Abstract
An undersowing system with additional intercropping of flowering plants was assessed in field trials in Germany and Japan to estimate regulating effects on pests and possible negative effects on white cabbage (Brassica oleracea var. capitata). In particular, we tested cabbage undersown with wheat (Triticum aestivum L.) and cabbage undersown with wheat plus additional sweet alyssum (Lobularia maritima L. Desv.) intercropping. Counts of the aphid species Brevicoryne brassicae (L.) and Myzus persicae (Sulzer), as well as related predators on cabbage plants, were determined. Abundance of Phyllotreta spp. flea beetles and their feeding damage on cabbage plants were recorded and cabbage yield was compared. In both countries, trials showed that wheat undersowing reduced the abundance of M. persicae but not B. brassicae. The occurrence of natural enemies on cabbage plants was not significantly affected by any of the companion plants. Additional sweet alyssum intercropping increased the abundance of adult hoverflies at the German but not at the Japanese location. However, it also significantly increased flea beetle infestation on cabbage plants at both locations. Neither wheat undersowing nor additional sweet alyssum intercropping significantly reduced cabbage harvest weight.
In conclusion, adding companion plants can be a promising method to improve pest control in vegetable crops. However, intercropping crucifer crops with sweet alyssum may not be recommended in regions where flea beetles are a relevant pest because of the observed enhancing effect on them. In contrast, to prove the positive effect of wheat undersowing on white cabbage, results from further years of investigation are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Companion plants are an important tool in integrated pest management in horticultural crop production (Begg et al. 2017; Parolin et al. 2012; Wezel et al. 2014). Adding companion plants to a cropping system may influence the abundance of pest arthropods by physically or chemically interfering with insect migration (Patt et al. 1997; Perrin and Phillips 1978; Pfiffner et al. 2009; Wäschke et al. 2013), changing host plant supply or quality (Root 1973; Seress et al. 2000) or increasing abundance of natural enemies (Letourneau 1987; Sheehan 1986). Currently, there is an urgent demand to reduce the use of synthetic insecticides worldwide due to political and social pressure and methods of biological control are gaining particular importance (Hulot and Hiller 2021). Consequently, more and more studies are evaluating the role of specific companion plant systems in horticultural crops as an alternative pest control method (Chen et al. 2020; Gómez-Marco et al. 2016; Gulidov and Poehling 2013; Meyling et al. 2013; Ponti et al. 2007; Sekine et al. 2021; Sun and Song 2019).
Companion plants are also an interesting tool for the sustainable production of white cabbage (Brassica oleracea var. capitata). White cabbage plants are affected by a number of different herbivorous pests that can lead to severe losses in yield and quality of the crop (Andow et al. 1986; Stoleru et al. 2012). Several studies have been conducted on companion plant systems to regulate pest arthropods in white cabbage production, most of them focusing on undersowing with Trifolium spp. (Andow et al. 1986; Costello and Altieri 1995; Lehmhus 2001; Meyling et al. 2013; Pfiffner et al. 2009; Sekine et al. 2021; Theunissen et al. 1995). Besides positive effects regarding pest control, a negative effect on yield was often reported (e.g., Lehmhus 2001).
Farmers will be more likely to integrate companion plants as biological control measures if they suppress herbivorous arthropods without negatively affecting yield or production processes (Barratt et al. 2018; Rezaei et al. 2020). But not every companion plant system leads to the desired effect from a grower’s perspective and potential plants have to be carefully selected to fit specific cropping systems and regional requirements (Brewer and Goodell 2012). Consequently, it is important to assess undesirable effects of companion plants such as increased incidence of pests or adverse effects on growth and development of the main crop because of resource competition.
In this study, we focused on two companion plants that have not been previously explored for their compatibility with white cabbage: wheat (Triticum aestivum L.) and sweet alyssum (Lobularia maritima L. Desv.). Wheat is already used in “banker plant” systems in greenhouse crops. There, it acts as a host plant for aphid and thrips species that serve as alternative hosts or prey for relevant natural enemies but rarely infest horticultural crops (Frank 2010; Jandricic et al. 2014; Nagasaka et al. 2010; Sun and Song 2019). In addition, wheat is cheap and seeds are easily available for most farmers. Regarding the intercropping of cabbage with other cereal plants, Masuda and Miyata (2008) and Sekine et al. (2021) reported an aphid suppressing effect of barley intercropping in white cabbage. In this study, we tested cabbage undersown with wheat drilled as a single strip between cabbage rows, because similar strip undersowing with clover was shown to be the least competitive to cabbage plants in a previous study by Lehmhus (2001). The second companion plant, sweet alyssum, is widely reported to increase adult hoverfly abundance and decrease aphid numbers in vegetable crops (Brennan 2013; Chaney 1998; Gontijo et al. 2015). The integration of such flowering plants into cabbage fields for pest regulation can provide pollen and nectar sources for natural enemies, especially hoverflies and parasitic wasps (Chaney 1998; Kopta et al. 2012; Pfiffner et al. 2009; Ponti et al. 2007). The long flowering period of sweet alyssum can provide these nutrients for adult hoverflies during the whole cultivation period of white cabbage (Brennan 2016; Picó and Retana 2003). Also, the white colour of sweet alyssum flowers is attractive for hoverflies and the morphological structure of flowers allows easy access (Ribeiro and Gontijo 2017). Tiwari et al. (2020) and Ribeiro and Gontijo (2017) further reported an aphid regulating effect of sweet alyssum interplants in crucifer crops. Although sweet alyssum is also a cruciferous plant, unintended promotion of pests in cruciferous crops with sweet alyssum intercropping has not yet been described. However, most studies to date have focused on one or a few pest species without investigating possible undesirable effects on others.
Our objective was to evaluate effects of wheat undersowing and additional sweet alyssum intercropping on cabbage yield and on various arthropods in white cabbage at two study locations, one in Germany and one in Japan. In both countries, cabbage is one of the major horticultural field crops, and pest as well as natural enemy communities are quite similar. Furthermore, research on improved pest control by measures of conservation biological control belong to the main priorities towards more sustainable agriculture in both countries. Weight of the harvested cabbage heads, grown with and without companion plants, was compared to derive the competitiveness of white cabbage against these companion plants. With regard to cabbage pests, we focused on the cabbage infesting aphid species Brevicoryne brassicae (L.) and Myzus persicae (Sulzer), and on crucifer-feeding flea beetles (Phyllotreta spp.). In addition, the effects of companion plants on the abundance of natural enemies of aphids such as ladybirds, hoverfly larvae, and spiders were studied. Based on these results, we estimate the overall potential of wheat undersowing and alyssum intercropping for pest management in white cabbage.
Methods
Field sites and experimental design
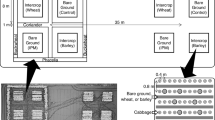
Field experiments were conducted in 2020 at two locations, one in central Germany near Brunswick (52° 12′ 17.3" N, 10° 36′ 17.6" E) and one in Morioka in the Tohoku region of northern Japan (39° 45′ 12.3" N, 141° 08′ 18.5" E). At each location, a randomized complete block design was installed with four blocks of four treatments: a non-insecticide control without companion plants [C], an insecticide control without companion plants [IC], cabbage undersown with wheat [W] and cabbage undersown with wheat plus sweet alyssum intercropping [WL] (Fig. 1). Insecticide applications in the [IC] treatment were applied according to local action thresholds (Supplementary table 1). If necessary, biopesticides based on Bacillus thuringiensis was applied to control feeding damage by Lepidopteran pests in all treatments. The size of the experimental plots was 4.0 m × 3.0 m.
Design of experimental plots with different treatments of white cabbage: (A) Control treatments: non-insecticide control [C] and insecticide control [IC], both without companion plants, (B) wheat undersowing treatment [W] with single-row wheat undersowing (grey bars) between cabbage rows, and (C) wheat undersowing plus sweet alyssum intercropping treatment [WL] showing wheat undersowing (grey bars) between cabbage rows and sweet alyssum intercropping (blossoms) replacing one cabbage plant per plot
In all four treatments, white cabbage (Brassica oleracea L. var. capitata, variety “Socrates”) was planted in six rows of eleven plants per plot on 18th May 2020 (Germany) and 21st May 2020 (Japan). Spacing of cabbage rows was 0.6 m, and distance between cabbage plants in each row was 0.4 m. Seedlings were pre-grown for five weeks in cell-trays in the greenhouse without heating, and then planted at the four-leaf stage. At the Japanese location, cabbage plants were planted on ca. 10 cm high ridges to improve drainage. At the German location, no ridges were needed. In [W] and [WL] treatments, wheat (Triticum aestivum, variety “Benchmark”) undersowing was drilled between cabbage rows as single strips with 300 seeds per m2. In both locations, wheat was drilled in early April, six weeks prior to the planting of cabbage. A winter wheat variety was used to avoid stem elongation in wheat plants without vernalisation. In the [WL] treatment, in addition to wheat undersowing, one cabbage plant of each row was replaced with alyssum seedlings. Sweet alyssum (Lobularia maritima L., variety “Benthamii”) was sown in the greenhouse with five seeds per tray and planted in the field five weeks later, together with cabbage plants.
Two meters of bare soil between plots were kept with regular tillage at both locations. Weeds that grew in plots were removed by hand during the experiment. Temperature was similar at both locations, but precipitation differed strongly (Supplementary table 2). The study location in Japan was influenced by heavy rainfall and short intervals of sun during the monsoon season, with the heaviest accumulated rainfall of 356 mm measured in July. The summer months in Germany were relatively dry with the heaviest accumulated rainfall measured in July at 58 mm. Therefore, the German field site was irrigated with 10 mm of water per week to avoid harvest losses to drought.
Assessment of wheat, alyssum and cabbage development and cabbage yield
The development stage of cabbage plants was documented using the BBCH scale (Meier et al. 2009) during the experimental season. Height of the undersown wheat plants was measured weekly at four spots of each plot. Flowering of sweet alyssum plants was also monitored weekly. On 12th August 2020 (Japan) and 13th October 2020 (Germany), ten cabbage plants per plot were harvested at BBCH 49 and 47, respectively. As an indicator of yield, the head weight of harvested cabbage plants was recorded after removing loose outer leaves.
Assessment of pest occurrence
Ten plants per plot were assessed weekly for pest occurrence and feeding damage from planting until harvest. Plants for assessment were selected before the start of the experiment and were distributed evenly over the plot, leaving out the border rows. Numbers of the aphid species M. persicae and B. brassicae (nymphs and adults together), as well as adult Phyllotreta spp. flea beetles, were counted up to ten individuals. Higher numbers were estimated in steps of ten up to 100 individuals and in steps of 100 up to 1000 individuals per plant. Feeding damage of flea beetles on cabbage plants was assessed by visually estimating the percentage of affected leaf area as proportion of the overall leaf area.
At the German location, two suction samples from sweet alyssum plants per plot were taken to identify the occurrence of flea beetles on this companion plant. Sampling was performed using an InsectaZooka Field Aspirator (BioQuip Products, CA, USA) each week during the experiment. Suction was performed for five seconds per sample and the trapped flea beetles per sample were counted. No additional flea beetle assessment on alyssum plants was carried out at the Japanese location because of low flea beetle density.
Assessment of natural enemies
Numbers of ladybirds (adults and larvae), spiders and hoverfly larvae were assessed weekly on ten cabbage plants per plot. The same plants as those for the pest assessments were selected. Due to rather low counts in weekly assessments, total numbers of each assessed cabbage plant were compared. Adult hoverflies were trapped using one transparent sticky trap (12 × 26 cm, Tripheron®, Trifolio-M GmbH, Germany) in the centre of each plot. The lack of colouring of the trap should prevent hoverflies from being attracted to specific colour schemes. Traps were exchanged weekly, and numbers of captured adult hoverflies were counted. Numbers of adult hoverflies were summed up over time for each trap and total numbers were compared. Additional visual counts of adult hoverflies were carried out at the German location. Visual counting was done by observing each plot for two minutes once per month, at the same time during the day and in comparable weather conditions. Such additional visual counts of adult hoverflies were not carried out at the Japanese location.
Statistical analysis
All statistical analyses were performed using R Statistical Software (v4.2.1; R Core team 2022) in the graphical user interface of R studio. Data were analysed separately between the German and Japanese location, because there were fundamental differences in climate and soil conditions as well as insecticide use in the [IC] treatment. Differences in pest arthropod numbers between treatments were analysed with generalized linear mixed-effects models using the function glmer (package lme4; Bates et al. 2015). Models included treatment and assessment week as fixed effects and repetition as a random effect. A Poisson distribution was assumed and models were tested for overdispersion. In the case of overdispersion, models were fitted using a negative binomial distribution. Tukey method was used for pairwise comparison of the four tested treatments, using the function emmeans (package emmeans; Lenth 2022). Counts of natural enemies (hoverfly larvae, ladybeetles, and spiders) on cabbage plants as well as of adult hoverflies on sticky traps were added up over the cropping season. Differences between the treatments were then analysed as described for pests, except excluding “week” as a factor, because count were summed up over time.
A linear mixed-effects model using the function lmer (package lme4; Bates et al. 2015) was fitted including treatment (trmt) and location (loc) as fixed effects and repetition (rep) as a random effect for the metric data of harvest weight. A pairwise comparison was carried out using the function emmeans (package emmeans; Lenth 2021).
Results
Wheat, alyssum and cabbage development and cabbage yield
Development of white cabbage, wheat and sweet alyssum differed between the German and the Japanese location (Supplementary table 3). Cabbage plants entered the head building phase eight weeks after cabbage planting at the German location and four weeks after cabbage planting at the Japanese location. Overall, the growing season was shorter in Japan, where cabbage was harvestable eleven weeks after it was planted. In Germany, plant development was completed after twenty weeks. At both locations, flowering of sweet alyssum continued for almost the whole growing season. Flowering of sweet alyssum started two weeks after cabbage planting in Germany, whereas it started before planting in Japan. In weeks eight and thirteen after cabbage planting, flowering intensity of sweet alyssum temporarily decreased in Germany. The mean height of undersown wheat increased to a maximum of 30.4 cm in week twelve and 28.5 cm in week five at the German and the Japanese location, respectively. Shortly after reaching the peak height, wheat plants started wilting at both locations.
When comparing weight of harvested cabbage heads as an indicator for cabbage yield, the weight was significantly higher at the German location compared to the Japanese location (Fig. 2). Neither insecticide use nor companion plants significantly influenced the weight of cabbage heads in both locations (p > 0.05).
Weight of harvested white cabbage heads on the German and Japanese location shown for different treatments: non-insecticide control [C], insecticide control [IC], wheat undersowing [W] and wheat undersowing plus sweet alyssum intercropping [WL]. Boxplots show the median value (solid line), the 25th and 75th percentile; error bars below and above the box indicate the 10th and 90th percentile, respectively. Jittered points indicate measures of single cabbage plants. Calculated means are marked as grey cross. Different letters indicate significant differences between treatments for each location
Effects of companion plants on pest occurrence on cabbage plants
Effects of companion plants and synthetic insecticide use on aphid numbers were different for the two aphid species B. brassicae (A) and M. persicae (B) (Fig. 3). At the German location, the highest aphid counts were observed six weeks and three weeks after cabbage planting for B. brassicae and M. persicae, respectively. Highest numbers of both aphid species at the Japanese location were counted seven weeks after cabbage planting. No significant effect of the companion plants treatments, [W] and [WL], on B. brassicae counts was detected compared to the non-insecticide control [C] in Germany (C/W: p = 0.7088, C/WL: p = 0.4485) or Japan (C/W: p = 0.1265, C/WL: p = 0.8082). The overall abundance of B. brassicae was lowest in the insecticide control [IC] at both locations (C/IC: p < 0.0001). Both companion plant treatments, [W] and [WL], showed significantly lower M. persicae counts as compared to the non-insecticide control [C] in Germany (C/W: p = 0.0169, C/WL: p = 0.0003) and Japan (C/W: p < 0.0001; C/WL: p = 0.0106). M. persicae numbers were also significantly affected by insecticide treatment [IC] compared to the non-insecticide control in Germany (C/IC: p < 0.0013) and Japan (C/IC: p < 0.0001).
Seasonal occurrence of aphids, B. brassicae (A and B) and M. persicae (C and D), on white cabbage plants shown for the two study locations in Japan and Germany. Four treatments were compared: non-insecticide control [C], insecticide control [IC], wheat undersowing [W] and wheat undersowing plus sweet alyssum intercropping [WL]. Data is shown for different weeks after planting of cabbage at the German and Japanese location (each n = 4). Symbols show mean of aphid counts; vertical lines show confidence interval given for the best-fitted model. Different letters indicate statistically significant differences between treatments for each location and aphid species
Wheat undersowing plus sweet alyssum intercropping [WL] increased flea beetle numbers on white cabbage, in comparison to all other treatments in Germany (WL/C, WL/IC, WL/W: p < 0.0001) and Japan (WL/C: p = 0.0361, WL/IC: p = 0.0221, WL/W: p = 0.0142) (Fig. 4A and B). At the German location, higher flea beetle counts in the [WL] treatment were especially observed from week eight until week seventeen after cabbage planting. Flea beetle counts at the German location were significantly lower in the insecticide control [IC], as compared to the non-insecticide control [C] (p = 0.0002) and the wheat undersowing treatment [W] (p = 0.0172). The overall flea beetle abundance at the Japanese location was much lower than at the German location. Consequently, there was no flea beetle feeding damage on cabbage plants at the Japanese location (data not shown). Conversely, feeding damage by flea beetles at the German location was significantly higher in the [WL] treatment compared to other treatments (WL/C, WL/IC, WL/W: p < 0.0001; Fig. 4C). Nevertheless, low feeding damage was also detected on harvested cabbage heads at the German location. There was 0.0 ± 0.0% (mean ± SE) leaf area damaged in both control treatments [C, IC] and only 0.2 ± 0.3% and 0.2 ± 0.1% leaf area damaged in wheat undersowing [W] and wheat undersowing plus alyssum intercropping [WL] plots, respectively (data not shown). Additional suction samples from sweet alyssum plants revealed the highest captures of flea beetles eight weeks after planting, with 96.87 ± 13.36 individuals per sample (Fig. 4D).
Seasonal occurrence of Phyllotreta spp. flea beetles on white cabbage plants shown for the two study locations in Japan (A) and Germany (B). Flea beetle feeding damage (%) on cabbage shown for the German location (C); no feeding damage was detected on cabbage plants at the Japanese study site (data not shown). Four treatments were compared (n = 4): non-insecticide control [C], insecticide control [IC], wheat undersowing [W] and wheat undersowing plus sweet alyssum intercropping [WL]. Symbols show mean of flea beetle counts or damage, vertical lines show confidence interval given for the best fitted model. Different letters indicate statistically significant differences between treatments. (D) For comparison, mean numbers of Phyllotreta spp. flea beetles in 5 s suction samples in sweet alyssum plants are shown for the German location; flea beetles on sweet alyssum plants were not assessed at Japanese location
Effects of companion plants on natural enemies
Total counts of three different groups of natural enemies (ladybirds, spiders and hoverfly larvae) were each summed up for each assessed cabbage plant (Fig. 5). At the German location, ladybird counts were significantly lower in the insecticide control [IC] than in the non-insecticide control [C] (p = 0.0003). Wheat undersowing [W] (p = 0.0723) and wheat undersowing plus additional sweet alyssum intercropping [WL] (p = 0.8741) did not significantly differ from the non-insecticide control [C]. At the Japanese location, numbers of ladybeetles were not significantly different between treatments (p > 0.05). Spiders and hoverfly larvae did not differ significantly between treatments at the German location (p > 0.05). At the Japanese location, counts of spiders and hoverfly larvae were significantly lower in the insecticide control [IC] than in the non-insecticide control [C] (spiders: p < 0.0001; hoverfly larvae: p = 0.0002). Japanese counts of spiders and hoverfly larvae in both undersowing treatments, [W] and [WL], did not significantly differ from untreated control (p > 0.05).
Seasonal occurrence of (A) ladybirds, (B) spiders and (C) hoverfly larvae on white cabbage plants shown for the two study locations in Germany and Japan. Four different treatments were compared: non-insecticide control [C], insecticide control [IC], wheat undersowing [W] and wheat undersowing plus sweet alyssum intercropping [WL]. Boxplots show the median value (solid line) and the 25th and 75th percentile; error bars below and above the box indicate the 10th and 90th percentile, respectively. Jittered points indicate measures of single cabbage plants. Calculated means are shown as grey cross. Different letters indicate significant differences between treatments for each location and natural enemy group
The total number of adult hoverflies caught on clear sticky traps was higher at the German location than at the Japanese location (Fig. 6A and B). The highest sticky trap captures were assessed in the [WL] treatment in Germany and in [W] treatment in Japan. Nonetheless, numbers of hoverfly captures did not significantly differ between treatments at both locations (p > 0.05). Visual counts of adult hoverflies at the German location were significantly higher in the [WL] treatment than in other treatments (WL/C, WL/IC, WL/W: p < 0.0001, Fig. 6C). These additional visual counts were only carried out at the German location.
Total count of adult hoverflies on clear sticky traps, summed up for each trap over cropping season shown for the two study locations in (A) Germany and (B) Japan. (C) Visual count of adult hoverflies in 2 min for each plot at the German location. All shown for four different white cabbage treatments: non-insecticide control [C], insecticide control [IC], wheat undersowing [W] and wheat undersowing plus sweet alyssum intercropping [WL]. Boxplots show the median value (solid line) and the 25th and 75th percentile; error bars below and above the box indicate the 10th and 90th percentile, respectively. Jittered points indicate single measures per trap or plot. In (C), different symbols indicate the four assessment dates. Calculated means are shown as grey cross. Different letters indicate statistically significant differences between treatments for each location and assessment
Discussion
Although Germany and Japan are geographically far apart, cabbage productions in both countries face similar pest management problems. Our study showed similar effects of the two tested companion plant systems on pests, natural enemies and yield of white cabbage at both study locations. Due to the warm and humid climate (Supplementary table 3), crop development was much faster at the Japanese location than at the German location. Also, Japanese white cabbage plants were harvested before reaching their full expected head size, reflecting different local consumer preferences. That is why the overall head weight of cabbage heads is lower at the Japanese location compared to the German location. Still, no effect of either of the companion plant systems on cabbage yield was found at either of the two locations.
Past studies reported yield loss due to competition when cabbage was undersown with different clover species, especially if clover was drilled before cabbage planting (Hamid et al. 2006; Lehmhus 2001). In this study, the proportion and arrangement of both companion plant species in field plots were planned to balance positive effects on pest regulation without compromising negative effects on yield based on the available literature. Lehmhus (2001) defined a one-row undersowing with clover between wide (> 60 cm) cabbage rows as the best compromise for undersowing arrangement. Brennan (2013, 2016) suggested a replacement of crop plants with sweet alyssum rather than additive intercropping to limit competition effects. Both were reflected in the current study design.
Wheat in the [W] and [WL] treatments developed faster at the Japanese location, reaching its maximum height in June, as compared to late July at the German location. This observation is in line with the differences in cabbage development in both countries. Wheat undersowing started to wilt around seven weeks (Japanese location) and eleven weeks (German location) after cabbage planting. Consequently, potential effects on arthropods were mainly expected in the first half of each cultivation period. However, the start of the wilting did not mark a change in the effects on aphids or flea beetles in this study. This is likely because the main attacks of the studied pests occurred early in the season. Some effects seem to last sometime after wheat wilting in both locations (Fig. 3A and C, Fig. 4A-C), but this could be a retained observation of earlier effects of undersowing on the initial pest infestations.
At both locations, the most prominent aphid species on cabbage plants were M. persicae and B. brassicae. A regulating effect of companion plants on aphid abundance could only be detected for M. persicae at both locations. Due to the shorter growing season in Japan, aphid abundance could only be assessed for eleven weeks after cabbage planting. At the German location, M. persicae aphids were only present on cabbage plants in the first nine weeks. B. brassicae aphids had a second migration phase later in the growing season. This second migration peak, though not comparable to the Japanese location, did not affect the statistical outcome for the German location (analysis not shown).
Regulating effects of different companion plants on M. persicae have been described for several horticultural crops (Andorno and López 2014; Chen et al. 2022; McKinlay 1985; Sun and Song 2019). In cruciferous crops, such as broccoli and radish, companion plants can reduce numbers of M. persicae and B. brassicae (Costello and Altieri 1995; Ponti et al. 2007). Intercropping with sweet alyssum in particular is reported to significantly reduce numbers of M. persicae in radish (Tiwari et al. 2020), and M. persicae and B. brassicae in collard greens (Ribeiro and Gontijo 2017). In our study, the regulating effect of companion plants on M. persicae, but not on B. brassicae, could be confirmed.
The natural enemy hypothesis, as described by Russell (1989), states that a higher abundance of natural enemies is a major factor for controlling pests in mixed cropping. Fidelis et al. (2018) revealed the most prominent natural enemies of B. brassicae to be predators such as hoverfly larvae, ladybirds, and spiders. In this study, no significant effects of companion plants on abundance of these natural enemies of aphids on cabbage plants were observed and the natural enemy hypothesis was not supported by our data. Nonetheless, the small spacing (2 m) between plots (3 × 4 m) in our study may have masked the differences between treatments in terms of hoverflies. The dispersal radius of female adults for egg laying is much wider and can be up to 250 m from their original pollen source (Gillespie et al. 2011; Sommaggio 1999; White et al. 1995). Enhancing effects on adult hoverflies by sweet alyssum intercropping were described for different horticultural crops, especially lettuce (Brennan 2013; Chaney 1998; Gillespie et al. 2011). At both locations, flowering of sweet alyssum was observed for almost the whole growing season (Supplementary table 3) and therefore provided a continuous pollen and nectar supply. In contrast to our study, an enhancing effect of sweet alyssum intercropping on hoverfly abundance and aphid regulation could be especially reported in studies with either larger plot sizes (7 × 16 m; Ribeiro and Gontijo 2017) or larger distances between plots (20 m; Tiwari et al. 2020). As visual counts at the German location (Fig. 4D) showed, higher numbers of adult hoverflies were observed in the [WL] treatment here. However, it is possible that female hoverflies in particular dispersed easily between plots for oviposition due to the short distance, resulting in similar activity of hoverfly larvae in all treatments.
Flea beetle numbers were not influenced by wheat undersowing alone but have been increased by additional intercropping of sweet alyssum. As sweet alyssum is a cruciferous plant, like all cabbage crops and oilseed rape, it is likely to operate as a habitat not only for beneficial insects, but also for crucifer herbivorous pests. Suction samples on sweet alyssum plants at the German location identified a high abundance of Phyllotreta spp. flea beetles on this companion plant throughout the growing season. Flea beetles could be observed feeding on flower buds and petals of sweet alyssum and presumably migrated to adjacent cabbage plants. Leavitt and Robertson (2006) reported that Phyllotreta spp. flea beetles can be found feeding on flowers of the crucifer plant Lepidium papilliferum (L.), especially on its petals. In combination with the relatively low distance between plots, enhancement of the flea beetle population by sweet alyssum may have obscured regulating effects of the [W] treatment alone on this beetle. As counting of flea beetles in suction samples was only carried out at the German location, no clear conclusion can be drawn for the Japanese location. A few flea beetles were observed on sweet alyssum plants in Japan, but they were not counted due to low overall numbers.
Significantly higher flea beetle numbers were detected on cabbage in the sweet alyssum intercropped treatment at both locations, although overall flea beetle numbers were much lower at the Japanese compared to the German location. Low beetle numbers may be linked to heavy rainfalls at the Japanese location. However, the location itself may also play an important role here. Gikonyo et al. (2019) reviewed that 137 species of the genus Phyllotreta, 118 of which had an endemic distribution, could be found in the Palearctic region, including Germany. Compared to this, only 25 species, including 18 endemic species, are found in the Oriental region including Japan. At the German location, an increased number of flea beetles resulted in increased feeding damage in the sweet alyssum intercropped plots. In contrast, there was almost no flea beetle feeding damage observed on cabbage due to the low overall infestation rates at the Japanese location. Enhancing effects on herbivorous insects have not yet been reported in studies that evaluated sweet alyssum intercropping in cruciferous crops (Ribeiro and Gontijo 2017; Tiwari et al. 2020). Brennan (2016) even describes that sweet alyssum intercropping is already a common practice in broccoli production in California. However, in the corresponding regions of these studies (Neotropical region, Oriental region and Nearctic region), species numbers of the genus Phyllotreta are much lower as compared to Germany and the Palearctic region (Gikonyo et al. 2019).
Although high flea beetle numbers and feeding damage were observed throughout the growing season at the German location, the final feeding damages on harvested cabbage heads was very low and no significant differences between treatments were observed. This is likely because feeding increased relatively late in the season and was concentrated on outer leaves, which were removed from the harvested cabbage head before assessment. However, any potential increase of pest infestation will certainly lower the acceptance of an intercropping strategy by farmers.
The different effects of insecticide use on pests and natural enemies at the German and Japanese locations may be related to differences in spray intensity and the insecticides used. Frequency of insecticide application and choice of active compounds were based on local farmers’ practice and action thresholds given by local advisors. Total numbers of all three assessed natural enemies on cabbage plants (ladybirds, hoverfly larvae and spiders) were significantly reduced in the insecticide control in at least one of the locations. Reasons for this reduction could include direct side-effects of insecticide applications on predators (Bacci et al. 2009; Bozsik 2006) or lower pest numbers as available prey on cabbage plants, or a combination of both.
In conclusion, this study showed that companion plants have similar effects on pests and natural enemies in white cabbage at two locations as far apart as Japan and Germany, despite profound environmental differences. Companion plants reduced numbers of M. persicae while having no effect on B. brassicae. Additional intercropping with sweet alyssum enhanced numbers and damage of herbivorous flea beetles on cabbage plants, but at different densities depending on the location. Regulating effects of wheat undersowing on flea beetles could not be detected in this study. Adult hoverfly visits were enhanced by additional sweet alyssum intercropping, but the effects on the densities of predatory hoverfly larvae as well as ladybeetles and spiders could not be proven in this study. No negative impact of wheat undersowing and sweet alyssum intercropping on cabbage harvest weight was observed.
Data availability
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
References
Andorno AV, López SN (2014) Biological control of Myzus persicae (Hemiptera: Aphididae) through banker plant system in protected crops. Biol Control 78:9–14. https://doi.org/10.1016/j.biocontrol.2014.07.003
Andow DA, Nicholson AG, Wien HC, Willson HR (1986) Insect populations on cabbage grown with living mulches. Environ Entomol 15(2):293–299. https://doi.org/10.1093/ee/15.2.293
Bacci L, Picanço MC, Rosado JF, Silva GA, Crespo ALB, Pereira EJG, Martins JC (2009) Conservation of natural enemies in brassica crops: comparative selectivity of insecticides in the management of Brevicoryne brassicae (Hemiptera: Sternorrhyncha: Aphididae). Appl Entomol and Zool 44(1):103–113. https://doi.org/10.1303/aez.2009.103
Barratt BIP, Moran VC, Bigler F, van Lenteren JC (2018) The status of biological control and recommendations for improving uptake for the future. Biocontrol 63(1):155–167. https://doi.org/10.1007/s10526-017-9831-y
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Begg GS, Cook SM, Dye R, Ferrante M, Franck P, Lavigne C, Lövei GL, Mansion-Vaquie A, Pell JK, Petit S, Quesada N, Ricci B, Wratten SD, Birch ANE (2017) A functional overview of conservation biological control. Crop Prot 97:145–158. https://doi.org/10.1016/j.cropro.2016.11.008
Bozsik A (2006) Susceptibility of adult Coccinella septempunctata (Coleoptera: Coccinellidae) to insecticides with different modes of action. Pest Manag Sci 62(7):651–654. https://doi.org/10.1002/ps.1221
Brennan EB (2013) Agronomic aspects of strip intercropping lettuce with alyssum for biological control of aphids. Biol Control 65(3):302–311. https://doi.org/10.1016/j.biocontrol.2013.03.017
Brennan EB (2016) Agronomy of strip intercropping broccoli with alyssum for biological control of aphids. Biol Control 97:109–119. https://doi.org/10.1016/j.biocontrol.2016.02.015
Brewer MJ, Goodell PB (2012) Approaches and incentives to implement integrated pest management that addresses regional and environmental issues. Ann Rev Entomol 57:41–59. https://doi.org/10.1146/annurev-ento-120709-144748
Chaney WE (1998) Biological control of aphids in lettuce using in-field insectaries. In: Pickett CH, Bugg RL (eds) Enhancing Biological Control: Habitat Management to Promote Natural Enemies of Agricultural Pests. University of California Press, Berkeley, CA, pp 73–84
Chen X, Jaworski CC, Dai H, Liang Y, Guo X, Wang S, Zang LS, Desneux N (2022) Combining banker plants to achieve long-term pest control in multi-pest and multi-natural enemy cropping systems. J Pest Sci 95(2):685–697. https://doi.org/10.1007/s10340-021-01428-6
Chen Y, Mao J, Reynolds OL, Chen W, He W, You M, Gurr GM (2020) Alyssum (Lobularia maritima) selectively attracts and enhances the performance of Cotesia vestalis, a parasitoid of Plutella xylostella. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-62021-y
Costello MJ, Altieri MA (1995) Abundance, growth rate and parasitism of Brevicoryne brassicae and Myzus persicae (Homoptera: Aphididae) on broccoli grown in living mulches. Agric Ecosyst Environ 52(2–3):187–196. https://doi.org/10.1016/0167-8809(94)00535-M
Fidelis EG, Santos AA, Sousa FF, da Silva RS, Dângelo RAC, Picanço MC (2018) Predation is the key mortality factor for Brevicoryne brassicae in cabbage crops. Biocontr Sci Tech 28(12):1164–1177. https://doi.org/10.1080/09583157.2018.1516735
Frank SD (2010) Biological control of arthropod pests using banker plant systems: past progress and future directions. Biol Control 52(1):8–16. https://doi.org/10.1016/j.biocontrol.2009.09.011
Gikonyo MW, Biondi M, Beran F (2019) Adaptation of flea beetles to brassicaceae: host plant associations and geographic distribution of Psylliodes Latreille and Phyllotreta Chevrolat (Coleoptera, Chrysomelidae). ZooKeys 856:51–73. https://doi.org/10.3897/zookeys.856.33724
Gillespie M, Wratten S, Sedcole R, Colfer R (2011) Manipulating floral resources dispersion for hoverflies (Diptera: Syrphidae) in a California lettuce agro-ecosystem. Biol Control 59(2):215–220. https://doi.org/10.1016/j.biocontrol.2011.07.010
Gómez-Marco F, Urbaneja A, Tena A (2016) A sown grass cover enriched with wild forb plants improves the biological control of aphids in citrus. Basic Appl Ecol 17(3):210–219. https://doi.org/10.1016/j.baae.2015.10.006
Gontijo LM, Beers EH, Snyder WE (2015) Complementary suppression of aphids by predators and parasitoids. Biol Control 90:83–91. https://doi.org/10.1016/j.biocontrol.2015.06.002
Gulidov S, Poehling HM (2013) Control of aphids and whiteflies on brussels sprouts by means of UV-absorbing plastic films. J Plant Dis Prot 120(3):122–130. https://doi.org/10.1007/BF03356463
Hamid HA, Montà LD, Battisti A (2006) Undersowing cruciferous vegetables with clover: the effect of sowing time on flea beetles and diamondback moth. Bull Insectol 59(2):121–127
Hulot JF, Hiller N (2021) Exploring the benefits of biocontrol for sustainable agriculture: A literature review on biocontrol in light of the European Green Deal. Institute for European Environmental Policy.
Jandricic SE, Dale AG, Bader A, Frank SD (2014) The effect of banker plant species on the fitness of Aphidius colemani Viereck and its aphid host (Rhopalosiphum padi L.). Biol Control 76:28–35. https://doi.org/10.1016/j.biocontrol.2014.04.007
Kopta T, Pokluda R, Psota V (2012) Attractiveness of flowering plants for natural enemies. Hort Sci 39(2):89–96. https://doi.org/10.17221/26/2011-hortsci
Leavitt H, Robertson IC (2006) Petal herbivory by chrysomelid beetles (Phyllotreta sp is detrimental to pollination and seed production in Lepidium papilliferum (Brassicaceae). Ecol Entomol 31(6):657–660. https://doi.org/10.1111/j.1365-2311.2006.00820.x
Lehmhus J (2001) Auswirkungen von Untersaaten in Weißkohlkulturen auf die Populationsdynamik der Schadinsekten, die Unkräuter und den Ertrag. Dissertation, University of Hannover.
Lenth R (2022) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.8.0. https://CRAN.R-project.org/package=emmeans
Letourneau DK (1987) The enemies hypothesis: Tritrophic interactions and vegetational diversity in tropical agroecosystems. Ecology 68(6):1616–1622. https://doi.org/10.2307/1939853
Masuda T, Miyata M (2008) Effects of cover cropping on occurence of insect pests in cabbage fields. Ann Rept Plant Prot North Japan 59:153–175
McKinlay RG (1985) Effect of undersowing potatoes with grass on potato aphid numbers. Ann Appl Biol 106(1):23–29. https://doi.org/10.1111/j.1744-7348.1985.tb03090.x
Meier U, Bleiholder H, Buhr L, Feller C, Hack H, Heß M, Lancashire P, Schnock U, Stauß R, van den Boom T, Weber E, Zwerger P (2009) The BBCH system to coding the phenological growth stages of plants-history and publications. J Kulturpflanzen 61(2):41–52. https://doi.org/10.5073/JfK.2009.02.01
Meyling NV, Navntoft S, Philipsen H, Thorup-Kristensen K, Eilenberg J (2013) Natural regulation of Delia radicum in organic cabbage production. Agric Ecosyst Environ 164:183–189. https://doi.org/10.1016/j.agee.2012.09.019
Nagasaka K, Takahasi N, Okabayashi T (2010) Impact of secondary parasitism on Aphidius colemani in the banker plant system on aphid control in commercial greenhouses in Kochi. Japan Appl Entomol Zool 45(4):541–550. https://doi.org/10.1303/aez.2010.541
Parolin P, Bresch C, Desneux N, Brun R, Bout A, Boll R, Poncet C, Antipolis FS (2012) Secondary plants used in biological control: a review. Internat J Pest Manag 58(2):91–100. https://doi.org/10.1080/09670874.2012.659229
Patt JM, Hamilton GC, Lashomb JH (1997) Foraging success of parasitoid wasps on flowers: interplay of insect morphology, floral architecture and searching behavior. Entomol Exp Appl 83(1):21–30. https://doi.org/10.1046/j.1570-7458.1997.00153.x
Perrin RM, Phillips ML (1978) Some effects of mixed cropping on the population dynamics of insect pests. Entomol Exp Appl 24(3):585–593. https://doi.org/10.1111/j.1570-7458.1978.tb02820.x
Pfiffner L, Luka H, Schlatter C, Juen A, Traugott M (2009) Impact of wildflower strips on biological control of cabbage lepidopterans. Agric Ecosyst Environ 129(1–3):310–314. https://doi.org/10.1016/j.agee.2008.10.003
Picó FX, Retana J (2003) Seed ecology of a mediterranean perennial herb with an exceptionally extended flowering and fruiting season. Bot J Linnean Soc 142(3):273–280. https://doi.org/10.1046/j.1095-8339.2003.00172.x
Ponti L, Altieri MA, Gutierrez AP (2007) Effects of crop diversification levels and fertilization regimes on abundance of Brevicoryne brassicae (L.) and its parasitization by Diaeretiella rapae (M’Intosh) in broccoli. Agric Forest Entomol 9(3):209–214. https://doi.org/10.1111/j.1461-9563.2007.00330.x
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rezaei R, Safa L, Ganjkhanloo MM (2020) Understanding farmers’ ecological conservation behavior regarding the use of integrated pest management - an application of the technology acceptance model. Global Ecol Conserv 22:e00941. https://doi.org/10.1016/j.gecco.2020.e00941
Ribeiro AL, Gontijo LM (2017) Alyssum flowers promote biological control of collard pests. Biocontrol 62(2):185–196. https://doi.org/10.1007/s10526-016-9783-7
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica Oleracea). Ecol Monogr 43(1):95–124. https://doi.org/10.2307/1942161
Russell EP (1989) Enemies hypothesis: a review of the effect of vegetational diversity on predatory insects and parasitoids. Environ Entomol 18(4):590–599. https://doi.org/10.1093/ee/18.4.590
Sekine T, Kanao K, Inawashiro S, Hori M (2021) Insect pest management by intercropping with leafy daikon (Raphanus sativus) in cabbage fields. Arthropod-Plant Interact 15(5):669–681. https://doi.org/10.1007/s11829-021-09848-y
Seress Z, McKinlay RG, Pénzes B, Slezák K (2000) What mechanisms are involved in cabbage-clover intercropping and a further proof of the ‘host plant quality’ hypothesis. Internat J Hort Sci 6(4):47–51. https://doi.org/10.31421/IJHS/6/4/220
Sheehan W (1986) Response by specialist and generalist natural enemies to agroecosystem diversification: A selective review. Environ Entomol 15(3):456–461. https://doi.org/10.1093/ee/15.3.456
Sommaggio D (1999) Syrphidae: Can they be used as environmental bioindicators? Agric Ecosyst Environ 74(1–3):343–356. https://doi.org/10.1016/S0167-8809(99)00042-0
Stoleru VV, Munteanu NC, Stoleru CMV, Rotaru LG (2012) Cultivar selection and pest control techniques on organic white cabbage yield. Not Bot Hort Agrobo 40(2):190–196. https://doi.org/10.15835/nbha4027993
Sun H, Song Y (2019) Establishment of a wheat banker plant system for the parasitoid Aphidius gifuensis against Myzus persicae in greenhouse chili pepper. Appl Entomol Zool 54(4):339–347. https://doi.org/10.1007/s13355-019-00624-2
Theunissen J, Booij CJH, Lotz LAP (1995) Effects of intercropping white cabbage with clovers on pest infestation and yield. Entomol Exp Appl 74(1):7–16. https://doi.org/10.1111/j.1570-7458.1995.tb01869.x
Tiwari S, Sharma S, Wratten SD (2020) Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J Asia-Pacific Entomol 23(3):634–640. https://doi.org/10.1016/j.aspen.2020.05.002
Wäschke N, Meiners T, Rostás M (2013) Foraging strategies of parasitoids in complex chemical environments. In: Wajnberg E, Colazza S (eds.). Chemical ecology of insect parasitoids, 1st edn. Wiley, New York, pp 37–63. Doi: https://doi.org/10.1002/9781118409589.ch3
Wezel A, Casagrande M, Celette F, Vian JF, Ferrer A, Peigné J (2014) Agroecological practices for sustainable agriculture. A Rev Agron Sustain Dev 34(1):1–20. https://doi.org/10.1007/s13593-013-0180-7
White AJ, Wratten SD, Berry NA, Weigmann U (1995) Habitat manipulation to enhance biological control of brassica pests by hover flies (Diptera: Syrphidae). J Econ Entomol 88(5):1171–1176. https://doi.org/10.1093/jee/88.5.1171
Acknowledgements
The work was conducted as a strategic international collaborative project promoted by the Ministry of Agriculture, Forestry and Fisheries, Tokyo, Japan (JPJ008837) and financially supported by the German Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany through the Federal Office for Agriculture and Food (BLE). The authors thank Anne-Kathrin Grashoff for technical assistance in the German field trial.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical Approval
This article does not contain any studies, performed by any of the authors, with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Köneke, A., Uesugi, R., Herz, A. et al. Effects of wheat undersowing and sweet alyssum intercropping on aphid and flea beetle infestation in white cabbage in Germany and Japan. J Plant Dis Prot 130, 619–631 (2023). https://doi.org/10.1007/s41348-023-00730-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00730-y