Abstract
Plasmopara viticola is controlled by fungicides with different modes of action, which include zoxamide. Zoxamide was developed in 1998 by Dow AgroSciences LIC (Indianapolis, IN) and has been commercialized since 2001 by Rohm and Haas Company. This fungicide is highly effective against oomycetes, used for foliar application in vine crops, potato, and other vegetables to control oomycete-induced diseases. Zoxamide acts by causing mitotic arrest by binding to β-tubulin, inhibiting tubulin polymerization and cell division of the pathogen. In the past three decades, the management of fungicide resistance has emerged as a significant concern for both growers and regulatory authorities; this is primarily due to the substantial increase in resistance, in terms of occurrence and spread, towards most fungicide groups. The monitoring of the effectiveness of fungicides is the only way to accurately identify as soon as possible the onset of resistance. In this study, we were interested in tracking the changes in the sensitivity of zoxamide to P. viticola populations collected during a 6-year monitoring (2017–2022) in two Italian locations: the autonomous province of Trento and the autonomous region of Friuli-Venezia Giulia. Bioassays on leaf discs were carried out, and EC50 and MIC values were elaborated. From our results, zoxamide showed for a long period of time high sensitivity, but over the last years we observed a change, as in 2022 most of the samples tested in these two regions showed MIC > 100 mg L−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Downy mildew caused by Plasmopara viticola (Berk. & Curt.) Berl. & de Toni is a disease affecting grapes worldwide, exerting a strong impact on production. In Italy, there is a wide range of products that can be used to control P. viticola. At present, this includes 23 approved active ingredients, covering 14 different modes of action.
Zoxamide RH-7281(3,5-dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-4-methylbenzamide) is one of the fungicides utilized in the control of P. viticola; its activity is due by arresting nuclear division and destroying the microtubule cytoskeleton of oomycete pathogens (Young and Slawecki 2001). To date, sixteen zoxamide-based products are approved in Italy (Table 1). Despite considerable progress in understanding the biology and aetiology of plant pathogens, plant disease management has not significantly changed for the last 50 years, relying primarily on fungicides (Beckerman et al. 2023). The utilization of single-site fungicides has led to the emergence of a new issue for the control of downy mildew: fungicide resistance that can be defined as the acquired and heritable reduction in the sensitivity of the pathogen to a specific molecules.
In order to avoid the risk of fungicides resistance, a proactive approach using different disease control tactics is the most effective. The degree of success of anti-resistance strategies is strongly influenced by the timing of the start of the monitoring activity (Brent 2012); therefore, it is crucial to plan multiyear monitoring to assess fungicide sensitivity. The objective of this study was to track the zoxamide sensitivity changes towards P. viticola populations in two Italian regions where the fungicide was employed for several years, in order to best manage the potential onset of resistance.
Material and methods
Sample collection
The samples collected for the monitoring studies were originating from three provinces of the region Friuli-Venezia Giulia (FVG), Pordenone (PN) Udine (UD), and Gorizia (GZ), located in the lowland area (Fig. 1), and from the Province of Trento (Fig. 2).
104 P. viticola populations were generated out of leaf samples showing typical symptoms of downy mildew being collected from the same number of commercial vineyards in FVG and Trento and then studied in leaf disc tests to determine EC50 and MIC values. Samples were dispatched to the laboratory from 2017 to 2022. Each sample constitutes around 25–40 leaves and considered to be representative for each vineyard.
Biological assays
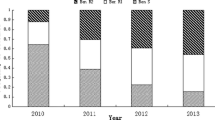
Bioassays were carried out utilizing grape leaf discs (cv. Chardonnay), being treated with three concentrations (1, 10, and 100 mg L−1) of zoxamide (Zoxium 240 SC) 24 h before inoculation. For each concentration tested, an untreated control was included. Fifteen leaf discs were soaked in fungicide suspensions of each concentration for 45 min. The inoculation was conducted by spraying a sporangial suspension (5 × 104 spores mL−1) onto the adaxial surface of leaf discs, which were then incubated at 23 °C and a 12-h photoperiod. The sporulation was assessed 8–10 days after the inoculation by evaluating the percentage of sporulated leaf area, the EC50 values (mg L−1) were calculated by probit analysis, and minimum inhibitory concentration (MIC) was derived (Figs. 3 and 4).
Results
Table 2 shows the EC50 and MIC values of the P. viticola populations coming from FVG and Trento. The zoxamide baseline was determined after 6 years of monitoring and shows a range of EC50 values from 0.00004 to 0.47.
Friuli-venezia giulia region
In the season 2017, only two populations were tested for zoxamide (448, 455), which showed an EC50 value and MIC of 0.009/ < 10 mg L−1 and 1.43/100 mg L−1, respectively. In 2018, again only two populations were tested (538, 567), showing EC50 values and MICs of 0.13/ < 10 mg L−1 and 0.001/ < 10 mg L−1, respectively. Population 610 tested in 2019 showed an EC50 and MIC of 0.93 / < 10 mg L−1. In 2020, three populations tested, 654, 655, and 679, showed EC50 and MIC values of 0.066/ < 100 mg L−1, 0.001/ < 1 mg L−1, and 0.001/ < 1 mg L−1, respectively. Three populations studied in 2021 showed EC50 values ranging from 0.38 to 9.44 mg/l, while MIC values were < 100 to > 100 mg L−1. In 2022, eight populations investigated showed EC50 values ranging from 0.89 to 100 mg/l, while MIC values were for all samples > 100 mg/l.
Trento province
Populations originating from Trento in 2017 showed an EC50 range between 0.001 and 0.15 mg L−1; MIC values were between < 1 to < 100 mg L−1.
In 2018, EC50 values did not exceed 0.51 mg L−1, and only two populations (495, 547) showed MIC values > 100 mg L−1. In 2019, EC50 values did not exceed 0.47 mg L−1, and MIC values were between < 1 to < 100 mg L−1. In the year 2020, populations showed EC50 values being always below 0.42 mg L−1, while the corresponding MIC values were ranging between < 10 and < 100. Only population (650) showed an EC50 of 5.08 mg L−1 and a MIC < 100. In 2021, EC50 values ranged between 0.11 and 11.8 mg L−1; in particular, sample (685) showed the highest EC50 with a MIC > 100; furthermore, seven samples out of eleven showed a MIC > 100 mg L−1. In 2022, EC50 values ranged between 0.041 and 2.56 mg L−1. Regarding the MIC values, nine populations out of eleven exhibited values above 100 mg L−1, one population (742) was below < 100 mg L−1, and population 748 showed a MIC of < 1 mg L−1.
Discussion
Zoxamide is a fungicide considered by the FRAC with a low to moderate risk of resistance. Regular sensitivity monitoring of fungicides is the best way to accurately identify as soon as possible the onset of resistance. In this study, we present a six-year monitoring (2017–2022) conducted in two regions of Northern Italy, Trentino-Alto Adige, in particular the province of Trento, and Friuli-Venezia Giulia, specifically the provinces of Gorizia (GZ), Pordenone (PN), and Udine (UD), where zoxamide was used to control downy mildew.
This study revealed that for the Friuli-Venezia Giulia region, from 2017 to 2020, two samples out of eleven exhibited an EC50 less than 0.5 mg L−1. However, starting in 2021, EC50 values began to exceed 0.5 mg L−1, and in 2022, EC50 values were generally higher than those in previous years, with MICs > 100 mg L−1 for all samples tested.
Data generated with samples coming from Trento showed high sensitivity towards zoxamide from 2017 to 2020, while in 2021 and 2022 15 out of 22 samples showed EC50 > 0.5 mg L−1 and 16 out of 22 samples showed a MIC > 100 mg L−1.
Based on these sensitivity monitoring programs, it is possible to state that P. viticola populations coming from Friuli-Venezia Giulia and Trento territories were sensitive to zoxamide over the years; starting from the 2021 and 2022 seasons, populations exhibited changes of sensitivity to zoxamide.
As we know the emergence of populations with reduced sensitivity to one fungicide could be caused by the repeated use of the molecule with a single-site mode of action, in order to prevent the onset of these issues, careful monitoring and technical assistance are necessary. Performing bioassays is well known to be time-consuming, but to our strongest belief and long-term experience, this method is necessary to closely understand the in vivo behaviour of the fungicide. Certainly, molecular tools to potentially detect mutations conferring fungicide resistance are very insightful in explaining sensitivity changes and should be added to in vivo methods when appropriate, providing an even more comprehensive overview of the phenomenon of fungicide resistance.
References
Beckerman J, Palmer C, Tedford E, Ypema H (2023) Fifty years of fungicide development, deployment, and future use. Phytopathology 113:694–706. https://doi.org/10.1094/PHYTO-10-22-0399-IA
Brent KJ (2012) Historical perspectives of fungicide resistance. In: Tarlochan S (ed) fungicide resistance in crop protection: risk and management. CABI, Wallingford, UK, pp 3–18
Young DH, Slawecki RA (2001) Mode of action of Zoxamide (RH-7281), a new oomycete fungicide. Pestic Biochem Physiol 69:100–111
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nanni, I.M., Oggiano, I., Laricchia, M. et al. Monitoring and tracking changes in sensitivity to zoxamide fungicide in Plasmopara viticola in Italy. J Plant Dis Prot 131, 1211–1216 (2024). https://doi.org/10.1007/s41348-024-00947-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-024-00947-5