Abstract
Production routes were recorded on available reactions for 111Ag production from nuclear reactors or cyclotrons using a natural palladium target based on 110Pd(n, γ) and 110pd(d, n) reactions, respectively. natCd(γ, x) based on 110Cd(γ, p) has also been studied as a prospective reaction for the production of 111Ag. Unfortunately, these nuclear reactions are difficult to utilize because, in some cases, they reduce the specific activity of 111Ag. This is a consequence of the stable silver isotopes produced in high concentrations. These isotopes include 107, 109Ag and, in other cases, the high impurity of silver radioisotopes, such as 110m, 106m, 105Ag, that are produced during parallel nuclear reactions. Due to a scarcity of data regarding the (γ, α) reaction, the gamma reaction on natural indium for 111Ag production based on the 115In(γ, α) reaction was calculated. The natIn(γ, α) reaction satisfies the criteria as a possible reaction to produce 111Ag with a sufficient yield and purity as consequence of the high 115In (95.7%) abundance as an enriched form and a relatively soft background caused by the parallel nuclear reactions.
Similar content being viewed by others
1 Introduction
Silver has long been recognized as an antimicrobial and therapeutic metal, frequently used for the treatment of a number of superficial wounds, bruises, and mild burns [1, 2]. The incorporation of silver into several pharmaceuticals is a common commercial in medical applications [3,4,5,6,7]. Several radionuclides, including 165Dy, 169Er, 198Au, 166Ho, 177Lu, 32P, 186Re, 153Sm, 89Sr, 182Ta 170Tm, and 90Y, have been used as therapeutic agents as a consequence of the linear energy transfer (LET) property resulting from beta particle decay [8,9,10]. Recently, radionuclide theranostic concepts in nuclear medicine have demonstrated that gamma-ray decay or positron emission, as well as beta particle emission, can be used for both diagnostic and therapeutic purposes [11,12,13]. Referring to 111Ag as a radionuclide (t1/2 = 7.45 d), it has β−- particle emission (Emax = 1.04 MeV) [14] and γ-rays at energies of 245 keV (1.3%) and 342 keV (6.7%) suitable for detection [15]; consequently, it has theranostic properties [16] owing to its characteristic decay state, as shown in Table 1. The length of beta emitting in tissue ranged from one to ten millimeters based on the quantity of energy emitted; consequently, it is ideal for medium to large tumors [17]. In addition, the decay of 111Ag produces low gamma rays, allowing simultaneous SPECT imaging. The combination of diagnostic and therapeutic uses in the same isotope enables parallel therapy as well as in vivo dose monitoring [18]. Furthermore, the relatively long half-life of 111Ag is compatible with the biological half-lives of antibodies; consequently, this isotope is attractive for application in radio-immunotherapy [19, 20]. 111Ag has been produced using a variety of nuclear reactions based on nuclear reactors [21, 22] and cyclotrons [23,24,25]. Thermal neutron irradiation of natural palladium targets produces an intermediate of 111Pd radionuclide via a 110Pd(n, γ)111Pd reaction and then the short-lived 111Pd (t1/2 = 23.4 min) decays to 111Ag [21, 22]. Unfortunately, the cross section of the 110Pd(n, γ) reaction is small (0.34 b) [26, 27], and the presence of six stable palladium isotopes permits the numerous simultaneous nuclear reactions that reduce the purity and activity of 111Ag. Deuteron-induced reactions have been studied to produce 111 Ag by 110Pd(d, n) and natPd(d, x) [23,24,25] resulting in an inescapable co-production of 110mAg (t1/2 = 249.83 d) in both reactions in addition to the presence of many silver radioisotopes produced due to a variety of nuclear reactions occurring on natural palladium. The yields of photonucleon reactions with different multiplicities that occur on a natural mixture of cadmium isotopes were measured in order to produce 111Ag via a natCd (γ, p) reaction based on the 112Cd (γ, p) reaction using Bremsstrahlung as a gamma-ray source with an endpoint energy of 55 MeV [28].
As a result, photon-induced reactions were emphasized as electromagnetic radiation with a moderate energy of roughly (20–25) MeV that perturbs the nucleons in the target nucleus, causing the product particles to be released [29, 30], and evidence for the regular threshold dependence of photon-induced reactions was observed. For the emission of alpha particles, reactions occur at the sum of the reaction threshold (Eth) and the Coulomb barrier (Bc). For many decades, researchers have investigated the (γ, α) reaction for light targets. However, literature data for medium-weight and heavy targets are limited. Cross sections of electro- and photonuclear reactions were studied on 58Ni and 60Ni targets [31, 32]. The product yields of (γ, α) reactions with antimony targets have been reported [33]. The yield of 117In in the 121Sb(γ, α) reaction has also been reported to be close to 0.8% of the (γ, n) yield, which is much greater than that obtained by Volkov et al., 1980 [31]. The yield of the (γ, α) reaction was found less than that of the (γ, p) reaction by a factor of 20, i.e., roughly 10–4 of the rate of the (γ, n) reaction [28]. Due to contradictory results in the literature, as well as a general lack of reliable evidence, it can be concluded that there is a need to investigate the relative yields of bremsstrahlung-induced reactions, particularly in the case of (γ, α) reactions to heavy targets such as natural indium for the production of 111Ag.
This work evaluates 111Ag production methods based on accessible nuclear reactions, taking into consideration the target selection that provides the lowest cost, largest yield, and best purity, by evaluating nuclear data for all feasible nuclear reactions.
2 Production route
The criteria for producing 111Ag as a theranostic radioisotope are based on reaction selection, which achieves high radionuclidic purity with high specific activity via projectile type, energy, and isotopic abundance in the target, as well as high chemical purity via chemical separation methods, as shown in Table 2. Although several nuclear reactions, including nuclear reactors via natPd (n, γ) reactions [21, 22] and cyclotrons via 232Th(p, f) and natPd (d, n) reactions [16, 34], are used for 111Ag production, an additional factor associated with the type of nuclear reaction is the chemical separation route. This is governed by yield, purity, and separation duration. The separation yield of 111Ag based on the (n, γ) reaction was performed with a yield of 80–82% and a suitable chemical purity for use in nuclear medicine, where the palladium concentration was measured to be 1–2 μg [21, 22]. Unfortunately, 111Ag was produced with low specific activity due to the high concentration of stable isotope 109Ag product (90 GBq/mg at 24 h irradiation time and 5 × 1013 n cm−2 s−1 neutron flux) [21]. According to the literature, two nuclear reactions, 232Th(p, f) and 110Pd(d, n), were used in the cyclotron to produce 111Ag. Although the activity of 111Ag produced in the (p, f) reaction is very significant, the fission reactions produce several radionuclides, resulting in a high proportion of radioactive impurities and the difficulty of separating 111Ag with high purity, as well as a separation method that requires significant time. Furthermore, a 110mAg long-lived radioisotope (t1/2 = 249.83 d) with a relatively high ratio of 0.1% (0.518 GBq) could not be separated. Deuterium reactions have been studied on the110Pd(d, n)111Ag [36], but these routes are limited by the small 110Pd deuterium capture cross section and the unavoidable co-production of the 110mAg. In the medical context, typical 111Ag radiotherapeutic doses range from 3700 to 7400 MBq (100 to 200 mCi) per patient [24, 37]. The properties of the nuclear reactions are discussed individually below.
2.1 natPd(n,x)111Ag reaction
Natural palladium was irradiated in a nuclear reactor [21, 22]. The 111Ag activity concentration in the irradiated target was determined using Eq. (1):
where A is the activity concentration of 111Ag by Becquerel (disintegration per second), Nnet is the net area under the peak of 695 keV, while ε is the absolute efficiency of the detector at the given gamma energy, Iγ is the branching ratio (7.1%) of the given gamma energy, and t is the measurement time. The quantity of produced stable 109Ag isotope (μg) may be calculated by using the Avogadro number to estimate the number of radionuclides (N) in 109Pd by knowing its activity (A) measured by Eq. (1) at a gamma energy peak of 88 keV, which has a branching ratio (Iγ) of 3.6%. Because both activity A and the half-life of 109Pd (13.7 h) are known, Eq. (2) can be used to determine the number of radionuclides for 109Pd.
where λ is the decay constant. The mass of 109Ag (g) was calculated using Eq. (3).
where NA is the Avogadro's number.
2.2 natPd(d,x)111Ag reaction
Excitation functions were accomplished by stacked-foil activation on natural palladium by deuteron-induced reactions in medium-energy cyclotron [23,24,25] using a small beam current, after which the irradiated targets were measured by high-resolution gamma-ray spectroscopy. The cross sections of 103, 104, 105, 106m, 110m, 111Ag were calculated using the standard activation Eq. (4).
where Nt is the surface density of the target atoms, Nb is the number of bombarding particles per unit time, Tγ is the photopeak number count, εd is the efficiency of the detector, εγ is the gamma-ray intensity, εt is the dead time of measurement, which is the ratio of live time to real time, λ is the decay constant, tb is the time of irradiation, tc is the time of cooling, and tm is the time of acquisition.
The data obtained from cross section reactions play a very important role in the production of radionuclides by cyclotrons, as described earlier [38, 39]. In order to be able to determine the yield with a good accuracy, one needs to know the full excitation functions. Therefore, the estimated yield of a product within a certain energy range, that is, the target thickness, may be determined using Eq. (5):
where Y is the yield of the activity concentration of the product by Becquerel, H is the enrichment (or isotopic abundance) of the target nuclide, M is the mass number of the target element, σ(E) is the cross section within a certain energy range, ρ is the density of the target material, and x is the projectile distance traveled through the target material.
2.3 Photonuclear reactions
Experimental photonuclear reaction data are typically collected by directly recording the number of particles released or by measuring the residual nuclear activity. In the case of energies that exceed the multi-particle emission threshold, more than one combination of emitted light particles may associate the same number of neutrons produced or may contribute to the same residual nucleus. The experimental data collection recorded for the (γ, n) cross section may contain charged particles released at the same time as a single neutron emission, that is, (γ, 1n) + (γ, 1np) + (γ, n2p)….etc., which depend on the incident photon energy. Continuous bremsstrahlung spectra are generated by impacting the target with an electron beam from the accelerator (initially betatrons and synchrotrons, and now, linear accelerators). The photon energy spectrum is continuous, and the yield of the reaction Y(Eo) can be measured as in Eq. (6): [28]
where Eγ is the photon energy, σ(Eγ) is the reaction cross section, W is the bremsstrahlung energy-dependent photon flux, E0 is the endpoint energy of the bremsstrahlung spectrum relative to the electron beam energy, Eth is the threshold energy, and NR is the standardized coefficient. Adjusting the E0 by simple changes allows the measurement of the yield curve, and then the use of the "unfolding" technique achieves a cross section for the photonuclear reaction. The benefit of bremsstrahlung measurements is the high photon beam intensity, which introduces the possibility of achieving sufficient statistics even with very limited cross section reactions. However, this technique has many challenges [28]. It is necessary to know more regarding the bremsstrahlung spectrum for all electron energies. Then, γ-analysis of the residual nucleus is performed after irradiation of the target by bremsstrahlung at varying endpoint energies [28,29,30]. From the γ-lines, the photonuclear reaction cross sections were calculated. The photon charged particle reaction cross sections, σ(γ, p) and σ(γ, α), respectively, are insufficient and limited in the literature and do not contain photon fission reactions.
2.3.1 natCd(γ,x)111Ag reaction
The RM-55 microtron (55 MeV) was used for the irradiation experiments, as illustrated in Fig. 1. A tungsten braking converter (2.2 mm tungsten thickness) was used to generate bremsstrahlung radiation. The CdO target powder occupied an area of 6.3 cm2 [28]. The cadmium target was placed directly behind the braking target. The gamma radiation dose was monitored with the aid of a Faraday cylinder. The target is irradiated for a known duration and then transported for gamma-ray spectrum measurement by the Hp-Ge detector. The reaction yield (Y) was calculated using Eq. (7):
where Sγ is the count rate of gamma rays under the peak, εγ is the efficiency of the gamma-ray detector at the specific energy, λ is the decay constant of a radionuclide produced, Iγ is the branching ratio of gamma rays for the radionuclide produced by that specific energy, and t1, t2, t3 are the irradiation, cooling, and the measurement times, respectively.
Photodisintegration of natural cadmium [28]
2.3.2 natIn(γ,x)111Ag reaction
The following equation governs the activity A(t) of a produced radioisotope when a target is irradiated in a nuclear radiation flux: [40]:
where R is the rate of nuclear interaction and can be expressed by Eq. (9), where λ is the decay constant of the radionuclide formed.
N is the number of atoms in the target for a given nuclide, as calculated by Eq. (10), σeff denotes the effective cross section of the reaction, and ϕ represents the radiation flux.
where \(a\) and \(M\) are the abundance and atomic mass of the nuclide, \(m\) is the mass of the element in the target.
The analysis assumes that a natural indium target is exposed to a bremsstrahlung radiation beam produced by an electron accelerator at the energy distributions provided in Ref. [41], particularly at the endpoint energy of 24 MeV for the production of 111Ag through the (γ, α) reaction. Indium has two naturally occurring stable isotopes, 113In and 115In, with natural abundances of 0.0429 and 0.9571, respectively. As a consequence of the (γ, α) reaction, the undesirable stable 109Ag is produced alongside the radioisotope 111Ag. The 109Ag production is based on the abundance of 113In on the natural indium target. Figure 2 illustrates the (γ, α) reaction cross sections of the two isotopes, and the threshold energy for both nuclides is approximately 10 MeV. The effective cross section can be calculated as follows:
where σ(E) represents the reaction cross section of energy E and ϕ(E) represents the energy-dependent flux. In this case, the bremsstrahlung radiation was calculated using the following equation:
3 111Ag production routes
Three nuclear reaction pathways were used by the nuclear reactor, cyclotron, and microtron RM-55, respectively, to produce 111Ag as a β−-emitter in a no-carrier-added form:
-
110Pd(n,γ)111Pd \(\stackrel{{\beta }^{-}}{\to }\) 111Ag [21, 22]
-
natCd(γ,p)111Ag [28]
-
natIn(γ,α)111Ag
The production methodology involves work in many different directions, such as nuclear data, irradiation technology, chemical separation, and product quality control.
3.1 Production of 111Ag by natPd(n,x) reactions
Palladium involves six stable isotopes of natural abundance; therefore, different radionuclides are produced by their activation using thermal neutrons in the nuclear reactor, as shown in Scheme 1. Activation of natural palladium targets by thermal neutrons produced 111,111mPd as an intermediate radionuclide, as shown in the first nuclear reaction pathway. Nuclear data were obtained from literature data [42,43,44,45,46]. Scheme 1 shows that 111Ag can be produced by pathway (1) accompanied by 103Pd and 109Pd. In the case of 103Pd, it decayed to 103Rh via electron capture, which could be easily separated chemically. However, the parallel reaction for the production of 111Ag using natural palladium is the neutron capture of 108Pd to form 109Pd, which eventually decays by β− emission into stable 109Ag. This reaction also limits the final specific activity of 111Ag due to the high concentration of 109Ag. Therefore, 111Ag is produced as a carrier, according to the data provided in Table 3 [21, 47]. An increase in the irradiation time from 24 to 960 h contributes to a decrease in the specific activity of 111Ag from 90 to 24 GBq/mg due to an increase in 109Ag resulting from the decay of the high 109Pd activity level based on a high natural abundance of 108Pd (26.46%) and a high neutron cross section reaction (8.68 b). According to Alberto et al., 1992 [21], the average yield of 109Pd is 1630 MBq at 3 × 1013 n cm−2 s−1 of neutron flux for 26 h of irradiation time, followed by 72 h of cooling to produce 100 MBq of 111Ag (1 μg of Ag). The specific activity of 111Ag was enhanced by decreasing the amount of 109Ag, which could be accomplished by decreasing the cooling time and rapid chemical separation of 111Ag from 109Pd after EOB. However, the enriched 110Pd could be used instead of the natural palladium to achieve a high specific activity for the production of 111Ag carrier-free. However, this type of nuclear reaction is not preferred because of the high target price.
3.2 Production of 111Ag by natPd(d, x) reactions
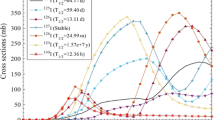
There are two long-lived states of the 111Ag radioisotope: the ground state (t1/2 = 7.45 d) and the excited state of 111mAg (t1/2 = 64.8 s), which decays to 111gAg by IT (99.3%). Excitation studies have been performed to determine the potential to produce 111Ag (theranostic applications) using deuteron-induced reactions on natPd [23, 25, 48,49,50,51]. The meta-stable and ground-state of 111Ag are occupied by the beta decay of meta-stable and ground-state of 111Pd (t1/2 = 5.5 h and 23.4 min, respectively. Experimental excitation functions have been studied using deuteron-induced reactions on natural palladium [23, 25], which have encountered difficulties in 111Ag production with high purity due to a variety of nuclear reactions that are synchronized with the main reaction (d,n), as shown in Table 4. Side nuclear reactions depend on the projectile energy (deuteron energy) to reach the reaction threshold energy and the abundance of each isotope. Table 4 reveals that 111Ag production uses a natPd(d,x) reaction based on a 110Pd(d,n) reaction as a direct nuclear reaction. However, there are parallel reactions to 106m,105,104,103Ag production that also consume the products of the 105Pd(d,n), 104Pd(d,n), 103Pd(d,n), and 102Pd(d,n) reactions, respectively. 104,103Ag does not pose a problem because of their short half-lives and decay by electron capture and positron emission to yield stable 104Pd and 103Rh isotopes, which can be easily separated by chemical methods. The experimental cross section data for 105,106m,110m,111Ag indicated the presence of their cross sections with high values within the deuteron energy range of 5–25 MeV [25, 51]. Half-lives of 110mAg, 106mAg and 105Ag are longer than the 111Ag half-life and are thus difficult to separate chemically. In the literature, experimental data for integral physical yields are rare and have only been found in Ditroi et al. [23], as shown in Fig. 3.
Integral yields for the natPd(d,xn)111,110m,106m,105,104,103Ag reactions [23]
Furthermore, as shown in Table 5, 111Ag can be produced indirectly via natPd(d, x) on the basis of a 110Pd(d, p) reaction to produce 111Pd, which decays by beta emission to 111Ag. The deuteron-induced reaction for 108Pd via (d, p) produces 109Pd, which decays by beta emission to stable 109Ag, reducing the specific activity of 111Ag. Although the cross section values for the production of 109Pd via natPd (d, p) are high [23], there is no documented existence of 109Ag in the literature.
3.3 Production of 111Ag based on natCd(γ,x) reactions
Figure 4 indicates that the maximum cross sections of (γ, n) and (γ, p) reactions on natural cadmium within the gamma energy range of 15–20 MeV are 200–250 mb [52]. Table 6 provides the threshold energies for various photon reactions to cadmium isotopes, which clarified that both (γ, n) and (γ, p) reactions begin within the energy range of 7–11 MeV, whereas the photon energy produced and required for the reaction to occur is within the range of 15–20 MeV, which is greater than the threshold energies for these reactions. Therefore, the generated γ-ray energy was able to produce all the radionuclides established in Table 6 [28, 30, 53, 54], as verified by the relative yield measurements for these radionuclides [28]. 111Ag is difficult to produce through natCd(γ, x) reactions because of simultaneous nuclear reactions that produce 105, 107, 109, 110m, 112, 113, 115Ag based on the direct reactions (γ, p) cadmium isotopes at the same γ-energy used. In contrast, there are parallel reactions based on indirect reactions such as 108Cd(γ, n) and 110Cd(γ, n) which produce 107Cd and 109Cd. These two isotopes which decay by electron capture to 107Ag and 109Ag stable isotopes. These two Ag isotopes decrease the specific activity of 111Ag. The literature has not definitively indicated the presence of silver isotopes, whether long-lived radioactive, such as 105Ag, 110mAg, or stable silver isotopes such as 107Ag and 109Ag, and concentrations can influence the specific activity of 111Ag.
Bremsstrahlung gamma-ray spectrum as in the solid curve and cross section [σ(γ, x) = σ(γ, n) + σ(γ, p) + …] (the dashed curve) for the reaction of the natural cadmium target according to data obtained from [40]
3.4 Production of 111Ag based on natIn(γ,x) reactions
Table 7 indicates that 111Ag may be produced by the natIn(γ, x) based on the 115In(γ, α) reaction when the photon energy is greater than the sum of the two energies of the threshold and the Coulomb barrier (14.1 MeV). Other channels, such as the (γ, n) and (γ, p) reactions, are opened at threshold energies of 9 and 6.8 MeV, respectively, less than that required for the (γ, α) reaction, to produce 114mIn (t1/2 = 49.51 d) and the stable 114Cd isotope, which can be easily separated by chemical methods. Parallel nuclear reactions produce a stable isotope of 109Ag, a radionuclide of 112mIn (t1/2 = 20.56 min) and a stable isotope of 112Cd, based on the reactions of 113In(γ, α), (γ, n), and (γ, p), respectively. Fortunately, 113In has a small abundance (4.3%) compared to 115In (95.7%), so the 109Ag concentration produced will not be a concern, as in other nuclear reactions.
Figure 5 shows the energy distributions of bremsstrahlung radiation, as quoted in the energy range of interest. The integrations in Eq. (5) for the effective cross section of 113In and 115In were calculated using the data in Figs. 4 and 5 (0.00532 and 0.00432 barns, respectively). In addition, the bremsstrahlung radiation flux in the range of concern was assessed, producing 10.37% of the overall bremsstrahlung radiation, as summarized in Table 8.
The bremsstrahlung photon flux in the range of interest will be 5.7 × 1013 cm−2 s−1 if the electron accelerator is set to 10 μA [40, 41]. Substituting the evaluated results in Eq. (2), the interaction rates for both nuclides per gram of target (6.94 × 107 and 1.24 × 109 s−1, respectively) are calculated, as shown in Table 8. Since the (γ,α) reactions of 113In and 115In produce 109Ag and 111Ag, respectively, the rate of 109Ag production to the rate of 111Ag production is approximately 1: 18. The generated activity of 111Ag was calculated as a function of irradiation time by substituting the interaction rate of 115In in Eq. (1), as shown in Fig. 6.
4 Conclusion
This study confirms that the available nuclear reactions, which rely on natural targets to minimize production costs using Pd and Cd targets by the natPd(n, x), natPd(d, x), and natCd(γ, x) reactions, based on the 110Pd(n, γ), 110Pd(d, n), and 112Cd(γ, n) reactions, respectively, to produce 111Ag have several issues. Some reactions have a poor specific activity due to the high quantities of 109Ag stable produced, while others have a low yield of 111Ag because of the existence of radio-silver isotopes such as 105, 106mAg. The calculated data indicated that 111Ag may be produced using the natIn(γ, x) reaction based on the 115In(γ, α) reaction as a promising reaction according to the achieved results of approximately 800 MBq 111Ag per gram of indium metal target during 12 days of irradiation time. The ratio of 109Ag production to 111Ag production is roughly one to 18, implying that 111Ag may be produced with a high specific activity.
References
H.J. Klasen, A historical review of the use of silver in the treatment of burns II. Renewed interest for silver. Burns 26(2), 131 (2000). https://doi.org/10.1016/s0305-4179(99)00116-3
H.J. Klasen, A Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 26(2), 117 (2000). https://doi.org/10.1016/s0305-4179(99)00108-4
S. Silver, L. Phung, G. Silver, Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol 33(1), 627 (2006). https://doi.org/10.1007/s10295-006-0139-7
M.A. Hollinger, Toxicological aspects of topical silver pharmaceuticals. Crit. Rev. Toxicol 26(3), 255 (1996). https://doi.org/10.3109/10408449609012524
A. Kascatan-Nebioglu, A. Melaiye, K. Hindi et al., Synthesis from caffeine of a mixed N-heterocyclic carbene-silver acetate complex active against resistant respiratory pathogens. J. Med. Chem. 49(23), 6811 (2006). https://doi.org/10.1021/jm060711t
A. Melaiye, R.S. Simons, A. Milsted et al., Formation of water-soluble pincer silver(i)−carbene complexes: a novel antimicrobial agent. J. Med. Chem. 47(4), 973 (2004). https://doi.org/10.1021/jm030262m
H.N. Abdelhamid, A. Talib, H.F. Wu, Facile synthesis of water soluble silver ferrite (AgFeO2) nanoparticles and their biological application as antibacterial agents. RSC Adv. 44(5), 34594 (2015). https://doi.org/10.1039/c4ra14461a
J.F. Chatal, C.A. Hoefnagel, Radionuclide therapy. Lancet 354(9182), 931–935 (1999). https://doi.org/10.1016/S0140-6736(99)06002-X
H.Y. Tan, C.H. Yeong, Y.H. Wong et al., Neutron-activated theranostic radionuclides for nuclear medicine. Nucl. Medic. Biol. 90–91, 55–68 (2020). https://doi.org/10.1016/j.nucmedbio.2020.09.005
E. Lopci, A. Chiti, M.R. Castellani et al., Matched pairs dosimetry: 124I/131I meta-iodobenzylguanidine and 124I/131I and 86Y/90Y antibodies. EUR. J. Nucl. Med. Mol. Imaging. 38(1), S28 (2011). https://doi.org/10.1007/s00259-011-1772-6
S.M. Qaim, Therapeutic radionuclides and nuclear data. Radiochim Acta. 89, 297 (2001). https://doi.org/10.1524/ract.2001.89.4-5.297
S. Del Vecchio, A. Zannetti, R. Fonti et al., Nuclear imaging in cancer theranostics. Q. J. Nucl. Med. Mol. Imaging 51, 152 (2007)
J.R. Ballinger, Theranostic radiopharmaceuticals: established agents in current use. Br. J. Radiol 91, 1091 (2018). https://doi.org/10.1259/bjr.20170969
S. Chattopadhyay, K.V. Vimalnath, S. Saha et al., Preparation and evaluation of a new radiopharmaceutical for radiosynovectomy, 111Ag-labelled hydroxyapatite (HA) particles. Appl. Radiat. Isot. 66, 334–339 (2008). https://doi.org/10.1016/j.apradiso.2007.09.003
T.P. Aweda, S. Zhang, C. Chiedza et al., Investigating the pharmacokinetics and biological distribution of silver-loaded polyphosphoester-based nanoparticles using 111Ag as a radiotracer. J. Labelled Comp. Radiopharm. 58(6), 234 (2015). https://doi.org/10.1002/jlcr.3289
K. Ooe, T. Watabe, Y. Shirakami et al., Production and separation of theranostic radionuclide Ag-111 from Pd target. J. Nucl. Med. 61, 1116 (2020)
J.A. Odonoghue, M. Bardies, T.E. Wheldon, Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J. Nucl. Med. 36, 1902 (1995)
S.S. Kelkar, T.M. Reineke, Theranostics: combining imaging and therapy. Bioconjugate Chem. 22, 1879 (2011). https://doi.org/10.1021/bc200151q
T.M. Illidge, S. Brock, Radioimmunotherapy of cancer: Using monoclonal antibodies to target radiotherapy. Curr. Pharm. Design 6, 1399–1418 (2000). https://doi.org/10.2174/1381612003399257
D.K. Hazra, G.T. Stevenson, K.S. Kan, Linkage of silver to antibodies through 2-imino thiolane. Cell Biophys. 26, 183–186 (1995). https://doi.org/10.1007/BF02791579
R. Alberto, P. Blauenstein, I. Novakhofer et al., An improved method for the separation of Ag-111 from irradiated natural palladium. Appl. Radiat. Isotopes 43, 869 (1992). https://doi.org/10.1016/0883-2889(92)90148-8
M. Khalid, A. Mushtaq, M.Z. Iqbal, Separation of Ag-111 from neutron irradiated natural palladium using alumina as an adsorbent. Appl. Radiat. Isotopes 52, 19 (2000). https://doi.org/10.1016/s0969-8043(99)00083-4
F. Ditroi, F. Tarkanyi, S. Takacs et al., Activation cross-sections of deuteron induced reactions on natural palladium. Nucl. Instrum. Meth. B 270, 61 (2012). https://doi.org/10.1016/j.nimb.2011.10.010
A. Hermanne, S. Takacs, F. Tarkanyi et al., Experimental cross sections for charged particle production of the therapeutic radionuclide Ag-111 and its PET imaging analogue Ag-104m,g. Nucl. Instrum. Meth. B 217, 193–201 (2004). https://doi.org/10.1016/j.nimb.2003.09.038
N. Ukon, M. Aikawa, Y. Komori et al., Production cross sections of deuteron-induced reactions on natural palladium for Ag isotopes. Nucl. Instrum. Meth. B 426, 13 (2018). https://doi.org/10.1016/j.nimb.2018.04.019
E. Cornelis, G. J. Vanpraet, C. Bastian, et al., Average capture cross section of the fission product nuclei Pd-104, Pd-105, Pd-106, Pd-108, and Pd-110, Conf. Nucl. Data for Sci. Technol. p. 222, (1982).
R.L. Macklin, J. Halperin, R.R. Winters, Pd-104,105,106,108,110 (n, γ) cross sections above 2.6 keV. Nucl. Sci. Eng. 71, 182 (1979). https://doi.org/10.13182/NSE79-A20409
S.S. Belyshev, B.S. Ishkhanov, A.A. Kuznetsov et al., Photodisintegration of cadmium isotopes. Phys. Atomic Nuclei 77(7), 809 (2014). https://doi.org/10.1134/S1063778814060039
S.A. Karamian, J.J. Carroll, N.V. Aksenov et al., Production of Isotopes and Isomers with Irradiation of Z = 47–50 Targets by 23-MeV Bremsstrahlung. Phys. Atom. Nucl. 78, 757 (2015). https://doi.org/10.7868/S0044002715090123
S. A. Karamian, Yield of bremsstrahlung induced reactions as a probe nucleon-nucleon correlations in heavy nuclei. NPAE-Kyiv2012: 4. International Conference on Current Problems in Nuclear Physics and Atomic Energy, Kyiv (Ukraine), 3–7 Sep 2012. Reference number: 45058523, INIS Vol. 45, INIS Issue 22.
Y.M. Volkov, A.I. Ignatiev, G.A. Kolomenskii et al., α-decay of giant resonances in 58, 60Ni nuclei. Phys. Atomic Nuclei. 32, 595–602 (1980)
B.S. Dolbilkin, Sh. Kan, T. Kim et al., 58Ni(e, e’α) reaction at excitation-energy range of 8–25 MeV // Bull. RAS. Phys. 55, 967 (1991)
I. N. Vishnevsky, V. A. Zheltonozhsky, I. N. Kadenko et al., Integral cross-sections of the photonuclear reactions on 118Sn and 121Sb nuclei // Ibid. 121.
T. Mastren, V. Radchenko, J.W. Engle et al., Chromatographic separation of the theranostic radionuclide 111Ag from a proton irradiated thorium matrix. Anal. Chim. Acta 998, 75–82 (2018). https://doi.org/10.1016/j.aca.2017.10.020
O.N. Kononova, N.G. Goryaeva, O.V. Dychko, Ion exchange recovery of palladium (II) from nitrate weak acid solution. Nat. Sci. 1(03), 166 (2009). https://doi.org/10.4236/ns.2009.13021
W.A. Volkert, T.J. Hoffman, Therapeutic radiopharmaceuticals. Chem. Rev. 99, 2269–2292 (1999). https://doi.org/10.1021/cr9804386
C. Waldherr, M. Pless, H.R. Maecke et al., The clinical value of [Y-90- DOTA]-D-Phe(1)-Tyr(3)-octreotide (Y-90-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 12, 941–945 (2001). https://doi.org/10.1023/A:1011160913619
S. M. Qaim, Nuclear data relevant to cyclotron produced short-lived medical radioisotopes. Radiochim. Acta, 30, 147 (1982). Reference number: EDB-83-026510.
S.M. Qaim, (editor), Nuclear data for medical applications: an overview. Spec. Issue Radiochemica Acta 89, 189 (2001)
J.R. Lamarch, Introduction to Nuclear Engineering (Addison-Wesley Publishing Company Inc, USA, 1983)
T.D. Thiep, T.T. An, N.T. Khai et al., Determination of the total bremsstrahlung photon flux from electron accelerators by simultaneous activation of two monitors. Phys. Part. Nucl. Lett. 9, 648–655 (2012)
T. Kawano, Y. S. Cho, P. Dimitriou et al., IAEA Photonuclear Data Library 2019. Report number: LA-UR-19-26964.
M. Krticka, R.B. Firestone, D.P. Mcnabb et al., Thermal neutron capture cross sections of the palladium isotopes. Phys. Rev. C 77, 054615 (2008). https://doi.org/10.1103/PhysRevC.77.054615
T.H. Nguyen, G.N. Kim, K. Kim et al., Measurements of the thermal neutron cross-section and resonance integral for the 108Pd(n, γ)109Pd reaction. Nucl. Instrum. Meth. B 424, 37–42 (2018). https://doi.org/10.1016/j.nimb.2018.03.031
P. Lantz, C. Baldock, L. Idom. Oak Ridge National Lab. Reports No. 3679, 10 (1964).
C.L. Duncan, K.S. Krane, Neutron capture cross section of 102Pd. Phys. Rev. C 71, 054322 (2005). https://doi.org/10.1103/PhysRevC.71.054322
IAEA, Manual for reactor produced radioisotopes, IAEA-TEC-1340, (2003).
F. Tárkányi, F. Ditrói, S. Takács et al., Activation cross sections of proton induced nuclear reactions on palladium up to 80 MeV. Appl. Radiat. Isot. 114, 128 (2016). https://doi.org/10.1016/j.apradiso.2016.05.022
A. Hermanne, S. Takács, F. Tárkányi, R. Bolbos, Cross section for the charged particle production of the therapeutic radionuclide Ag-111 and its PET imagins analogue Ag-104g, Annales Universitatis Turkuensis, Seria, Turku, Finland, 14., (2002).
A. Hermanne, S. Takacs, F. Tarkanyi et al., Experimental cross sections for charged particle production of the therapeutic radionuclide Ag-111 and its PET imaging analogue 104m, gAg. Nucl. Instrum. Meth. B 217, 193 (2004). https://doi.org/10.1016/j.nimb.2003.09.038
A. Hermanne, F. Tárkányi, S. Takács et al., Experimental determination of cross section of alpha-induced reactions on natPd, in: Haight, R.C., Talou, P., Kawano, T. (Eds.), International Conference on Nuclear Data for Science and Technology. AIP, Santa Fe, USA, 961 (2004b).
A. Leprêtre, H. Beil, R. Bergère et al., A study of the giant dipole resonance of vibrational nuclei in the 103 ≦ A ≦ 133 mass region. Nucl. Phys. A 219, 39 (1974)
S.S. Belyshev, B.S. Ishkhanov, V.N. Orlin et al., Photodisintegration of the isotope 116Cd. Phys. At. Nucl. 76, 931 (2012). https://doi.org/10.1134/S106377881308005X
B.S. Ishkhanov, V.N. Orlin, Description of cross sections for photonuclear reactions in the energy range between 7 and 140 MeV. Phys. Atomic Nuclei 72, 410 (2009). https://doi.org/10.1134/S1063778809030041
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through a fast-track research funding program.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Khaled Mohamed El-Azony, Nader M. A. Mohamed and Dalal A. Aloraini. The first draft of the manuscript was written by Khaled Mohamed El-Azony, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Azony, K.M., Mohamed, N.M.A. & Aloraini, D.A. Advantages and disadvantages of nuclear reactions used in reactors or cyclotrons, in addition to a theoretical study based on photodisintegration on natural indium for 111Ag production. NUCL SCI TECH 33, 14 (2022). https://doi.org/10.1007/s41365-022-00991-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-022-00991-6