Abstract
Cyst producing nematodes are persistent soil-borne organisms causing severe damage to cultivated plants. Persistence of the economically relevant cyst nematode species Globodera pallida, G. rostochiensis and Heterodera schachtii was investigated at different stages during a large-scale industrial composting process to evaluate its efficiency to prevent spread of these nematodes into natural and agricultural habitats. Using reference cyst nematodes incorporated into organic waste from households and the processing industry, the effect of anaerobic fermentation and aerobic composting processes were investigated. Treated cysts were analysed for viability and reproductive potential by performing hatching tests and bioassays on susceptible host plants. The investigated composting plant showed temperatures between 40 and 72 °C at aerobic composting conditions depending on the sample position (bottom, middle, top) within the pile. We found no viable juveniles or reproductive potential of Globodera spp. (Loof and Bakker 1992) and less than 5 per cent reproduction of H. schachtii. Additionally to temperature conditions, we presume that competition of the microbial community and their released biodigestants like fatty acids also play a major role in successful treatment of these severe pest organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Besides rice (Oryza sativa L.), grain and maize (Zea mays L.), the potato (Solanum tuberosum L.) is one of the major crops worldwide (Marks and Brodie 1998). Potato crops are used for direct consumption, supply of starch or animal feed. Sugar beet (Beta vulgaris subsp. vulgaris) is of great importance in the food industry as one of the main sources of sucrose worldwide and could also be used as a high-quality animal food (Anonymus 2022).
The potato cyst nematodes Globodera pallida and G. rostochiensis, as well as the beet cyst nematode Heterodera schachtii, are considered the main damaging species for those crops on a global scale. Potato and sugar beet plants infested by cysts nematodes show primarily unspecific symptoms like stunted plants with yellowing or wilting leaves which finally die off if nematode pressure becomes severely high (Radtke et al. 2000). Infestation by cyst nematodes has been reported to cause yield damage as high as 60% in potato (Brodie and Mai 1989) and up to 25% in sugar beet (Daub 2021).
Potato cyst nematodes have their origin in the Andes of South America and show distribution rates of 14 and 22% of the annual monitoring area in Germany (0.5% of the potato growing production sites, data from annual reports to DG Sante; JKI). Both of the Globodera species are invasive species and therefore under plant quarantine regulation in the European Union (EU Commission 2022). In contrast to the situation of Globodera species, H. schachtii is not considered as quarantine pest in Central Europe, due to its endemic origin, whereas it is non-endemic in some regions such as South America, South Africa and Asia (Gamel et al. 2017) and thus being treated as regulated quarantine pest there.
There is a high risk for the spread of cyst nematodes via soil particles due to the long persistence in the soil for up to 20 years (Been and Schomaker 1996; Grainger 1964; Turner 1996) and the existence of different pathotypes with different levels of virulence (Hockland et al. 2012; Niere et al. 2014). A mayor goal to undermine this risk is to prevent transfer of soil from field to field which is attached to farm machinery used for tillage and harvest. A considerable amount of soil previously attached to tubers or beets accumulates during post-harvest processing of crops. For Belgium, Ruysschaert and colleagues used more than 1000 soil tare data of potato processing factories calculating an amount of 2.2 to 45 tons per hectare of soil loss due to harvesting which is depending on soil texture (Ruysschaert et al. 2007). Tare soils from processing factories compile adhering material from tubers or beets where roots with attached cysts can be expected to occur in very high densities. Consequently, introduction of untreated residual soil from processing of tubers and beets into agroecosystems is considered a main potential route of nematode spread. Hence, transfer of non-treated tare soil from potato or sugar beet processing with unknown pest status of G. pallida or G. rostochiensis back to any agricultural used field site is prohibited following to the European commission’s implementing regulation 2022/1192 EU (European Commission 2022).The ongoing accumulation of tare soils in processing factories put some pressure on the identification of ecological sound as well as technically and economically efficient methods for treatments of tare soils to minimise these risks.
It has been shown that composting is suitable for killing harmful organisms (Bøen et al. 2006; Papajová et al. 2007; Termorshuizen et al. 2005). Composting is a process of biological decomposition of organic biodegradable material under controlled conditions that are predominantly aerobic. Microbial activity leads to the development of temperatures above 60 °C so that the remaining solid particulate material becomes stable and sanitary free of most pathogenic organisms. The elevation of temperature is generated in the first mesophilic or maturation phase starting with temperatures around 20 °C but not exceeding 40 °C. In this phase of aerobic composting of crop residues and organic household waste, the microbial activity is high (Zibilske 1998). Generally, the microbial activity is higher under mesophilic conditions at temperatures around 38–55 °C than at temperatures of 60–70 °C, where the most active groups of the microbiota necessary for the composting process are depleted from the complex microbial community. Above 70 °C, cyst nematodes cannot survive due to reaching their thermal death point (Womersley et al. 1998).
According to the legal standard requirements as stated in the German biowaste regulation (BioAbfV 2013), the treatment of biodegradable material should achieve uninterrupted thermophilic temperature conditions (55 °C for 2 weeks, 60 °C for 6 days or 65 °C for three days) at a sufficient moisture (> 40%) and pH value (≈ 7) and sufficient nutrient contents for high biological activity (as defined in the BioAbfV 2013).
Focusing on treatments of tare soils, this study aims to determine whether the different processes of composting and fermentation could provide effective protection against the spread of viable stages of the cyst nematode species G. pallida, G. rostochiensis and H. schachtii. Three independent analyses were implemented within regular runs of the composting plant Biokompostwerk (BKW) Bützberg close to Hamburg (Germany). Major experimental questions were (i) whether each of the processes (anaerobic fermentation and/or aerobic composting) could inactivate cyst nematodes, (ii) which temperatures were reached during the different process runs (iii) are there differences in process efficiency in order to achieve inactivation of the cyst nematodes between different seasons, and (iv) are there differences between samples rotated/not rotated in their position within the composting pile.
Materials and methods
Fermentation and composting process in an industrial plant
Experiments to test for the potential of aerobic composting processes as well as anaerobic fermentation in order to achieve inactivation of cyst nematodes were conducted at industrial scale in the composting plant Bützberg situated in the German federal state Schleswig Holstein. Operator of this plant is the municipal owned company Stadtreinigung Hamburg.
The fermentation and composting processes at the composting plant in Bützberg comprise several stages after delivery of organic waste (Fig. 1).
In most composting plants, biodegradation of organic material comprises two main processes, anaerobic fermentation and aerobic composting. Images of both process are given in Fig. 1, and a schematic overview of the material workflow during both processing steps is depicted in Fig. 2A. Processing starts with the (i) preliminary separation of impurities (e.g. non-degradable contaminations such as plastic, metallic debris), shredding of the material and sieving through a mesh of 80 mm (Fig. 1B). Then the material is subjected to (ii) anaerobic fermentation (Fig. 1C) and the remaining digestate will be entered into the (iii) aerobic post-treatment (Fig. 1D–F). The discontinuous dry fermentation process will separate the water (percolation) from the treated substrates in so-called rotting tunnels to set up a compost pile (hybrid fermenter, Eggersmann Recycling Technology, Bad Oeynhausen, Germany). By changing the mode of operation and thus the process control, the hybrid fermenter can be used for both aerobic composting and anaerobic fermentation. Usually, the material after fermentation is composed of 75% organic waste, 5% structure-forming green waste, 10% digestate for recycling and 10% processed sieve overflow. To enhance cost efficiency of the composting plant, biogas generated during fermentation is usually used for electric energy production.
Workflow of the processes in the composting plant Bützberg (A) and experimental set-up in parallel to the processes within the composting plant for three independent experiments examined in autumn 2020, winter 2021, and summer 2021 (B). Each experiment contained 12 replicates with four of them in each position (top, middle, bottom) within anaerobic composting pile and two times 12 gauze bags separately containing each cyst nematode species in anaerobic fermentation experiments 3a and 3b
After staying approximately one day in the fermenter the material reaches temperatures of about 40–42 °C under anaerobic conditions for which the remaining air (N2 and O2) is expelled from the gas space (including the pore volume in the substrate pile) by CO2 purging. The process takes about 15 days in total, then fresh air will be supplied to start the aerobic phase for the subsequent composting process, which will lead to further degradation of the material. The whole pile is converted into an empty hybrid fermenter in a weekly manner. Each time new material with coarse structure is used to aerate the material again. Temperature monitoring using temperature probes ensure that legal requirements are fulfilled (as stated in the German law BioAbfV 2007). The process normally takes three weeks with two repositionings, thereafter material is brought to the external warehouse. In the fine processing the compost is sieved through a 10 mm mesh, checked for quality and released for marketing. Coarser material is reused as structural material in the fermenter or used as fuel.
Multiplication of reference cyst material and sample preparation
Multiplication of reference nematode material (representative of populations mostly occurring in German field sites) and sample preparation was conducted at the JKI locations in Braunschweig and Elsdorf (Germany). The potato cyst nematode populations of G. pallida pathotype 2 “Kalle”, G. rostochiensis pathotype 5 “Harmerz”, and the beet cyst nematode H. schachtii pathotype Schach0 “MS” were used as references for testing the treatment’s efficiency in terms of nematode viability and reproductive potential over all phases of the biowaste degradation process. Potato cyst nematodes were propagated on highly susceptible potato cultivar “Desiree” (Agropa GmbH, Brunnen, Germany). Tubers were pre-germinated at room temperature, planted in 9*9.5 cm plastic pots filled with loess with low nutrient content and free from plant parasitic nematodes. The loess was fertilised with 1.5 g of Osmocote long term fertiliser (Scotts Miracle Gro, Marysville; OH, USA) per kilogram loess. Nematodes were introduced into the pots using 1*1 cm nylon gauze bags with 100 μm mesh size, containing fifty nematode cysts, and placed close to the tuber. Cultivation was done in climate chambers with 16 h light at a temperature of 21 °C and 8 h in dark with a temperature around 16 °C. Water was added as required. Every pot was set into a separate coaster with 15 cm diameter to avoid nematode transfer after hatching. After reaching a daily mean temperature sum of 1800 °C, the plants were dried for five days and the sprouts were cut off. Newly formed cysts were separated from the substrate with a 200 μm sieve (Seinhorst 1964), dried and stored at 4 °C for three months to simulate a natural diapause.
Beet cyst nematodes were propagated on highly susceptible winter oilseed rape cultivars in the greenhouse at JKI field station in Elsdorf. The rearing photoperiod for BCN propagation was 12 h (6 am to 6 pm) to meet long-day conditions for the host plants. Heterodera schachtii second stage juveniles (J2) were acquired after submission of cysts for seven days in a 5 mmol ZnCl2 solution to induce hatching. J2 were inoculated in pots with oilseed rape seedlings and cysts were harvested after development of two generations by reaching approximately 830 degree days (sum of daily mean temperatures in pots above 10 °C). For experiments, 80 intact cysts of each cyst nematode species were sealed in gauze bags (100 μm mesh size), and two individual bags used for every treatment point.
Experimental design
Three different experiments were conducted covering several scenarios in the process management of the industrial composting plant Bützberg (Germany). As a standard procedure organic waste was first fed to fermenters (Fig. 2A) for biogas production and then transferred to the composting process (Fig. 2A, iii) in large-scale hybrid fermenters. G. pallida, G. rostochiensis and H. schachtii cyst samples passed through both processes successively. Since material may only be fed to either the biogas fermenter or the large-scale hybrid fermenter, samples were also placed separately in both fermenters in order to individually assess the efficiency to inactivate juveniles inside cysts. The reason for this experiment was that in future the material might just pass through composting solely as part of a short recycling process without preliminary fermentation. Therefore, the digestion of the material in the hybrid fermenter should also result in a product that is safe from a phytosanitary point of view.
The cyst samples were introduced by Plancotec Analytic and Consulting (Neu-Eichenberg, Germany) into the pile of compost material using perforated stainless steel containers filled with the same compost material (Fig. 3A). Containers were incorporated into the pile at different positions (“bottom”, “middle”, “top”, Fig. 3B) and attached to chains allowing easy repositioning in the compost pile. Temperature loggers (Gemini Typ TK-4014, Gemini Data Loggers Ltd., Chichester, West Sussex, United Kingdom)) within each of the steel containers recorded the process temperature.
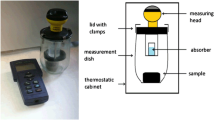
A Perforated stainless steel container and gauze bags containing nematode cysts of Globodera pallida (red paper label, with purple “plus” sign embedded) and G. rostochiensis (grey paper label, with black “minus” sign embedded) as used for reference sample introduction into the composting pile; cysts of H. schachtii were added on site of the composting plant; B schematic drawing of the hybrid fermenter used for composting of organic waste. A total 12 samples were placed on four horizontal positions (arrows above) in each of the three vertical rotting zones top, middle and bottom with two gauze bags each nematode species in each combined position
In detail, experiments were implemented with a duration of 3 to 4 weeks each (Fig. 2B).
In a first experiment examined in autumn 2020, only aerobic composting was analysed (8th to 29th October, Figs. 1 and 2B) with placement of 12 cyst samples at three different positions of the compost pile (Fig. 3A). This was done to elucidate the effect of sample position without turning the pile and elucidate the potential for blind spot development inside the hybrid fermenter. Blind spots with inefficient rotting conditions due to non-homogeneous rearrangement of the pile potentially appear at the edges where microbial mediated temperature increase does not reach the required minimum temperature to inactivate nematodes.
Under regular conditions, the samples were rotated weekly, together with the surrounding material, in an empty hybrid fermenter. Regular conditions were therefore investigated in a second experiment. This experiment was conducted during winter 2021 (18th February to 11th March) with placement of the samples in a way that reproduces the turn mode together with its surrounding material, which is a standard procedure of industrial composting. Figure 3B shows the positions of the samples at the starting point of the composting run. Ideally, the samples and the surrounding substrate remain in the test positions (“top”", “middle”, “bottom”, see Fig. 3B) that are present when introduced into the hybrid fermenter and rotate from front to back in the rotting tunnel when transferred.
In a third experiment, conducted in summer 2021 (8th July to 3rd August), one series of reference samples comprising 4 replicates for each vertical position was incorporated into the whole process of anaerobic fermentation and aerobic composting (experiment 3a), in parallel a second set of 12 replicates was separately subjected to fermentation (experiment 3b). Fermentation was conducted for 15 days between 24th June to 8th July 2021 without further processing of composting (Fig. 2) and the second subset of samples passed through the whole process (fermentation + composting) and was therefore additionally treated for another 27 days in the subsequent composting run of the organic material.
Evaluation of treatment success—nematode viability testing using hatching tests
To measure the viability of the treated cysts, a hatching test according to EPPO Standard PM 7/40(4) (EPPO 2017) was used for potato cyst nematodes. Twenty cysts were randomly chosen out of each gauze bag and 1 mL hatching medium (potato root diffusate, PRD; obtained from the susceptible potato cultivar “Desiree”, stored at 4 °C) was added. Treated samples and non-treated controls from the same multiplication batch were incubated at room temperature in 10-mL glass vials with a snap-lid. The number of hatched juveniles was counted in weekly intervals using a stereo zoom microscope (Wild Heerbrugg, Heerbrug, Switzerland). The hatching test was performed over seven weeks and PRD was changed each week.
For beet cyst nematodes, 20 cysts were randomly isolated form gauze bags and incubated on petri dishes filled with 5mmol ZnCl2 solution for 14 days at room temperature. Juveniles were separated using a 20-μm sieve and counted under a stereo microscope (Leica MZ APO, Wetzlar, Germany) at 50× magnification. Juveniles that did not hatch and inactive eggs were separated from remaining cysts using a cyst homogeniser and released juveniles and eggs were counted.
Bioassays for testing nematode reproduction
To determine whether there were differences for the number of hatched juveniles and the production of female nematodes in the population, bioassays were conducted from end of March to the beginning of July 2021 and from the mid of February to the end of May 2022. For bioassays of potato cyst nematodes the procedure described by Kort et al. (1977) using nematode cysts instead of hatched juveniles was applied. Therefore, the reproduction ratio of new cysts produced (Pf) to the number of cysts added initially (Pi) was calculated. Whole cysts were used from the replicates of each position in the three composting runs and compared to untreated controls from the same series of reference cyst multiplication (2018/2019). In the case of Globodera spp., the bioassays were conducted under the same parameters as described for the production of reference nematode cysts stated above. Newly developed cysts were counted with a stereo zoom microscope (M8, Wild Heerbrugg, Germany) at ten times magnification.
In case of Heterodera, gauze bags containing either fifty treated or untreated (control) cysts were placed in a 400-mL pot and filled with 350 g of loess enriched with fertiliser (3 g kg−1 Osmocote Exact 3-4 M). Four seeds of a susceptible oilseed radish (cv. Siletina) were seeded per pot and thinned to two remaining plants after seedling emergence. All pots were drenched with 30 mL of the fungicide Previcur (1.5 mL L−1, 530 g L−1 propamocarb, 310 g L−1 fosetyl) per pot to prevent seed born damping off disease. All variations of combined vertical and horizontal positions plus untreated controls were set up as complete random blocks. Greenhouse temperature was set to 20 °C/16 °C (day/night temperature) for 12 h each in additional light (2700 K). Data logger continuously recorded soil temperature, and test was terminated after 390 degree days (sum of daily mean temperatures in pots above 10 °C) allowing reproduction of one H. schachtii generation. This was monitored by specific controls with untreated cysts that achieved reproduction rates using the ratio of new cysts produced to the number of cysts added initially at Pf/Pi > 2. After removing original gauze bags used for inoculation, newly developed cysts were extracted from loess in individual pots using a sieve combination (1000/100 μm) and a subsequent centrifugal flotation method (Hallmann et al. 2021). Cysts were counted and contents (juveniles and eggs) were released using a cyst homogeniser. Juveniles and eggs were quantified per 100 g of soil for each sample using a stereo microscope at 50× magnification. Reproduction rate was calculated from Pf to Pi relation, where Pi was the total number of nematodes in 50 cysts for inoculation.
Statistics
Data of the hatching tests and bioassays were analysed using nonparametric Kruskal–Wallis tests with pairwise Dunnett’s post hoc test and subsequent type I error Bonferroni correction of post hoc test results (Sokal and Rohlf 2012) as implemented in R-package MultBiplotR (Vicente-Villardon 2021) running under R version R-4.03 (R Core Team 2021). Normal distribution of daily temperatures were confirmed by using R-function “hist” and Shapiro-Wilks test and therefore analysed by ANOVA (analysis of variance) with Tukey’s post hoc test for pairwise comparisons of composting run season and treatment position in the pile using R. Two-sided Student’s t-tests, as implemented under R-basic package stats, were used to explore the differences in hatching test and bioassay for non-treated controls between the composting runs conducted in autumn 2020 (experiment 1) and summer 2021 (experiment 3).
Results
Observed composting process parameters
We observed significant differences in maximal temperatures reached for at least five days in aerobic composting conducted in winter 2021 (experiment 2) compared to the remaining runs in autumn 2020 (experiment 1) and summer 2021 (experiment 3; Fig. 4). This was due to the fact that the oxygen-rich air was additionally temperated with exhausted air from a warehouse, whereby without additional heating of the air, seasonal fluctuations ultimately also occur. Temperatures at the three different positions varied significantly, generally being highest at the top and lowest at the bottom (Fig. 4).
Box and whisker plots of daily temperatures measured in four replicates for each sample position, bottom (B), middle (M), top (T). A Temperature in all three composting runs for each sample position; B temperature at all positions for the three different seasons, and C combination of position and season. Box and whisker plots show the median of temperatures (black bold horizontal line), mean (red bold horizontal line), and 25th and 75th percentiles (bottom and top of the box), while whiskers (vertical dashed lines) represent approximately the twofold standard deviation. Circles indicate potential outliers. Results of analyses of variance (ANOVA) are shown for each of the analysed comparisons and differences between the sub-groups calculated by Tukey’s post hoc tests are given in different lowercase letters above each box and whisker plot
In experiment 1, the temperature profile was clearly distinctive between the vertical levels with maximum temperature at top position of higher 65 °C for 6 days and maximum temperature at basis level 40–42 °C for 6 days. The daily temperature amplitude between lowest temperature measured at the bottom position and highest at the top position varied between 19.3 °C and 68.1 °C. The clear temperature stratification between the layers was not given in the other composting runs. In winter, the highest temperature was measured at the bottom position with 72.4 °C, followed by the Top position with 69 °C and 64.1 °C at the middle position. The daily temperature amplitude varied between 32.2 °C measured at the top layer and 72.4 °C at the bottom layer. The lowest maximum temperatures of the examined composting runs were reached in the summer of 2021. The maximum temperature measured in the top layer was 62 °C, followed by the middle layer at 57.2 °C and the bottom layer at just 43.8 °C. The minimum temperature was measured at 22.9 °C in both the bottom and middle layers. Over all composting runs, the discrepancy for daily temperatures at the horizontal level in the rotting tunnel was very low (Fig. 5).
Nematode cyst inactivation in aerobic composting as evaluated in hatching tests
Hatching tests for three independent runs of composting showed that no hatching of juveniles was found for both Globodera species nor the beet cyst nematode H. schachtii. This was observed for composting runs conducted without proceeding anaerobic fermentation.
Since there could be a potential risk that blind spots of untreated material can occur within the compost pile, resulting from non-homogeneous turning, it was examined in one experiment in October 2021, if turning the material enhances the efficiency of the composting process. Material was rotated according to the routine procedure, but samples were placed by hand at the same position where they were placed in the beginning. No influence of turning the material was detected and efficiency of cyst inactivation was 100% (Fig. 6C).
Number of hatched juveniles in the second stage of nematode development shown as the number of hatched juveniles per cyst (hatching tests) and reproduction of adult female nematode cysts (bioassays) conducted on host plants of Globodera spp. and Heterodera schachtii. Results show composting runs at the different positions of the hybrid fermenter (n = 4 for each of the positions) under aerobic conditions in A autumn 2020 (experiment 1), B winter 2021 (experiment 2), and C summer 2021 (experiment 3b). Standard errors are shown in whiskers. Statistical differences were calculated using Kruskal–Wallis tests for the hatching tests and bioassays and differences between the sub-groups were calculated by Dunnett’s post hoc test and are presented using different lower case letters above each bar graph
In order to better understand morphological changes in cysts, eggs and juveniles affected by the treatment in relation to the non-treated control we used dissection (up to 100× magnification) and light microscopy (at 400× magnification, see Fig. 7). It was observed that the colour of cuticle in the treated cysts had changed from light brown to extremely dark brown to black compared to the control cysts. The content of the cysts was mainly destroyed and most of the unhatched eggs were found to contain a brownish mass (Fig. 7D, middle). After cutting of the cyst and removal of the eggshell by slight pressure, juveniles showed characteristics of mortality, like vacuole formation in their abdominal intestine and body deformations. Over time, the content of eggs darkened and the collapse of J2 could be observed.
A Untreated Globodera pallida cysts, eggs and juveniles. B–D Treated G. pallida cysts including four reference cysts, distinguishable by their light brown colour, for comparison B eggs and hatched juveniles treated by anaerobic fermentation. C G. pallida cysts, eggs and juveniles after aerobic composting in the hybrid fermenter D G. pallida cysts, eggs and juveniles after anaerobic fermentation and subsequent composting. Scale bars shown under D measuring 1 mm (left) under 20× magnification and 100 μm under 400× magnification (middle and right side)
Hatching of juveniles per cyst in controls of H. schachtii remained relatively constant during all seasons (t = 0.088518, df = 4.1572, P = 0.9336). Whereas hatching of juveniles of G. pallida decreased from 224 juveniles per cyst in the autumn composting run (experiment 1) to less than 17 hatched J2 in experiment 3 (summer 2021; t = 18.9, df = 2.5081, P < 0.001) in relation to approximately 500 eggs present per cyst (Fig. 6). This effect could also be observed for G. rostochiensis with a lower rate of decrease ranging from 170 in autumn 2020 to 57 in summer 2021 (t = 12.692, df = 2.3528, P = 0.0032).
Nematode cyst inactivation as evaluated in bioassays
In concordance to hatching tests, the bioassays showed no reproductive activity for the potato cyst nematode species at all positions in the composting pile and in all three composting runs (Fig. 6). Nevertheless, some reproduction (4%) for the treated cysts of H. schachtii could be observed, with resulting Pf/Pi between 1.00 and 1.04 for samples from the middle position during the winter run. Compared to the control samples of H. schachtii, which showed multiplication rates of 17 and higher, the multiplication observed in treated samples was negelectible. Since H. schachtii is a non-regulated, wide spread nematode and well-established control strategies are available which are very useful to keep population densities below the threshold level, a minimum survival in treated soil seems to be manageable and thus acceptable. Non-treated controls of H. schachtii showed no differences in reproduction between the experiment conducted in autumn 2020 (experiment 1) and experiment 3 (summer 2021) with a mean reproduction factor (Pf/Pi) of 20.5 in experiment 1 and 16.4 observed in experiment 3 (t = 0.8282, df = 15.825, P = 0.42). G. rostochiensis showed a weak decrease in reproduction from 79.1 in experiment to 75.5 observed in experiment 3 (t = 0.5511, df = 5.692, P = 0.603). Comparable to the hatching behaviour, G. pallida showed in non-treated controls a remarkable decreased reproduction factor from 51.8 to 25.8 (Fig. 6, t = −4.9131, df = 5.1084, P < 0.01).
Cyst inactivation in anaerobic fermentation and combination of fermentation and aerobic composting
The processes of anaerobic fermentation, aerobic degradation of the organic material by composting and the complete process of fermentation and composting as routinely conducted at the composting plant Bützberg were analysed separately for their efficacy regarding inactivation of cyst nematodes.
In one out of three experiments conducted in summer 2021 (Experiment 3b), anaerobic fermentation with a reduced residence time of the material of 14 to 16 days in comparison 25 days in “conventional” biogas plants as well as additional fresh air exchange by means of CO2 flushing was included in our analysis. Anaerobic fermentation was the second step of the whole recycling process after sorting. A subset of twelve samples passed solely through fermentation while the remaining twelve samples passed through the whole process of fermentation and subsequent composting. The temperature in the fermenter was observed to be constantly between 40 and 42 °C during the whole treatment. The results of the hatching tests of juveniles showed that using only fermentation is sufficient for successful inactivation of cyst nematodes (Fig. 8A). The same result was observed for these samples passing through both of the processes (Fig. 8B).
A Hatching of Globodera spp. juveniles under treatment with potato root diffusates (PRD) during a period of 7 weeks from cysts exposed only to the fermenter (n = 12 independent replicates) for 15 days, and B from cysts passing through the complete process of fermentation and composting (n = 12 replicates, that means 4 replicates for each position top, middle, bottom). Twenty cysts (< 500 μm) per recovered gauze bag and position in the hybrid fermenter (B = bottom, M = middle, T = top) were tested for hatching. Technical replicates of the control (20 intact cysts, < 500 μm) were also set up (n = 3), and standard error is shown as whisker
Discussion
A recent risk-based assessment presented by the Norwegian Scientific Committee for Food and Environment (Alsanius et al. 2021) globally analysed the reliability of pathogen treatments in biogas and composting plants in order to avoid spread of pathogenic organisms into natural and agricultural habitats. This study completely depended on literature data and concluded that potato cyst nematodes are expected to completely withstand both aerobic mesophilic fermentation and anaerobic mesophilic digestion as well as vermicompost processes and basket composting. In conclusion, infectivity and reproduction remain to be investigated especially with regard to composting in actively and passively aerated piles. Therefore, survival rates will depend mainly on the temperature reached and duration of high-temperature conditions. The risk analysis estimated a treatment with temperature within the pile of 55 °C for four weeks not to be sufficient for eradication of nematodes from genera Globodera sp. and Meloidogyne sp..
In contrast, the results in our study showed that both, the composting process in the hybrid fermenter and the anaerobic fermentation process are highly efficient methods to inactivate cyst nematodes and thus avoid spread of these pests. We evaluated viability and infectiveness for reference samples of the cyst nematodes G. pallida, G. rostochiensis and H. schachtii introduced into mesophilic to thermophilic aerobic composting and mesophilic fermentation processes at the composting plant Bützberg. Mesophilic fermentation with temperatures below 50 °C was analysed independently from aerobic composting in summer 2021. Temperatures around 40–42 °C were achieved during this process and were found to be sufficient to successfully inactivate eggs inside the cysts by 100%. This confirms the results of Spaull et al. (1989) who observed a complete loss of viability of the eggs from G. rostochiensis and G. pallida in laboratory experiments during anaerobic fermentation within 30 min at 35 °C using sewage sludge or 9 weeks without temperature elevation (cold anaerobic digestion). In numerous other recent studies, this effect could be attributed to the production of nematode suppressive by-products during microbial fermentation processes (Eberlein et al. 2022; Jothi et al. 2003) rather than an elevation of the temperature. Nevertheless, the identification of secondary metabolites with nematicide effects and their practical or commercial use remains difficult due to the high variability of the organic source material and the variation of operating parameters during fermentation (Oldani et al. 2022, oral presentation). Besides O2-depletion, a number of volatiles e.g. volatile fatty acids (Davis et al. 1997), other organic acids and to some extent elevation of CO2 and NH3 seem to have a relevant impact on nematode inactivation while others (e.g. CH4 and H2S) could not be correlated with such effects (Runia et al. 2014). In sum, the anaerobic conditions, CO2 elevation and microbial secondary metabolites (digestates) with potential nematicidal effects provided a proper control of cyst nematodes within a rather short period of 10 to 15 days in our analysed fermentation run.
The temperatures in the composting process during our study reached between 40 and 70 °C, and therefore, minimum temperatures for effective treatment were lower in some areas of the composting pile than previously recommended. The test design used in our analyses allowed for a differentiation of the temperature effects in the different zones of the pile (top, middle, bottom), with the lowest mean temperature being observed in the bottom area. The highest temperature was found in the top zone and not as expected in the core. These findings differed between seasons. During autumn and summer, the differences between the rotting zones were significant while the mean process temperature was most constant during the run conducted in the winter 2021 with no significant differences between the rotting zones (Fig. 4). Daily measured temperatures in the autumn composting run showed the highest differences in their amplitude within each of the vertical levels accompanied by a clear vertical stratification of the layers in the composting pile (Fig. 5). This shows the huge variability in process regulation and the importance of repeated analyses for phytosanitary control of the processes in such plants.
In the bottom rotting zone, temperatures did not exceed 42 °C in the composting runs conducted in autumn 2020 and summer 2021 which should, according to previous literature (Alsanius et al. 2021; van Loenen et al. 2003; Wallace 1963), not be sufficient to inactivate cyst nematodes without turning of the material. In the literature, a number of partially conflicting studies can be found which were mostly conducted on cysts of G. rostochiensis. Wallace observed that cysts of G. rostochiensis survive less than one hour when submerged in water warmer than 47 °C, whereas van Loenen showed that less than 3 min in 60 °C aerated steam are not sufficient to inactivate cysts of this species safely (van Loenen et al. 2003; Wallace 1963). Furthermore, there is a difference between moist cysts and dry cysts. While pre-soaked cysts contained no viable eggs and juveniles after 30 min at 58–59 °C (Evans 1991) dried cysts required temperatures of 90 °C for more than 30 min or 65–70 °C for up to 24 h for inactivation (Lindhardt 1959; Stone and Wesley 1975). Reports on the effect of temperature on cyst nematodes within the composting process are scarce and inconsistent. The Soil Association Standard for Organic Farming and Production (Soil Association, 2002) emphasises the beneficial effects of composting to reduce pathogen loads and to produce a product that is stable in pH value and not further degradable or showing any phytotoxic effects. The authors of this standard recommend a temperature higher than 55 °C for at least three days, achieved by turning the material and supplying forced oxygen enriched air to destroy most weed seeds, pathogens, chemical residues and antibiotics. Another study (Gale 2002) suggests a minimum of 60 °C for two days where composting piles should be turned at least three times within 14 days during the composting process. Christensen et al. (2002) reported that composting requires 70 °C for two days or 65 °C for four days with at least five turns of the pile, and Nishinome and colleagues (1997) showed composting at 40 °C for ten days or at 50 °C for five days is sufficient for the inactivation of cyst nematodes.
If ventilation of oxygen-rich air is not optimal, unfavourable fermentation residue conditions or too little structure, anaerobic stains can occur in the compost heap. However, this would be noticeable through low temperatures. Therefore, these initial factors play an important role in the process but it is largely ruled out by the weekly moving and ventilation. Turning the pile enables oxygen flow to avoid anaerobic gaps accompanied by temperatures lower than 45 °C of up to 20% of the composting mass (Noble and Roberts 2004). We analysed the effect of turning versus not turning of the pile in autumn 2020 and did not see any differences in terms of cyst nematode inactivation between the runs.
In case of the composting process, some important process parameters recommended in the literature regarding temperature and retention time of the organic material in the hybrid digester were not fully achieved, but the treatment of the cyst nematodes was nevertheless successful. Fermentation and composting in an aerobic hybrid fermenter are parts of the same process chain as conducted in the composting plant Bützberg. This means that the resulting material from anaerobic fermentation will contain numerous nematicidal organic compounds as described above and will subsequently be used for aerobic composting to produce a fine-grained finished compost at the end. The hypothetical presence of soluble nematicidal compounds in the starting material for composting could explain the successful inactivation of cyst nematodes by daily temperatures as low as 40 °C. Therefore, effects of anaerobic and aerobic processes cannot be disentangled.
The different nematode species showed slight differences in their ability to endure temperature. It is known from the literature that G. rostochiensis is more heat-resistant than G. pallida (Stone and Wesley 1975). H. schachtii has been reported to be able to persist in the first maturation phase for up to six weeks at temperatures lower than 55 °C. Only in the thermophilic sanitisation phase at higher temperatures they die (Noble and Roberts 2004). Although there remained a few hatched juveniles from H. schachtii not able to start population growth in absence of the sugar beet host, the composting processes tested here were safe in relation to the deletion of the tested reference cyst nematode species. H. schachtii is not part of quarantine regulation in Europe. Nevertheless, the hatch of a non-quarantine cyst nematode species after fermentation and composting indicated inconsistencies in the treatment process of the composting plant and should therefore kept into consideration for further improvement of technical process management.
Hatching tests and bioassays are routine testing procedures to investigate viability and infectivity of nematodes (for Globodera sp. concluded in the standard PM 7/40(4) EPPO 2017 and for H. schachtii see Whitney 1970). The number of hatched juveniles in non-treated controls was observed to decrease for both of the potato cyst nematode species but not for H. schachtii. This effect could be observed in a weakened form for reproduction of G. rostochiensis and G. pallida in bioassays ongoing in time when the experimental runs were conducted (Fig. 6). Therefore, a number of endogenous factors could affect hatching and reproductive success. Reduced hatching activity could result from differences in the response of nematodes to individual hatching factors (HF) as α-solanine and α-chaconine as present in potato root diffusates due to an evolutionary divergence for eggshell receptors even in the closely related species such as G. pallida and G. rostochiensis. It was shown that G. rostochiensis is more sensitive to low concentrations of HF than G. pallida (concluded in Jones et al. 1998). In our study, potato root diffusates were collected in August 2020, aliquoted in plastic vials, and stored at − 20 °C until use in October 2020, March 2021 and August 2021. Therefore, changes in hatching behaviour of G. pallida might be due to a decomposition and decrease in HF concentration in PRD during storage. However, the differences between control and treated cysts are statistically significant in all cases and provided proof of successful treatment of compost during composting and anaerobic fermentation in the composting plant Bützberg.
Based on the results generated in the present study, we can assume that maximum inhibitory effects can be achieved at low temperatures of < 50 °C and that other factors, not analysed in our study as microbial activity could play a key role. Based on the literature, this would also be true for seeds of weeds and harmful organisms. We conclude that at this temperature level the risk of compost matter for the spread of PCN and BCN into environment is negligible from a phytosanitary point of view. In consequence, numerous factors as organic material composition in interaction with process control both responsible for microbial community and subsequently bio digestants composition have to be considered for a safe composting process. Our study showed that a safe product in regards to G. pallida, G. rostochiensis and H. schachtii could be produced with our test conditions in the composting plant in Bützberg. Further investigations are needed to understand which specific conditions should be used for sterilising processes during composting of biodegradable material.
References
Alsanius B, Magnusson C, Nicolaisen M, Wright SAI, Wendell M, Krokene P, Stenberg J, Thomsen IM, Rafoss T (2021) Assessment of treatment methods and validation criteria for composting and biogas facilities in relation to plant health risks and the risk of spreading alien organisms: Scientific Opinion of the Panel on Plant Health of the Norwegian Scientific Committee for Food and Environment. VKM Report. Norwegian Scientific Committee for Food and Environment (VKM), Oslo
Anonymus (2002) Soil association standards for organic farming and production. Soil Association, Bristol
Anonymus (2022) Crop information sugarbeet. https://www.fao.org/land-water/databases-and-software/crop-information/sugarbeet
Anonymus (2013) Bioabfallverordnung - Verordnung über die Verwertung von Bioabfällen auf landwirtschaftlich, forstwirtschaftlich und gärtnerisch genutzten Böden: BioAbfV
Been TH, Schomaker CH (1996) A new sampling method for the detection of low population densities of potato cyst nematodes (Globodera pallida and G. rostochiensis). Crop Prot 15:375–382. https://doi.org/10.1016/0261-2194(96)89822-X
Bøen A, Hammeraas B, Magnusson C, Aasen R (2006) Fate of the potato cyst nematode Globodera rostochiensis during composting. Compost Sci Util 14:142–146. https://doi.org/10.1080/1065657X.2006.10702275
Brodie BB, Mai WF (1989) Control of the golden nematode in the United States. Annu Rev Phytopathol 27:443–461
Christensen KK, Carlsbaek M, Kron E (2002) Strategies for evaluating the sanitary quality of composting. J Appl Microbiol 92:1143–1158. https://doi.org/10.1046/j.1365-2672.2002.01648.x
Daub M (2021) The beet cyst nematode (Heterodera schachtii): an ancient threat to sugar beet crops in Central Europe has become an invisible actor. In: Sikora RA, Desaeger J, Molendijk L (eds) Integrated nematode management: state-of-the-art and visions for the future. CABI, Wallingford, pp 394–399
Davis EL, Meyers DM, Dullum CJ, Feitelson JS (1997) Nematicidal activity of fatty acid esters on soybean cyst and root-knot nematodes. J Nematol 29:677–684
Eberlein C, Edalati A, Zhang R, Westphal A (2022) Effects of substrate and processing conditions on nematode suppressiveness of anaerobic biogas digestates. PhytoFrontiers, The American Phytopathological Society
EPPO (2017) PM 7/40 (4) Globodera rostochiensis and Globodera pallida. EPPO Bull 47:174–197. https://doi.org/10.1111/epp.12391
EU Commission (2022) Commission Implementing Regulation (EU) 2022/1192 of 11 July 2022 establishing measures to eradicate and prevent the spread of Globodera pallida (Stone) Behrens and Globodera rostochiensis (Wollenweber) Behrens: (EU) 2022/1192
Evans K (1991) Lethal temperatures for eggs of Globodera rostochiensis, determined by staining with new blue r. Nematologica 37:225–229. https://doi.org/10.1163/187529291X00204
Gamel S, Letort A, Fouville D, Folcher L, Grenier E (2017) Development and validation of real-time PCR assays based on novel molecular markers for the simultaneous detection and identification of Globodera pallida, G. Rostochiensis and Heterodera Schachtii. Nematology 19:789–804. https://doi.org/10.1163/15685411-00003086
Grainger J (1964) Factors affecting the control of eelworm diseases. Nematologica 10:5–20
Hallmann J, Daub M, Wesemael W (2021) Estimating numbers. In: Perry RN, Hunt DJ, Subbotin SA (eds) Techniques for work with plant and soil nematodes. CABI, Wallingford, pp 42–59
Hockland S, Niere B, Grenier E, Blok V, Phillips M, Den Nijs L, Anthoine G, Pickup J, Viaene N (2012) An evaluation of the implications of virulence in non-European populations of Globodera pallida and G. rostochiensis for potato cultivation in Europe. Nematology 14:1–13. https://doi.org/10.1163/138855411X587112
Jones PW, Tylka GL, Perry RN (1998) Hatching. In: Perry RN, Wright DJ (eds) The physiology and biochemistry of free-living and plant-parasitic nematodes. CABI Publishing, Wallingford, pp 181–211
Jothi G, Pugalendhi S, Poornima K, Rajendran G (2003) Management of root-knot nematode in tomato Lycopersicon esculentum, Mill., with biogas slurry. Biores Technol 89:169–170. https://doi.org/10.1016/S0960-8524(03)00047-6
Kort J, Ross H, Rumpenhorst HJ, Stone AR (1977) An international scheme for identifying and classifying pathotypes of potato cyst nematodes Globodera rostochiensis and G. pallida. Nematologica 23:333–339
Lindhardt K (1959) Kartoffelål – en samlet oversigt. Statens Plantetilsyn, København
Loof PAA, Bakker J (1992) Authorities of specific names in, and transfer to, the nominal genus Globodera Skarbilovich, 1959. Nematologica 38:385–391
Marks RJ, Brodie BB (ed) (1998) Potato cyst nematodes: biology, distribution and control. CAB Internat, Wallingford
Niere B, Krüssel S, Osmers K (2014) Auftreten einer außergewöhnlich virulenten Population der Kartoffelzystennematoden. J Kult 66:426–427
Noble R, Roberts SJ (2004) Eradication of plant pathogens and nematodes during composting: a review. Plant Pathol 53:548–568. https://doi.org/10.1111/j.0032-0862.2004.01059.x
Oldani E, Cabianca A, Dahlin P, Ruthes AC (2022) Biogas digestate as potential source for nematicides. DPG Nematology Meeting, Braunschweig
Papajová I, Sasanelli N, D’Addabbo T, Renčo M (2007) The effect of five composts of different origin on the survival and reproduction of Globodera rostochiensis. Nematology 9:537–543. https://doi.org/10.1163/156854107781487260
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
Radtke W, Rieckmann W, Brendler F (2000) Kartoffel: Krankheiten - Schädlinge - Unkräuter. Mann, Gelsenkirchen
Runia WT, Thoden TC, Molendijk L, van den Berg W, Termorshuizen AJ, Streminska MA, van der Wurff A, Feil H, Meints H (2014) Unravelling the mechanism of pathogen inactivation during anaerobic soil disinfestation. Acta Hortic 1044:177–193. https://doi.org/10.17660/ActaHortic.2014.1044.21
Ruysschaert G, Poesen J, Auerswald K et al (2007) Soil losses due to potato harvesting at the regional scale in Belgium. Soil Use Manag 23:156–161. https://doi.org/10.1111/j.1475-2743.2006.00077.x
Seinhorst JW (1964) Methods for the extraction of Heterodera cysts from not previously dried soil samples. Nematologica 10:97–94
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research, 4th edn. W. H. Freeman, New York
Spaull AM, McCormack DM, Pike EB (1989) Effects of various sewage sludge treatment processes on the survival of potato cyst-nematodes (Globodera spp.) and the implications for disposal. Water Sci Technol 21:909–916. https://doi.org/10.2166/wst.1989.0293
Stone LEW, Wesley DP (1975) The effect of heat on the hatch of potato cyst eelworms. Plant Pathol 24:74–76. https://doi.org/10.1111/j.1365-3059.1975.tb01866.x
Termorshuizen AJ, van Rijn E, Blok WJ (2005) Phytosanitary risk assessment of composts. Compost Sci Util 13:108–115. https://doi.org/10.1080/1065657X.2005.10702226
Turner SJ (1996) Population decline of potato cyst nematodes (Globodera rostochiensis, G. pallida) in field soils in Northern Ireland. Ann Appl Biol 129:315–322. https://doi.org/10.1111/j.1744-7348.1996.tb05754.x
van Loenen MC, Turbett Y, Mullins CE, Feilden NE, Wilson MJ, Leifert C, Seel WE (2003) Low temperature–short duration steaming of soil kills soil-borne pathogens, nematode pests and weeds. Eur J Plant Pathol 109:993–1002. https://doi.org/10.1023/B:EJPP.0000003830.49949.34
Vicente-Villardon JL (2021) MultBiplotR: multivariate analysis using biplots in R. https://CRAN.R-project.org/package=MultBiplotR
Wallace HR (1963) The biology of plant parasitic nematodes. Edward Arnold Publishers Ltd., London
Whitney ED (1970) Large scale hatching, disinfestation, and storage of Heterodera schachtii larvae. Phytopathology 60:1191–1194. https://doi.org/10.1094/Phyto-60-1191
Womersley CZ, Wharton DA, Lynne MH (1998) Survival Biology. In: Perry RN, Wright DJ (eds) The physiology and biochemistry of free-living and plant-parasitic nematodes. CABI Publishing, Wallingford, pp 271–298
Zibilske LM (1998) Composting of organic wastes. In: Sylvia DM (ed) Principles and applications of soil microbiology. Prentice Hall, Upper Saddle River, pp 482–497
Acknowledgements
The authors of this study are very thankful to Lucas Dülfer (Plancotec), Young-Sung Kim, and the Employees of the VKW-Bützberg for conducting the experimental tests on site at the composting plant. We thank Katja Reimann, Christine-Maria Gottwald, Marlies Schmitz, Edita Mösges and Claudia Aukamp-Timmreck for conducting reference nematode reproduction, sample preparation, hatching tests and bioassays at JKI locations Braunschweig and Elsdorf. The authors are very thankful to Kathleen Gärtner for English language editing and revision of the manuscript. The authors would like to thank two anonymous reviewers for their valuable advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the Federal Programme for Ecological Farming and Other Forms of Sustainable Agriculture. The research grant number (FKZ) was 2815NA120.
Author information
Authors and Affiliations
Contributions
All authors agree with the content of this publication and consent to submit this article to the journal. The authors declare that they obtained content from the responsible authorities of the Julius Kühn Institute for the article. The authors declare that they have no conflict of interest with the data published here. All authors whose names appear on this submission made substantial contributions to the conception or design of the work or interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no conflict of interest.
Ethical standard
The authors declare that (i) the manuscripts has not been submitted to another journal simultaneously, (ii) the submitted work contains original data not published earlier, (iii) the data published are not split up into several parts to increase the personal number of publications, (iv) the current publication is not a secondary publication with the same content, (v) the presented data were not at any manipulated, and (vi) no data, text or theories by others not explicitly cited for their origin are presented in the current publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schumann, L., Berger, B., Daub, M. et al. Industrial-scale composting process as a successful method for inactivation of potato cyst nematodes (Globodera spp. Skarbilovich) and sugar beet cyst nematode (Heterodera schachtii Schmidt). J Plant Dis Prot 130, 1317–1330 (2023). https://doi.org/10.1007/s41348-023-00801-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00801-0