Abstract
Leaf rust, caused by the pathogenic biotrophic rust fungus Puccinia triticina (Pt), is one of the most destructive wheat diseases worldwide; its negative impact on crop yields is exacerbated by increasing temperatures due to climate change. Ascarosides are nematode pheromones that induce resistance to microbial pathogens and pests in a wide range of crops, making them valuable components in biocontrol scenarios. We investigated the effect on infection of various wheat (Triticum aestivum) genotypes with the virulent Pt race 77W × R by ascr#18, the major ascaroside secreted into the rhizosphere by plant-parasitic nematodes. Spraying the leaves with ascr#18 24 h before inoculation with fungal uredospores slowed disease development and resulted in a reduction of the number of rust pustules on treated compared to untreated leaves. Dose–response analysis over the nano- and micromolar range revealed a broad optimum concentration down to 0.01nM ascr#18. Microscopic analysis showed very early arrest of the fungus at the appressorial stage, with associated enhanced local accumulation of H2O2 and abortive stoma penetration. Similarly, ascr#18 also induced strong resistance to Pt race PKTTS, confirming its race-unspecific biocontrol activity. The results of this study are consistent with and extend previous research that has shown that ascr#18 activates plant immunity and thus protects plants from pathogens even at very low doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the face of climate change, political crises, and a growing population, there is an urgent need to secure and even increase wheat production to ensure sustainable food security (Poore and Nemecek 2018; OECD 2020). Leaf rust caused by Puccinia triticina (Pt) is one of the most common diseases affecting wheat worldwide (Bolton et al. 2008; Huerta-Espino et al. 2011; Kolmer 2013) and is exacerbated by temperature increases (Helfer 2014; Junk et al. 2016;Caubel et al. 2017). Although widely successful in the past, control of leaf rust using conventional measures involving resistance genes (R genes) and/or synthetic pesticides becomes ineffective over time because of strong selection pressures on fungal populations in intensive agricultural production systems to overcome R gene function or develop resistance or tolerance to pesticides (Andersen et al. 2018; Hawkins et al. 2019; van Esse et al. 2020).
Modern integrative crop protection strategies include methods that rely on the plant’s natural immune system (Galli et al. 2024). Plants, like animals, have evolved various innate and acquired immune strategies to combat microbial diseases (Sharrock and Sun 2020; Mermigka et al. 2020). Plants depend primarily on two interconnected layers of the innate immune system to recognize and respond to pathogens (Jones and Dangl 2006; Spoel and Dong 2012; Han 2019). Firstly, pattern-recognition receptors (PRRs) recognize microbe-associated molecular patterns (MAMPs) from a wide spectrum of microbes, resulting in pattern-triggered immunity (PTI). A second layer involves disease resistance (R) proteins that recognize pathogen effector molecules or their activities on host targets, resulting in effector-triggered immunity (ETI). In addition, plants can acquire disease resistance by previous encounters with microbes or natural compounds such as plant hormones resulting in systemic acquired resistance (SAR) or induced systemic resistance (ISR), depending on whether the salicylic acid or jasmonate defense pathway is activated (Ryals et al. 1996; Pieterse et al. 2014; Klessig et al. 2018). This knowledge has led to the commercial development of synthetic resistance inducers such as Benzothiadiazole (BTH) or Probenazole that mimic the activity of a natural inducer and help to protect plants against various diseases (Nakashita et al. 2002; Kogel et al. 1994; Görlach et al. 1996; Vlot et al. 2021). More recently, it was discovered that some synthetic resistance inducers and natural compounds such as salicylic acid, β-aminobutyric acid (BABA), or acyl-homoserine lactones (AHLs) at low concentrations trigger induced resistance via defense priming in plants leading to a physiological state that enables plants to respond more rapidly and/or more robustly to a challenge inoculation after exposure to biotic or abiotic stress (detailed reviews see Conrath et al. 2015; Balmer et al. 2015; Baccelli and Mauch-Mani 2016; Cooper and Ton 2022). An early response of primed plants is their production of reactive oxygen species (ROS) at the site of attack when they recognize a pathogen or pest, which is associated with the termination of the invasion or infestation (Balmer et al. 2015). Since a primed plant has only a very limited part of its defense system activated, priming could be an approach to protecting plants from diseases and pests while minimizing energy expenditure and thus yield losses (Westman et al. 2019; Schenck et al. 2014; Jung and Cecchini 2023).
Several recent studies have shown that a family of nematode-derived pheromones called ascarosides induces resistance in many plants against a broad spectrum of pathogens and pests by upregulating specific defense signaling pathways (Manosalva et al. 2015; Ali et al. 2018; Klessig et al. 2019; Ning et al. 2020). The term ascaroside originally referred to a distinct type of lipid first detected in parasitic roundworms of the family Ascaridia more than 100 years ago (Flury 1912). Ascarosides serve a wide range of biological functions which is facilitated by a great diversity of ascaroside chemical structures (Ludewig and Schroeder 2013). These are based on the sugar ascarylose, which is linked to fatty acid-like side chains of varying lengths and often decorated further with building blocks derived from amino acids, folate, and other primary metabolites. Plants can metabolize ascarosides and thereby change their chemical message, generating ascaroside mixtures that repel diseases and pests and reduce infection (Manohar et al. 2020; Yu et al. 2021).
Here, we report on the biocontrol effect of ascr#18, the most abundant ascaroside secreted by plant-parasitic nematodes into the plant rhizosphere (Manosalva et al. 2015), on P. triticina infections of various wheat (T. aestivum) genotypes. Importantly, ascr#18 was effective in the nano- and micromolar range, indicating a broad optimal concentration for controlling Pt. Our finding identifies a novel mode of ascr#18-induced resistance by triggering the accumulation of H2O2 at attacked stomata, a characteristic also observed with other resistance inducers (Schenck et al. 2015).
Materials and methods
Plant material, fungal inoculation, and ascr#18 treatment
The P. triticina-susceptible wheat varieties T. aestivum cv. Chinese Spring (spring wheat) and winter wheat Arina LR (both provided by the Julius Kuehn Institute (JKI) Kleinmachnow, Germany), Zentos, Chinofuz (provided by the JKI Quedlinburg, Germany), and Boolani (Seed and Plant Improvement Institute, Karaj, Iran) were used. The leaf rust Pt race 77W × R (Serfling et al. 2013) was a gift of the JKI Quedlinburg. The virulence/avirulence profile of race 77W × R used in field trials and seedling test is found in Rollar et al. (2021). The wheat cv. Boolani is susceptible to Pt race PKTTS (Delfan et al. 2022). The profile of virulence/avirulence of race PKTTS is shown in Table S1.
The ascaroside ascr#18 was a gift from Ascribe Biosciences, 95 Brown Rd, Ithaca, NY, USA. For all experiments, wheat plants were grown in a pot containing fine-structured soil (Fruhstorfer Erde type T; HAWITA Gruppe GmbH, Vechta, Germany) in a growth chamber under controlled conditions with the temperature set to 18/20 °C (night/day), light period of 16 h, and 65% relative humidity. Ten-day-old seedlings were sprayed with ascr#18 in an aqueous solution containing 0.1% ethanol until run-off using a hand sprayer (Carl Roth, Germany); control plants were sprayed with 0.1% ethanol. After 24 h, leaves were inoculated by brushing with two-week-old Pt uredospores isolated from T. aestivum cv. Kanzler, using a mix of rust uredospores and talcum powder (Alliance Chemical, Germany) in a concentration of 1:4 (McIntosh et al. 1995). The inoculated seedlings were grown at 18/20 °C (night/day) with 16 h of photoperiod and 95% relative humidity for three days, followed by 65% relative humidity for seven days. The number of uredinia was evaluated on one leaf per plant in an area of 0.5 cm2 after 10 days post-inoculation (dpi) by use of a binocular (Leica Microsystems GmbH, Wetzlar, Germany).
Test on direct toxicity of ascr#18
To test whether there is a direct effect of ascr#18 on the germination of P. triticina, 9-cm petri dish plates of water agar (3% w/v agar) were pretreated with 2 mL of 1 µM ascr#18 dispensed in 0.1% v/v ethanol using a sprayer (Preval, Art.-Nr. YC44.1). Subsequently, a suspension of Pt isolate 77W × R (5 mg of uredospores in 25 mL 0.1% w/v agar) was sprayed onto the agar plates either 15 min or 24 h after ascr#18 application. Inoculated plates were incubated in dark at 25 °C for 10 h at 100% relative humidity. Three plates were prepared for each treatment, and 100 uredospores were examined for germination on each plate. Uredospores were rated as germinated when germ tubes were visible and at least five times the size of the uredospore. Ethanol (0.1%) was used in the absence of ascr#18 as control. Water agar with 0.16 g/L prothioconazole (Proline©, Bayer CropScience) was used as positive control for inhibition of Pt germination.
Statistics
For statistical analysis, data were checked for normality. The t test for normalized data and Mann–Whitney test for unnormalized data were performed in experiments with two groups to compare. For dose effect experiments, the analysis was done by one-way ANOVA, and multiple comparisons were carried out using Tukey’s post‐hoc test (p < 0.05). All statistical analyses and graphs were done with GraphPad Prism 8 software. For the germination test, data were fitted to a linear model using the function aov (Chambers et al.1992) in R. Tukey honest significant difference test was conducted on the fitted model using the TukeyHSD function (p < 0.05; Miller 1981).
Microscopy
Pt-infected leaves (10 dpi) of ascr#18-treated and mock-treated (0.1% ethanol) control plants were fixed in 4% paraformaldehyde (in PBS buffer) or 0.15% trichloroacetic acid (in chloroform:ethanol 20:80, v/v). Fungal structures were visualized using chitin-specific staining with WGA-AF488 (wheat germ agglutinin; Molecular Probes, Karlsruhe, Germany). Leaves were investigated under an epifluorescence microscope (Axio Imager.A2, Carl Zeiss, Oberkochen, Germany) and a confocal laser scanning microscope (CLSM; TCS SP8, Leica Microsystems GmbH, Wetzlar, Germany) by use of ZEISS ZEN 3.8 and Leica LAS X software, respectively. WGA-AF488 was visualized at λexc 494 nm, λem 515, and fluorescence control settings were set to λexc 631 nm, λem 642. For H2O2 detection, Pt-infected leaves of ascr#18- and mock-treated plants were collected: 12 hpi, 24 hpi, 48 hpi, and 96 hpi, and samples were stained with 3,3′-diaminobenzidine (DAB)-tetrahydrochloride (Hückelhoven et al. 1999) and subsequently kept in 0.15% trichloroacetic acid (in chloroform:ethanol 20:80, v/v). To evaluate the DAB-stained area, the average size of precipitates on the attacked stomata was quantified using ImageJ free software (https://imagej.net/ij/).
Results
Ascr#18 induces resistance against Puccinia triticina in all tested wheat cultivars
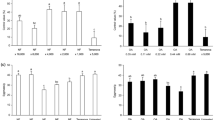
The effect of ascr#18 on wheat against leaf rust was first tested with the four cultivars (cvs.): Zentos, Chinese Spring, Arina LR, and Chinofuz. Leaves of 10-day-old seedlings were sprayed with 1µM ascr#18 and, 24 h later, inoculated with uredospores Pt race 77W × R. Ascr#18 significantly reduced the number of Pt uredinia on all four wheat genotypes as compared to mock treatment (t test for normalized data, Mann–Whitney test for unnormalized data; p < 0.05): Zentos (70%), Chinese Spring (71%), Arina LR (77%), and Chinofuz (81%) (Fig. 1; Supplementary Fig. 1). To exclude the possibility that the effect on Pt development was due to direct toxic effects of ascr#18, we exposed the fungus to 1 µM ascr#18 on water agar plates for 10 h. Consistent with previous reports on other fungal pathogens (Manosalva et al. 2015; Klessig et al. 2019), Pt’s germination rate was unaffected by ascr#18 and was comparable to the control treatments (Table 1). We concluded that ascr#18 induces resistance to leaf rust fungus in the wheat cultivars tested.
The ascaroside ascr#18 reduces the number of uredinia in wheat leaves inoculated with the leaf rust fungus Puccinia triticina. Leaves of 10-day-old wheat cvs. Zentos, Chinese Spring, Arina LR, and Chinofuz were sprayed with 1 µM ascr#18 in 0.1% ethanol and, 24 h later, inoculated with uredospores of Pt race 77W × R. Controls (Con) were treated with 0.1% ethanol. The number of uredinia was counted at 10 dpi. Per experiment, 15 seedlings were treated, each represented by a single dot in the boxplot. The experiment was repeated twice. Minimum/maximum values are represented by whiskers, and center represents the median in the boxplot. Statistics was performed with t test for normalized data and Mann–Whitney test for unnormalized data (p < 0.05). Asterisks indicate significant differences to the control group (***p ≤ 0.0001).
Ascr#18 induces resistance against Puccinia triticina in the nM range
Next, we conducted a dose–response experiment in the concentration range between 0.000001 and 10 µM ascr#18. Leaves of 10-day-old seedlings of cvs. Chinese Spring and Zentos were sprayed with the respective concentrations of ascr#18 and, 24 h later, inoculated with Pt race 77W × R. Ascr#18 significantly reduced the number of uredinia on both wheat genotypes down to a concentration of 0.01 nM (one-way ANOVA, Tukey’s post‐hoc test; p < 0.05) (Fig. 2).
Dose–response analysis of the effect of ascr#18 on the formation of uredinia in wheat. Leaves of 10-day-old seedlings of cvs. Chinese Spring and Zentos were sprayed with the indicated concentration of ascr#18 dissolved in 0.1% ethanol and, 24 h later, inoculated with uredospores of Puccinia triticina race 77W × R. Control seedlings were treated with 0.1% ethanol. The number of uredinia was counted at 10 dpi. Treatments were done on 15 seedlings, each represented by a single data point in the boxplot. The experiment was repeated twice. Minimum/maximum values are represented by whiskers, and center represents the median in the boxplot. Statistics was performed with one-way ANOVA, and boxplots with different letters are significantly different according to Tukey’s post‐hoc test (p < 0.05).
To broaden the agronomic relevance, we extended our investigation to the wheat cv. Boolani, which is susceptible to Pt race PKTTS. Ten-day-old seedlings were sprayed with ascr#18, and the dose effect on the number of uredinia at 10 dpi was analyzed. As expected, the number of uredinia was greatly reduced over a wide range of concentrations, suggesting that the effect of ascr#18 on Pt is not race-specific (Supplementary Fig. 2).
Ascr#18 induces impaired appressorial stoma penetration
Next, we examined microscopically how fungal growth was inhibited in response to ascr#18 treatment. To this end, cv. Chinese Spring was inoculated with Pt race 77W × R and, 10 days later, stained with chitin-specific WGA-AF488 to detect fungal hyphae and infection structures. Fluorescence microscopy at low magnification showed that fungal mycelium formation was greatly reduced and the density of uredinia on the examined leaf section was consistently very low after treatment with ascr#18 (Fig. 3a, b). Moreover, hyphae on the leaf surface were much shorter and barely branched (Fig. 3c, d). Further analysis using confocal laser microscopy (CLSM) showed that penetration of the fungus from an appressorium into the substomatal cavity often failed. Examples of the penetration failure on ascr#18-treated leaves are shown in Fig. 3e–h. Either the fungus did not penetrate the leaf at all (Fig. 3e, f) or, in rare cases, was arrested at the stage of substomatal vesicle formation (Fig. 3g, h). In agreement with this, 3D analysis of leaves using CLSM at 10 dpi showed a strong fungal invasion in the mesophyll of the control plants (Fig. 3i), whereas hardly any fungal structures were found in the mesophyll after ascr#18 treatment (Fig. 3j).
Pt structures on leaves of wheat cv. Chinese Spring as visualized by WGA-AF488 staining. Plants were treated with 0.1% ethanol (a, c: control) or 1 µM ascr#18 in 0.1% ethanol (b, d, e, g, f, and h) and, 10 days later, harvested for microscopic investigation. On ascr#18-treated leaves, only a few or no uredinia were formed; hyphal development was impaired, and germ tubes were short and barely branched (b, d). Many appressoria in ascr#18-treated leaves were not able to penetrate the stomata as revealed by CLSM inspection of the fungal infection structures in different layers under the appressorium (e, g, f, and h). In the rare cases, penetration of stomata in ascr#18-treated leaves was successful; substomatal vesicles were visible in layers below the appressorium, but formation of primary infection hyphae was not seen (g, h). Three-dimensional analysis of infected leaves by CLSM revealed heavily infected mesophyll tissue in control plants (i), while in ascr#18-treated leaves, barely any fungal structures were found in the mesophyll tissue (j). White arrows: uredinia on control leaf.
Ascr#18-mediated resistance is associated with enhanced early H2O2 accumulation at stomata
A previous study on the priming activities of AHLs showed that N-3-oxo-tetradecanoyl-l-homoserine lactone (oxo-C14-HSL) primed plants for accumulation of phenolic compounds, lignification of cell walls and promoted closure of stomata in response to Pseudomonas syringae infection (Schenck et al. 2014). For wheat leaf rust, R gene-mediated prehaustorial resistance in Triticum monococcum was also reported to be associated with H2O2 accumulation at sites of attempted infection (Serfling et al. 2016). Since we did not detect a hypersensitive reaction (HR) of epidermal or mesophyll cells, nor papillae formation in ascr#18-treated leaves at sites of attempted stomata penetration, we tested the possibility that the arrest of the fungus at this early stage of infection is associated with enhanced H2O2. Indeed, DAB-stained leaf samples collected at different times after inoculation with race 77W × R uredospores showed much more H2O2 accumulation as revealed by brown precipitate at attacked stomata of plants treated with 1 µM ascr#18 than at stomata of control plants at 12, 24, and 48 hpi (Table 2). Notably, at later time points (96 hpi), little H2O2 was detected by DAB staining only, suggesting that the accumulation of hydrogen peroxide is only transient and limited to the site of attempted penetration (Fig. 4).
DAB-mediated visualization of hydrogen peroxide accumulation at the sites of attempted penetration, indicated by a brown precipitate. Pt was stained with WGA-AF488. For each sample, brightfield images (top row) show the DAB-based brown precipitate, and fluorescence images (bottom row) show WGA-AF488 fluorescence of the fungal chitin structures. Plants were treated with 0.1% ethanol (Con) or 1 µM ascr#18 in 0.1% ethanol, inoculated 24 h later with Pt race 77W × R, and sampled at the time indicated in the image. After 12, 24, and 48 h, a higher accumulation of hydrogen peroxide was observed in the leaves treated with ascr#18 compared to the control samples. After 96 hpi, only little accumulation of hydrogen peroxide was detected in ascr#18-treated plants. White arrows indicate attacked stomata.
Discussion
Here, we demonstrate broad and highly efficient resistance-inducing activity of the currently best-studied and most active ascaroside, ascr#18, using a representative set of wheat cultivars and two P. triticina races with very different virulence spectra. Recording Pt infection on infected leaves showed that spray-pretreatment with ascr#18 significantly reduced the number of uredinia as compared to mock-pretreated Pt-inoculated plants. A dose–response analysis over the nano- and micromolar concentration range revealed a unusually broad optimum concentration down to 0.01 nM for the control of wheat leaf rust indicating that ascr#18 is a very potent resistance inducer. Moreover, microscopic analysis showed very early abortion of the fungus in the prepenetration stage. This was associated with local accumulation of H2O2 as visualized by DAB staining at attacked stomata. It is noteworthy that no papilla formation or HR of epidermal or mesophyll cells could be detected at the site of the attempted penetration. Instead, the fungus did not overcome the appressoric stage in many penetration attempts with the formation of substomatal vesicles in only rare cases. Overall, our results are consistent with the current view that H2O2 accumulation and the resulting strengthening of the cell wall and regulation of stomata play a key role in the very early defense responses of plants triggered by resistance inducers (Schenck et al. 2014; for review Balmer et al 2015).
Previous reports showed the strong resistance-inducing effect of ascr#18 in plant protection against a virus (Turnip Crinkle Virus), a bacterium (P. syringae pv. tomato), a fungus (e.g., Blumeria graminis f. sp. hordei), an oomycete (Phytophthora infestans), and two nematodes (Heterodera schachtii and Meloidogyne incognita) in four plant species (barley, potato, tomato, and Arabidopsis) (Manosalva et al. 2015). In another report, ascr#18 was shown to induce resistance to four crops (wheat, soybean, rice, and tomato) against eight pathogens/pests, including one virus, bacteria, fungi, an oomycete, and a nematode (Klessig et al. 2019), overall suggesting that ascarosides are effective tools that can be used in crop production. References Hoogkamp et al. (1998), Schabdach et al. (2014), Schmittgen and Livak (2008), Yandell (1997). are given in list but not cited in text. Please cite in text or delete them from list. corrected
There are only a few reports on the mode of action of resistance-inducing agents that are effective in controlling rust fungi on cereal crops. The bacterium Ensifer (syn. Sinorhizobium) meliloti induces resistance via priming against Puccinia hordei (Matros et al. 2023). Interestingly, the authors compared the priming activity of strain E. meliloti expR + chthat which produced large amounts of the AHL 3-oxo-C14-HSL with a transformed strain E. meliloti attM that does not accumulate AHL, suggesting an AHL-induced P. hordei resistance. Interestingly, oxo-C14-HSL in Arabidopsis can induce the oxylipin/SA signaling pathway and thus a stomata defense response and cell wall strengthening, preventing pathogen invasion (Schenck and Schikora 2015). This mode of action is similar to the effect of ascr#18 in our analysis, although a more detailed molecular investigation of the similarities and differences between AHL and ascr#18 is required.
7-oxo- and the 7-hydroxysterols also can induce resistance toward Puccinia striiformis and P. hordei in barley and wheat when sprayed onto primary leaves using 10–4 M in 1% ethanol (Schabbach et al. 2014) two days prior to challenge inoculation with the pathogen. It was suggested that the sterol derivatives selectively activate plant defense mechanisms that impair the development or differentiation of infection structures. Thus, changes in the morphology or chemistry of the cuticle that prevent the formation of appressoria at the stomata could suppress the fungus.
Induced resistance against P. triticina has also been achieved by treating wheat (cv. Arina) with the beneficial bacterium Pseudomonas protegens CHA0 (by seed coating) and the compound β-aminobutyric acid (BABA) (soil drenching) (Bellameche et al. 2021). BABA was tested at high concentrations (10–20 mM), and a dose-dependent reduction of pustule formation was observed with greatest protection at 20 mM. In light of these results, previous and our current work shows that ascr#18 acts at many orders of magnitude lower concentrations (Fig. 4; Manosalva et al. 2015; Klessig et al. 2019).
Similar to our study, accumulation of H2O2 in both CHA0- and BABA-treated plants was mostly detected in host guard cells at penetration sites, and both treatments reduced fungus penetration and haustorium formation. The authors suggested that during recognition or formation of appressoria, generation of H2O2 in guard cells is induced, possibly following secretion of rust effectors, and mechanical forces during adhesion of appressoria over stomata may also elicit H2O2 generation in guard cells (Bellameche et al. 2021). In Arabidopsis, H2O2 accumulation in guard cells was involved in signal transduction during ABA-mediated stomatal closing (Sun et al. 2017). Similarly, appressorium formation of P. triticina also caused stoma closure in wheat leaves (Bolton et al. 2008).
In conclusion, ascr#18 enables induction and modulation of different signaling pathways to activate immune responses in plants at very low concentrations. Thus, ascr#18 has an interesting potential as biological control agent to reduce disease damage and increase sustainable food security.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Ali MA, Anjam MS, Nawaz MA, Lam HM, Chung G (2018) Signal transduction in plant–nematode interactions. Int J Mol Sci 19(6):1648
Andersen EJ, Ali S, Byamukama E, Yen Y, Nepal MP (2018) Disease resistance mechanisms in plants. Genes 9(7):339
Baccelli I, Mauch-Mani B (2016) Beta-aminobutyric acid priming of plant defense; the role of ABA and other hormones. Plant Mol Biol 91(6):703–711
Balmer A, Pastor V, Gamir J, Flors V, Mauch-Mani B (2015) The ‘prime-ome’: towards a holistic approach to priming. Trends Plant Sci 20(7):443–452. https://doi.org/10.1016/j.tplants.2015.04.002
Bellameche F, Jasim MA, Mauch-Mani B, Mascher F (2021) Histopathological aspects of resistance in wheat to Puccinia triticina, induced by Pseudomonas protegens CHA0 and β-aminobutyric acid. Phytopathol Mediterr 60(3):441–453
Bolton MD, Kolmer JA, Garvin DF (2008) Wheat leaf rust caused by Puccinia triticina. Mol Plant Pathol 9:563–575. https://doi.org/10.1111/j.1364-3703.2008.00487.x
Caubel J, Launay M, Ripoche D, Gouache D, Buis S, Huard F, Huber L, Brun F, Bancal MO (2017) Climate change effects on leaf rust of wheat: implementing a coupled crop-disease model in a French regional application. Eur J Agr 90:53–66
Chambers JM, Freeny A, Heiberger RM (1992) Analysis of variance designed experiments. In: Chambers JM, Hastie TJ (eds) Statistical models in S. Wadsworth and Brooks/Cole, Pacific Grove
Conrath U, Beckers GJ, Langenbach CJ, Jaskiewicz MR (2015) Priming for enhanced defense. Annu Rev Phytopathol 53(1):97–119
Cooper A, Ton J (2022) Immune priming in plants: from the onset to transgenerational maintenance. Essays Biochem 66(5):635–646
Delfan S, Bihamta MR, Dadrezaei ST, Abbasi A, Alipour H (2022) Identification sources of resistance for leaf rust (Puccinia triticina Erikss.) in Iranian wheat genotypes. Iran J Plant Prot Sci. 52(2):115–133
Flury F (1912) On the chemistry and toxicology of ascarides. Arch Exp Path Pharmak 67:275–392
Galli M, Feldmann F, Vogler UK, Kogel KH (2024) Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J Plant Dis Prot 131:265–291. https://doi.org/10.1007/s41348-024-00878-1
Görlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH et al (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8(4):629–643
Han GZ (2019) Origin and evolution of the plant immune system. New Phytol 222:70–83. https://doi.org/10.1111/nph.15596
Hawkins NJ, Bass C, Dixon A, Neve P (2019) The evolutionary origin of pesticide resistance. Biol Rev 94(1):135–155
Helfer S (2014) Rust fungi and global change. New Phytol 201:770–780. https://doi.org/10.1111/nph.12570
Hückelhoven R, Fodor J, Preis C, Kogel KH (1999) Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol 119(4):1251–1260. https://doi.org/10.1104/pp.119.4.1251
Huerta-Espino J, Singh RP, German S, McCallum BD, Park RF, Chen WQ et al (2011) Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 179(1):143–160
Jones J, Dangl J (2006) The plant immune system. Nature 444:323–329
Jung HW, Cecchini NM (2023) Editorial: systemic resistance and defense priming against pathogens. Front Plant Sci. https://doi.org/10.3389/fpls.2023.1267513
Junk J, Kouadio L, Delfosse P et al (2016) Effects of regional climate change on brown rust disease in winter wheat. Clim Change 135:439–451
Klessig DF, Choi HW, Dempsey DA (2018) Systemic acquired resistance and salicylic acid: past, present, and future. Mol Plant Micr Interact 31(9):871–888
Klessig DF, Manohar M, Baby S, Koch A, Danquah WB, Luna E et al (2019) Nematode ascaroside enhances resistance in a broad spectrum of plant–pathogen systems. J Phytopath 167(5):265–272
Kogel KH, Beckhove U, Dreschers J, Münch S, Rommé Y (1994) Acquired resistance in barley’ the resistance mechanism induced by 2,6-dichloroisonicotinic acid is a phenocopy of a genetically based mechanism governing race-specific powdery mildew resistance. Plant Physiol 106:1269–1277
Kolmer J (2013) Leaf rust of wheat: pathogen biology, variation and host resistance. Forests 4(1):70–84
Ludewig AH, Schroeder FC (2013) Ascaroside signaling in C. elegans (January 18, 2013), WormBook, ed. The C. elegans Research Community, WormBook. https://doi.org/10.1895/wormbook.1.155.1. http://www.wormbook.org
Manohar M, Tenjo-Castano F, Chen S, Zhang YK, Kumari A, Williamson VM et al (2020) Plant metabolism of nematode pheromones mediates plant-nematode interactions. Nature Comm 11(1):1–11
Manosalva P, Manohar M, Von Reuss SH, Chen S, Koch A, Kaplan F et al (2015) Conserved nematode signaling molecules elicit plant defenses and pathogen resistance. Nature Comm 6(1):1–8
Matros A, Schikora A, Ordon F, Wehner G (2023) QTL for induced resistance against leaf rust in barley. Front Plant Sci 13:1069087. https://doi.org/10.3389/fpls.2022.1069087
McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. CSIRO publishing, Sydney
Mermigka G, Amprazi M, Mentzelopoulou A, Amartolou A, Sarris PF (2020) Plant and animal innate immunity complexes: fighting different enemies with similar weapons. Trends Plant Sci 25(1):80–91
Miller RG (1981) Simultaneous statistical inference. Springer, Cham (ISBN 10:0387905480)
Nakashita H, Yoshioka K, Yasuda M, Nitta T, Arai Y, Yoshida S, Yamaguchi I (2002) Probenazole induces systemic acquired resistance in tobacco through salicylic acid accumulation. Physiol Mol Plant Pathol 61(4):197–203
Ning S, Zhang L, Ma J, Chen L, Zeng G, Yang C et al (2020) Modular and scalable synthesis of nematode pheromone ascarosides: implications in eliciting plant defense response. Org Biomol Chem 18(26):4956–4961
OECD (2020) COVID-19 and International trade: issues and actions, https://read.oecd-ilibrary.org/view/?ref=128_128542-3ijg8kfswh&title=COVID-19-and-international-trade-issues-and-actions.
Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52(1):347–375. https://doi.org/10.1146/annurev-phyto-082712-102340
Poore J, Nemecek T (2018) Reducing food’s environmental impacts through producers and consumers. Science 360(6392):987–992
Rollar S, Serfling A, Geyer M, Hartl L, Mohler V, Ordon F (2021) QTL mapping of adult plant and seedling resistance to leaf rust (Puccinia triticina Eriks.) in a multiparent advanced generation intercross (MAGIC) wheat population. Theor Appl Genet 134(1):37–51
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8(10):1809–1819
Schabdach H, Johne S, Steiner U, Seifert K (2014) Plant disease resistance inducing activity of 7-Oxo- and 7-Hydroxysterols. Z Naturforsch C J Biosci 50(3–4):257–262. https://doi.org/10.1515/znc-1995-3-415. (PMID: 37978790)
Schenk S, Schikora A (2015) AHL-priming functions via oxylipin and salicylic acid. Front Plant Sci 5:784. https://doi.org/10.3389/fpls.2014.00784
Schenk S, Hernández-Reyes C, Samans B, Stein E, Neumann C, Schikora M, Reichelt M, Mithöfer A, Becker A, Kogel KH, Schikora A (2014) N-Acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell 26(6):2708–2723
Serfling A, Krämer I, Perovic D, Ordon F (2013) Broadening the genetic base of leaf rust (Puccinia triticina f. sp. tritici) resistance in wheat (Triticum aestivum). J Kult 65(7):262–272
Serfling A, Templer SE, Winter P, Ordon F (2016) Microscopic and molecular characterization of the prehaustorial resistance against wheat leaf rust (Puccinia triticina) in Einkorn (Triticum monococcum). Front Plant Sci 7:1668. https://doi.org/10.3389/fpls.2016.01668
Sharrock J, Sun JC (2020) Innate immunological memory: from plants to animals. Curr Opin Immunol 62:69–78. https://doi.org/10.1016/j.coi.2019.12.001
Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immun 12(2):89–100
Sun L, Li Y, Miao W, Piao T, Hao Y, Hao FS (2017) NADK2 positively modulates abscisic acid-induced stomatal closure by affecting accumulation of H2O2, Ca2+ and nitric oxide in Arabidopsis guard cells. Plant Sci 262:81–90
van Esse HP, Reuber TL, van der Does D (2020) Genetic modification to improve disease resistance in crops. New Phytol 225:70–86
Vlot AC, Sales JH, Lenk M, Bauer K, Brambilla A, Sommer A et al (2021) Systemic propagation of immunity in plants. New Phytol 229(3):1234–1250
Westman SM, Kloth KJ, Hanson J et al (2019) Defence priming in Arabidopsis – a Meta-Analysis. Sci Rep 9:13309
Yu Y, Zhang YK, Manohar M, Artyukhin AB, Kumari A, Tenjo-Castano FJ, Nguyen H, Routray P, Choe A, Klessig DF, Schroeder FC (2021) Nematode signaling molecules are extensively metabolized by animals, plants, and microorganisms. ACS Chem Biol 16(6):1050–1058
Acknowledgments
We thank Christina Birkenstock and Anne Witzke for technical assistance. This work was funded in the project PrimedWeizen FKZ 2818409B19 of the Bundesministerium für Ernährung und Landwirtschaft (BMEL), Germany, to KHK and the project WheatInterfere of the Bundesministerium für Bildung und Forschung (BMBF), Germany, to KHK.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
K.H.K. designed the study. A.K. conducted the experimental analysis of ascr#18 effects on uredinia formation in wheat. J.T. and A.K. conducted the microscopic analysis of ascr#18 activity on Pt infections. S.D. conducted experiments with cv. Boolani. T.K. and P.S. conducted the toxicity analysis. A.K., J.T., K.H.K., MM., DK, and FS analyzed the data and wrote the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The research described in the manuscript was not funded by private partners or industry. Authors Akshita Kamboj (she), Jennifer Thielmann (she), Saba Delfan (she), and Karl-Heinz Koge (he) declare that they have no conflict of interest. Authors Murli Manohar, Frank Schroeder, and Daniel Klessig are co-founders of Ascribe Bioscience, a company that develops plant treatments based on small molecules from microbiota.
Consent for publication
All authors declare consent of publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
41348_2024_950_MOESM1_ESM.pptx
Supplementary file 1 (PPTX 41 KB). Figure S1 The ascaroside ascr#18 induces resistance to Puccinia triticina on wheat (Triticum aestivum). Leaves of ten-day-old wheat cvs. Zentos, Chinese spring, Arina LR, and Chinofuz were sprayed with 1 µM ascr#18 in 0.1% ethanol and, 24 h later, inoculated with uredospores of Pt race 77W×R. Controls (Con) were treated with 0.1% ethanol. Images of infected leaves were taken at 10 dpi. Figure S2 Dose–response analysis of the effect of the ascaroside ascr#18 on uredinia numbers in wheat cv. Boolani. Leaves of ten-day-old seedlings were sprayed with the indicated concentration of ascr#18 in 0.1% ethanol and, 24 h later, inoculated with uredospores of Puccinia triticina race PKTTS. Controls were treated with 0.1% ethanol. The number of uredinia was counted at 10 dpi. Treatments were done on 10 seedlings, each represented by a single dot in the boxplot. Minimum/maximum values are represented by whiskers, and center represents the median in the boxplot. Statistics was performed with one-way ANOVA, and boxplots with different letters are significantly different according to Tukey’s post‐hoc test (p < 0.05).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamboj, A., Thielmann, J., Delfan, S. et al. The nematode signaling molecule ascr#18 induces prepenetration defenses in wheat against a leaf rust fungus. J Plant Dis Prot (2024). https://doi.org/10.1007/s41348-024-00950-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-024-00950-w