part in the longitudinal direction and another is the aged or undeformed part in the cross section of the screw thread. This part is believed to show less or even no plastic deformation from the creep test. The selected specimens were mounted in PolyFast. After the basic grinding and polishing, the sample was diamond polished with 9/3 µm suspension and finished by oxide polishing with 0.02 µm colloidal silica suspension.
A ΣIGMA VP FEG-SEM with Gemini column equipped with multiple detectors was used to examine the microstructure of the aged and creep-tested specimens. The path of the analysis can be seen as the red lines in Fig. 1b. In this way, the microstructures from the fracture surface to the inside, and from the surface to the center of the samples can be investigated. Energy dispersive X-ray spectroscopy (EDS) analysis was performed on precipitates or phases of interest to estimate their chemical compositions. Low voltage (< 7 kV) to medium voltage (10 kV) was used with BSD/SE-detectors to detect fine precipitates/particles. Electron backscattered diffraction (EBSD) technique was used to identify phases/precipitates, to obtain information about grains and grain boundaries and to calculate the volume fraction of each phase/precipitate. The post-processing of the EBSD data was performed in CHANNEL5 data processing software with the Tango module. Electron channel contrast image (ECCI) technique in a FIB SEM was used to study the deformation or fracture behavior in the sample.
For thermodynamic simulations, the Thermo-Calc software was used with additional modules for precipitation simulation, TC-PRISMA. Thermo-Calc is used to theoretically determine possible phases or precipitates present at certain temperatures, the stability of these and the expected volume fraction of each one. The databases used are TCS Steels/Fe-alloys Database (TCFE10) and TCS Steels/Fe-alloys Mobility Database (MOBFE2). The results from the theoretical thermodynamical simulations are compared with experimental results.
Results and Discussion
Creep Testing Results
Sandvik 3R60™ sample was creep tested at 700 °C with a set load of 45 MPa. The predicted rupture time for the creep specimen was estimated to be 100,000 h; however, the specimen broke first after 240,131 h, a 140% longer creep life. Figure 2 shows a comparison of the real creep life of the sample from the creep test, the expected value and the ECCC data for AISI 316 with similar composition (ECCC 2014). The expected data at 700 °C is similar to that of the ECCC data. The real data is much longer than that of the ECCC data at 700 °C. This indicates that the material can be designed to be use at higher temperatures with the same creep life or longer creep life with the same applied stress. The data are, therefore, valuable for long-term creep design for the component in coal-fired power plant or nuclear power plant.
The creep-tested result, expected data and comparisons with the ECCC data (ECCC 2014)
Oxidation Behaviors
An overview of the oxidation and precipitation behaviors in the crept Sandvik 3R60™ sample at 700 °C for 240 131 h is presented in Fig. 3a. Figure 3b presents the EDS line scan (path is marked in yellow, from the inside to the sample surface), and the EDS mapping data are shown in Fig. 3c. The oxide layer is approximately between 20 and 30 µm thick and varies in thickness depending on the location of measurement (Fig. 3b). The oxide is built up in layers, where regions of the matrix can be seen in it. With EDS phase mapping and EDS point analysis, the compositions of the different layers in the oxide have been characterized. The outer layer of the oxide is rich in Cr, but high Fe and Ni contents have also been observed (Fig. 3c). It is assumed that it could possibly be spinel oxides such as Fe3O4, FeCr2O4 and NiCr2O4 (Nomura 1990). In addition, it is also noted that the amount of Mn in this layer is very low (below the content of Mn in the alloy i.e. 1.79 wt%). Hence, it can be concluded that no Mn spinel oxide was observed in the analyzed oxide layer. This is similar to that recently reported in 316L austenitic stainless steel, which was long term exposed to air at high temperatures (Huang et al. 2020). The inner layer adjacent to the matrix; however, is rich in Cr and has been identified to be eskolaite (Cr2O3). Moreover, in the boundary between the matrix and the oxide, SiO2 has been observed and is shown in Fig. 3c.
In the cross section of the screw thread (aged part), oxide layers at the sample surface/edge can also be observed although it had less contact with the air and less stress applied. They are similar to those in the crept part but with a smaller thickness of about 10–15 µm. This indicates that oxygen could diffuse into the screw thread and caused the occurrence of oxidation.
Precipitation Behavior
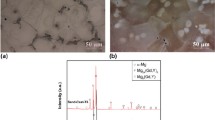
The phase identification was done using SEM EDS and EBSD techniques. SEM BE and EDS were used to study the phase morphology and identify the possible chemical compositions. Figure 4a shows SEM BE and EDS mapping images of visible phases/precipitates and compositions in a point at the center of the aged Sandvik 3R60™ sample. Figure 4b shows an enlarged part of Fig. 4a. Table 2 shows some typical chemical compositions of different phases analyzed by EDS. Together with EBSD, sigma phase, χ-phase, Eta phase, M23C6 and Cr2N have been identified. Sigma phase and Eta phase have mainly precipitated along grain boundaries, where these two phases most of the time form adjacent to each other. Sigma phase is thought to act as a nucleation site for Eta phase, where the growth possibly is controlled by up-hill diffusion (Johansson et al. 2015). Coarse sigma phase precipitates can also be observed in the matrix, along grain boundaries and in triple points, where it has grown into the matrix. Moreover, finer sigma phase particles have been observed in the matrix. Furthermore, precipitates of M23C6 have been seen along grain boundaries and at triple point, see enlarged micrograph, Fig. 4b. χ-phase have been found to precipitate randomly in the matrix and in the grain boundaries. In the crept sample, finer χ-phase can be found randomly dispersed in the matrix. Cr2N (phase with dark contrast) has been identified in some regions near the sample surface or edge of the crept sample, where it precipitated mainly along the grain boundaries or the phase boundaries of the other phases such as sigma phase and Eta phase, as shown in Fig. 5.
As shown by the EDS linear scan in Fig. 3, there is an area where the matrix close to the oxide layer is depleted in Cr (9 wt%) and enriched in Ni (17 wt%), respectively, when moving towards the bulk of the specimen. These elements will gradually increase and decrease. This Cr depleted region favors the precipitation of Mo-rich χ-phase and depress the formation of sigma phase that contains high amount of Cr. χ-phase has been found to precipitate at the oxide, in the boundary between the oxide and the matrix, and in the matrix. Due to the low level of Cr within about a distance of 10 µm to the oxide layer, no Cr2N phase has formed either. Table 3 shows a summary of the phases or precipitates observed in both the aged and crept samples near the edge/surface and at the center of the samples.
SEM EBSD technique was used to identify the phases and possibly to evaluate volume fraction of the phases. In this study, crystallographic data from reference (Liu 2004) were used to identify the phases in the material. Figure 6 shows the phases identified in both aged and crept samples. In the center of the aged sample (Fig. 6a, b), FCC, sigma phase, Eta phase, M23C6 and χ-phase have been identified. The matrix was identified to have an FCC crystal structure and can therefore be considered to be austenitic phase. Sigma phase, Eta phase, M23C6 and χ-phase have been found to precipitate along grain boundaries and randomly in matrix. Fine particles in the matrix are either sigma phase or χ-phase. In the center of the crept sample (Fig. 6c, d), FCC, sigma phase, Eta phase, and χ-phase have been observed in this analysis/point. Coarse sigma phase in a large quantity has been identified to have precipitated along grain boundaries and triple points. Eta phase has been identified to precipitate adjacent to sigma phase. χ-phase have been found to precipitate randomly in the matrix. Finer particles in the matrix are mainly χ-phase but some sigma phase. It was found that more Eta and χ-phases can be observed in the crept sample comparing with that in the aged sample. In this crept sample, sub-grain structure can be observed. This indicates that plastic deformation occurred during the creep test, which has led to the formation of dislocation sub-cells, and consequently small sub-grains.
Table 4 shows a summary of volume fraction (%) of identified phases with EBSD. The EBSD indexing rate is not high, especially for the aged sample. However, the results may be used to provide a qualitative discussion on the precipitation behaviors in the aged and crept samples. It can be found that the volume fractions of sigma phase, Eta phase and χ-phase in the crept sample are higher than that in the aged sample since the occurrence of plastic deformation during the creep process would have increased both nucleation and growth rates of these phases as expected. This has also led to obviously more coarse sigma phase and Eta phase in the crept specimen. Since no statistical study has been done, no clear trend can be predicted for the size change of χ-phase in the crept sample. However, it is interesting to mention that finer χ-phase can be observed in the crept sample. One explanation can be that plastic deformation during the creep process has increased the nucleation rate of χ-phase, and low diffusion rate of Mo atom has limited its growth rate, which could still keep fine size.
Thermodynamical Simulations
Thermodynamical simulations with Thermo-Calc have been performed to estimate and predict the stable phases present at 700 °C and how they develop over time. Figure 7a shows the prediction of stable phases in Sandvik 3R60™ alloy in the temperature range between 600 °C to 800 °C. At 700 °C, possible phases that could be noted are FCC, Laves phase, M23C6, sigma phase, Eta phase and χ-phase. χ-phase replaces Laves phase at 776 °C. Table 5 is a summary of amount of each phase evaluated by Thermo-Calc.
Figure 7b shows the TC-PRISMA simulation of sigma phase and M23C6 phases/particles in Sandvik 3R60™ material aged at 700 °C for 240 131 h. Sigma phase is continuously growing over time with a steady increase of the volume fraction for the phase, measuring approximately 8% before the end of the simulation. M23C6, however, is reaching a steady state in less than 1 h. The predicted volume fraction of M23C6 is approximately 0.5% in the specimen.
Comparing with the real values in Table 4, the volume fraction of M23C6 phase predicted by TC-PRISMA is close to that in the aged material. The predicted volume fraction of sigma phase, however, is higher than that in the aged material, but close to that in the crept material. The values of these two phases estimated by Thermo-Calc simulations are reasonable. The maximum amount of χ-phase by Thermo-Calc simulations is about 2.13 mol% at 776 °C. This may provide some explanation why large number of fine dispersed χ -phase have been observed in the matrix.
It has been reported that sigma phase, M23C6, M6C, χ-phase and Laves phase can precipitate in AISI 316L-type grades under creep conditions in the temperature range between 600 °C to 750 °C (Sourmail 2001; Lai 1981). In this study, no M6C or Laves phase were found. Instead the phases that have been identified are sigma phase, M23C6, χ-phase, Cr2N and Eta phase. Laves phase was reported to precipitate in AISI 316L alloy in the temperature range of 625 °C to 800 °C after approximately 1000 h (Sourmail 2001). However, the amount of Laves phase will decrease over time (Rieth et al. 2004). On the other hand, χ-phase can only precipitate at a temperature higher than 776 °C according to the Thermo-Calc evaluation, but they have been observed in the samples aged or creep tested at 700 °C for 240 131 h. Time–temperature–precipitation diagram for 316L (NIMS 2003) shows that at a very long ageing time, χ-phase can precipitate at 700 °C. If Laves phase is suspended for the Thermo-Calc evaluation, then χ-phase can precipitate at lower temperature. This indicates that Laves phase may be replaced by χ-phase in these samples in this study due to both thermodynamic and kinetic factors by such a long time treatment (240 131 h). M6C, G-phase and Eta phases often get mixed up when identified (Sourmail 2001). By comparing the results of EDS, EBSD data and recent TEM investigations, M6C and Eta phases have both diamond structure, but no C has been identified or M6C indexed at sites. The difference between G-phase and Eta phase is that G-phase has a FCC structure and Eta phase has a diamond structure. Actually, Eta phase is a nitride. With TEM diffraction and EDS study, some suspected G-phase particles have finally been identified as Eta phase.
As mentioned previously, Cr2N has been observed in the sample near the surface/edge. According to the Thermo-Calc evaluation, no Cr2N will form in this alloy. This is probably due to the fact that the nitrogen in the air has diffused into the sample that causes the formation of nitride in the sample. The results indicate that for long-term ageing or creep testing, the influence of environments/air on precipitation in material should be considered for application designs or material characterization.
Strengthening and Fracture Mechanisms
This material shows an extremely long creep life. Creep crack initiation is a key factor. Using SEM ECCI technique, two types of short creep cracks were observed as shown in Fig. 8. One is that the crack initiation started at the oxide layer and then propagated along the original grain boundary with sigma phase or Eta phase as shown in Fig. 8a. This crack propagation can be combined with the oxidation assisted crack propagation since high oxygen content could be identified at the crack tip. As discussed previously, a thin dense Cr2O3 oxide film formed between the matrix and oxide layer can prevent from continuous oxidation and reduces the possibility for crack initiation at the oxide layer. Another is an internal crack initiation. It is mainly at sigma phase boundary, especially at grain boundary triple point as shown in Fig. 8b. This is mainly due to the high stress concentration at these points during the creep process. In this alloy, sigma phase does not appear a continuous layer along the grain boundary. They are separated either by Eta phase or precipitated and grew separately. This will reduce stress concentration at the sigma phase and prevent a rapid crack propagation. Also, the presence of homogeneously dispersed fine χ-phase particles in the matrix can contribute to the strengthening, and consequently increases creep life by increasing the difficulty for crack initiation. This needs to be further investigated.
Conclusions
Sigma phase, χ-phase, Eta phase, M23C6 and Cr2N will precipitate in Sandvik 3R60™ material for long-term ageing or creep-tested at high temperature. Thermo-Calc evaluation can reasonably predict precipitation of sigma phase, Eta phase and M23C6, but not χ-phase and Cr2N phases. Long-term creep will increase amounts of sigma phase, Eta phase, and χ-phase.
For long-term ageing or creep testing at high temperature, environment will affect precipitation. Nitrogen in the air can diffuse into the material and leads to the formation of nitrides.
Sandvik 3R60™ material can have an extra-long creep life. Presence of homogeneously dispersed fine χ-particles in the matrix and small sub-grains can contribute to the strengthening.
Surface oxidation and coarse sigma particles at grain boundary or triple point can cause creep crack initiation. A thin dense Cr2O3 layer between the oxide and matrix and a discontinuous sigma phase distribution at grain boundary will reduce the risk for creep crack initiation.
References
Babakr AM et al (2008) Sigma phase formation and embrittlement of cast Iron–Chromium–Nickel (Fe–Cr–Ni). J Minerals Mater Char Eng 7:127–145
ECCC data sheet (2014) https://vdocuments.mx/eccc-eccc-data-sheets-approved-on-behalf-of-eccc-date.html
Féron D (2012) Nuclear corrosion science and engineering. Woodhead Publishing, Duxford
Gianfrancesco AD (2017) Materials for Ultra-supercritical and advanced ultra-supercritical power plants. Woodhead Publishing, Duxford
Horak JA, Sikka VK, Raske DT (1983) Mechanical properties of types 304 and 316 stainless steel after long-term aging and exposure. IAEA specialists meeting on mechanical properties of structural materials; Chester (UK); 10–14 Oct 1983; Available from NTIS, PC A03/MF A01; 1 as DE84003325
Huang X et al (2020) (2020) Oxidation behavior of 316L austenitic stainless steel in high temperature air with long-term exposure. Mater Res Express 7:066517
Johansson C, Lind M (2015) Evaluation of the η (Eta) nitride with three laboratory melts. MSc Thesis MH205X, KTH, Sweden
Lai J, Chastell D, Flewitt P (1981) Precipitate phases in type 316 austenitic stainless steel resulting from long-term high temperature service. Mater Sci Eng A 49(1):19–29
Liu P (2004) Phase analysis in steel using analytical transmission electron microscopy. Sandvik
AZO Materials (2019) https://www.azom.com/article.aspx?ArticleID=17267. Accessed November 27 2019
Meetham GW, Van de Voorde MH (2000) Materials for high temperature engineering applications, 1st edn. Springer, Berlin
NIMS (2003) NRIM-Metallographic Atlas of long-term crept materials No. M-2, national research institute for metals, Tokyo, Japan
Nomura K, Ujihira Y (1990) Analysis of oxide layers on stainless steel. J Mater Sci 25:1745–1750
Pilloni G, Quadrini E, Spigarelli S (2000) Interpretation of the role of forest dislocations and precipitates in high-temperature creep in a Nb-stabilised austenitic stainless steel. Mater Sci Eng A 279:52–60
Rainforth WM et al (2002) Precipitation of NbC in a model austenitic steel. Acta Mater 50:735–747
Rieth M et al. (2004) Creep of the Austenitic Steel AISI 316L(N). Wissenschaftliche Berichte FZKA 7065, Forschungszentrum Karlsruhe
Sandvik AB (2019) Sandvik TM3R60 Tube and pipe, seamless. www.materials.sandvik/en/materials-center/material-datasheets/tube-and-pipe-seamless/sandvik-3r60/. Accessed 20 June 2019
Sourmail T (2001) Precipitation in creep resistant austenitic stainless steels. Mater Sci Tech 17:1–14
Was GS et al (2017) Corrosion and stress corrosion cracking in supercritical water. J Nucl Mater 371:176–201
Zhou YH et al (2017) Precipitation and hot deformation behavior of austenitic heat-resistant steels: a review. J Mater Sci Tech 33:1448–1456
Zinkle SJ, Busby JT (2009) Structural materials for fission & fusion energy. Mater Today 12:12–19
Acknowledgements
This work is published by permission of Sandvik Materials Technology. The assistance of Mr. Jerry Lindqvist is acknowledged. Fredrik Gustavsson from SWERIM is thanked for fruitful discussions and carrying out TEM investigation in short time.
Funding
Open access funding provided by Linköping University. Sandvik own project.
Author information
Authors and Affiliations
Contributions
All the experiments and analysis were mainly conducted by OÖ, GC and RS. Manuscript was prepared jointly by GC, OÖ and RS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests in this paper.
Availability of data and materials
Data will be shared on request.
Ethics approval
NA.
Consent to participate
Yes.
Consent for publication
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Öhlin, O., Chai, G. & Siriki, R. Structural Stability of Sandvik 3R60™ After 240 131 Hours Ageing and Creep Test at 700 °C. Trans Indian Natl. Acad. Eng. 7, 625–633 (2022). https://doi.org/10.1007/s41403-021-00298-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-021-00298-9