Abstract
The addition of compost, green waste biochar, and wood biochar to a pathosystem consisting of Sclerotinia sclerotiorum (Lib.) de Bary and two susceptible host plants, sunflower (Helianthus annuus L.) and soybean (Glycine max L.), was investigated in a greenhouse study. Plant growth characteristics, disease incidence and the mycelial growth of S. sclerotiorum in root exudates of plants inoculated and not inoculated with this pathogen were determined. Both plants showed different responses in terms of shoot and root weight. Disease incidence in sunflower was lowest in the substrate consisting of green waste biochar and compost. The disease incidence in this substrate was approximately half that in the control treatment. In soybean, the highest disease suppression was achieved with this combined substrate too. Mycelial growth in sunflower root exudates from uninoculated plants was higher than that in sunflower plants inoculated with S. sclerotiorum. The substrate did not influence mycelial growth in root exudates. Soybean root exudates did not show this effect of the pathogen. This study proves that the addition of organic matter inputs such as biochar and compost can improve plant growth and can also have favorable effects against S. sclerotiorum infection. In addition, it is demonstrated that both the pathogen and host plant are instrumental in determining the mode of action of the supplemented organic material. The addition of the same organic material can lead to different responses in certain pathogens, such as S. sclerotiorum, depending on the host plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, organic amendments such as compost and biochar have gained increasing attention. While compost is the aerobic biological decomposition of different organic materials (Epstein 1997), biochar is a valuable carbon-rich product produced by the degradation of organic materials via pyrolysis (Lehmann and Joseph 2009). Due to the type of organic material, both amendments are never a completely standardized product and may vary in their physicochemical properties and nutrient contents. Nevertheless, numerous studies have shown that compost and biochar have proven positive effects on important agronomic factors, such as water-holding capacity (Curtis and Claassen 2005; Glaser et al. 2002), cation exchange capacity (Glaser et al. 2002; Pedra et al. 2008), soil acidity (Butler et al. 2008; Chan et al. 2009), nutrient retention (Lehmann et al. 2003; Steiner et al. 2008), and soil microbial activity (Paulin and O`Malley 2008; Thies and Rillig 2009).
The incorporation of compost into the soil not only affects plant growth but might be a promising strategy to combat a wide range of soil-borne fungal pathogens, e.g., shown for Fusarium sp., Phytophthora sp., Pythium sp., Rhizoctonia solani, and Verticillium dahliae (Termorshuizen et al. 2006). There is also some evidence that biochar can reduce fungal and fungal-like plant pathogens, such as Botrytis, Colletotrichum, Fusarium, Leveillula, Pythium, and Phytophthora species (Graber et al. 2014; Bonamomi et al. 2015). Furthermore, Hassan (2017) found a reduction in S. sclerotiorum in eggplants upon biochar application. Combining compost and biochar could not only enhance soil quality and crop performance (Agegnehu et al. 2017) but also can result in positive effects in some pathosystems (Akhter et al. 2015); however, the host plant effect has not yet been elucidated.
Various mechanisms may be responsible for the disease suppressive effect of compost (Mehta et al. 2014). These not only include the physicochemical properties of compost, chemical signaling affecting pathogen proliferation, as well as the systemic acquired and induced systemic resistance but also alterations in the microbial community due to competition for nutrients and infections courts, antibiosis, and hyperparasitism. Termorshuizen et al. (2006) found variability in pathogen response in several pathosystems; however, they used different host plants for each pathogen. Therefore, the extent of host plant contribution to these effects remains unknown.
Sclerotinia sclerotiorum (Lib.) de Bary is a widespread plant pathogen capable of attacking more than 400 host plants, primarily dicotyledonous species (Boland and Hall 1994). The necrotrophic pathogen, S. sclerotiorum, can remain viable as sclerotia of varying sizes for several years in the soil. Under favorable weather conditions, the fungus can spread following myceliogenic or carpogenic germination of the sclerotia, depending on the host plant (Saharan and Mehta 2008). Myceliogenic infection plays a major role in sunflower (Huang and Dueck 1980), and the carpogenic path of infection is common in soybean (Grau et al. 1982; Cline and Jacobsen 1983). Seed infection has been documented, albeit insufficiently described, for both crops (Saharan and Mehta 2008).
In sunflower, the root and hypocotyl may serve as infection courts for S. sclerotiorum (Hancock 1972; Huang and Dueck 1980). However, conflicting data are available on the necessity of exogenous nutrients to establish the successful infection of crop plants. According to Abawi and Grogan (1975), exogenous nutrient sources are a prerequisite for a successful infection, and it has also been reported that no other nutrient sources are required (Saharan and Mehta 2008). Biological activity in the rhizosphere is strongly influenced by a variety of plant compounds released into the rhizosphere. In particular, the components of plant root exudates have a profound impact on soil-borne pathogens, ranging from stimulatory and inhibitory to attractants as well as repellent effects (Baetz and Martinoia 2014), depending on the plant species, growth stages, and growth conditions. For instance, myceliogenic germination of S. sclerotiorum is generally favored by the root exudates of sunflowers but not during flowering. Monteiro et al. (2012) found that root exudates of cover crops, depending on the plant species, affect mycelial growth, but not the mycelial germination of the pathogen. Soybean root exudates affect apothecial formation (Chaves et al. 1996), but there is no information available on their impact on myceliogenic development.
In this study, we investigated the addition of municipal compost and biochar to a pathosystem consisting of S. sclerotiorum and two susceptible host plants, sunflower and soybean. Following the myceliogenic infection path, we aimed to elucidate the impact of both soil amendments on plant performance and disease incidence, as well as on the development of S. sclerotiorum in root exudates of plants inoculated and non-inoculated with this pathogen.
Materials and methods
Fungal isolate
S. sclerotiorum was routinely cultured on potato dextrose agar medium (PDA; Carl Roth GmbH + Co. KG, Karlsruhe, Germany) at 24 °C in the dark. The isolate is stored at the Institute of Plant Protection, University of Natural Resources and Life Sciences, Vienna, Austria. For the inoculation of soybean and sunflower plants, a mycelial suspension was used. Therefore, two mycelial plugs (approximately 8 mm2) from the edge of a 1 week-old S. sclerotiorum culture were added to 200 mL potato dextrose broth (PDB) in 500-mL Erlenmeyer flasks. The flasks were incubated at 24 °C on a rotary shaker at 160 rpm in the dark. After 1 week, the mycelial suspension was mixed in a sterile blender and adjusted to a final concentration of 37 mg mycelium (dry weight) per mL. A freshly prepared inoculum bottle was used for each replicate.
Plant material
Soybean (Glycine max L. cv. Gallec, kindly provided by Raiffeisen Ware Austria AG, Vienna, Austria) and sunflower (Helianthus annuus L. cv. NK Delfi, kindly provided by Saatbau Linz eGen, Linz, Austria) were used in the present study. The seeds were surface-sterilized by soaking in 50% household bleach (2.8% NaOCl) for 5 min and then washed thrice with autoclaved distilled water for 2 min.
Potting mix
For the pot experiments, a mixture of autoclaved (20 min at 121 °C; 2.1 bar) sand (Quarzsand 0.5–2.0 mm; Quarzwerke Österreich GmbH, Melk, Austria), soil (Aussaaterde; Gramoflor GmbH and Co. KG, Vechta, Germany) and expanded clay (Liapor fit; 1–4 mm, Lias Österreich GmbH, Fehring, Austria) (1:1:1, v/v/v) were used. Compost (Comp) at 20% and/or biochar at 3% (v/v) were added for plant cultivation, depending on the treatment. The compost was obtained from the municipal compost works in Klosterneuburg (Austria) and met the A + classification according to the Austrian compost regulation (BGBl. II Nr. 929/2001). Furthermore, two types of biochar from different raw materials were used: Wood biochar (WB) was made from beech wood chips and green waste biochar (GWB) from garden waste residues, both at a pyrolysis temperature of 500 °C. The physicochemical characteristics of the compost and wood and green waste biochars are shown in Table 1.
Greenhouse experiment
The experimental setup included 12 different variants for each crop plant: (i) control, (ii) WB, (iii) GWB, (iv) Comp, (v) WB + Comp, and (vi) GWB + Comp, each with (+ Scl) and without (− Scl) S. sclerotiorum. Each treatment consisted of six pots for each crop. The entire trial was carried out three times. For inoculation with S. sclerotiorum, seeds were dipped in the above-mentioned mycelial suspension for 2 h. Seeds that were not inoculated were treated in distilled water. Sets of three (due to irregular germination) soybean and sunflower seeds were planted into 1-L pots (Göttinger round pot; Lamprecht Verpackungen GmbH, Göttingen, Germany) filled with the aforementioned potting mix. Ten days after sowing, the seedlings were counted for germination and then thinned from 3 to 1 per pot to ensure uniform growth conditions. The plants were grown for 6 weeks in a greenhouse under supplemental light with a 14 h photoperiod at 24 °C day/night and ~ 60% RH and regularly irrigated with a nutrient solution (Steinkellner et al. 2005).
Diseases assessment
The assessment of S. sclerotiorum on sunflower and soybean plants was performed 20 d after planting. Plants showing lesions at the base of the stem were recorded. Disease incidence was calculated as the percentage of infected plants to the total number of plants in the corresponding treatment.
Root exudate extraction and agronomic characteristics
Six weeks after sowing, the plants were removed from the substrate by washing the roots under running tap water. Afterward, six plants per treatment were submerged in acetate buffer (25 mM, pH = 5.5) for 6 h as described by Hage-Ahmed et al. (2013) and then filtered through 0.22 µm sterile filters (EMD Millipore Corporation, Billerica, USA). The concentration of the root exudates was adjusted to a concentration of 10 mL/g root fresh weight, and the exudates were stored at − 80 °C until further use. One exudate comprised six plants, and three replicates (i.e., exudates) per treatment were prepared. Roots and shoots were then separated, and the dry matter content was recorded after drying at 105 °C for 24 h.
Mycelial growth assay
The mycelial development assay for S. sclerotiorum in root exudates was performed in 96-well plates (NUNCLON™ D Surface, F96 MicroWell™ Plates, NUNC™ Brand Products, Roskilde, Denmark). A 1-week-old mycelial suspension of S. sclerotiorum in PDB was blended under sterile conditions for 1 min and poured through a filter paper (disk filters for cans, ∅200 mm, Albert Kerbl GmbH, Buchbach, Germany). The resulting mycelial suspension was adjusted to an optical density of 0.02 at 600 nm. Next, 175 µL aliquots of the root exudate and 35 µL aliquots of the mycelial suspension were added to each well of the plates. Three replicates were made for each root exudate, and the plates were incubated on a rotary shaker at 200 rpm at 24 °C in the dark. Mycelial growth was determined using a spectrophotometer (FLUOstar Omega, BMG LABTECH GmbH, Ortenberg, Germany) by measuring the optical density at 600 nm after 72 h, according to the method described by Steinkellner and Mammerler (2007).
Statistical analysis
Data analyses (joint analysis for the repeated experiment) were performed after testing the homogeneity of variance using Levene’s test. One-way analysis of variance (ANOVA) followed by a post hoc comparison using Tukey’s test (P < 0.05) was performed for disease incidence. For all other parameters, two-way ANOVA considering the factors soil amendment (substrate), S. sclerotiorum (Scl) for –Scl and + Scl, and Substrate × Scl interactions followed by a post hoc comparison using Tukey’s test (P < 0.05) was performed using IBM SPSS Statistics V21.0.0 (SPSS Inc, Chicago, IL, USA).
Results
Evaluation of plant growth parameters
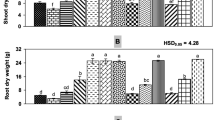
The treatments affected shoot dry matter in sunflower and soybean plants. The results of the two-way ANOVA revealed that both factors, i.e., ‘Substrate’ and ‘Sclerotinia,’ significantly influenced the shoot dry weight of sunflower, whereas, for soybean, only the ‘Substrate’ showed effects. No interactions were found between ‘Substrate’ and ‘Sclerotinia’ in either plant (Table 2). In sunflower, plants inoculated with S. sclerotiorum showed a reduction in shoot dry matter compared with the non-inoculated plants (Table 3). The compost only amendment (‘Comp’) or in combination with biochar (‘WB + Comp’ and ‘GWB + Comp’) increased the shoot weights in both Sclerotinia variants (–Scl and + Scl) significantly compared with that of the control and both biochar variants (‘WB’ and ‘GWB’). In soybean, no differences were found between –Scl and + Scl. While compost alone showed no effect on shoot growth, the addition of biochar alone or in combination with compost enhanced shoot dry weight (Table 3).
The root dry matter was significantly affected by the main factors ‘Substrate’ and ‘Sclerotinia,’ both in sunflower and in soybean, with significant interactions of both factors in both plants (two-way ANOVA, Table 2). In –Scl sunflowers, the root dry weight was not influenced by the substrate; in contrast, it increased in + Scl plants cultivated in substrates including compost, where compost alone gave the best result. Inoculation of sunflower with S. sclerotiorum lowered root dry weight (Table 2 and 3). In soybeans, the picture differed altogether. Compost alone (–Scl and + Scl) decreased root dry matter compared with the other variants. In –Scl plants, compost in combination with biochar also reduced the dry matter of soybean roots. The S. sclerotiorum inoculation did not alter the root dry matter relative to uninoculated plants in the respective substrate, except in ‘WB’ and ‘GWB’ (Table 2 and 3).
Germination rate
The seed germination rate of sunflower and soybean plants was determined 10 d after planting. The results of the two-way ANOVA showed significant effects and interactions for the main factors ‘Substrate’ and ‘Sclerotinia’ in sunflower, whereas, in soybean, the germination rate was only affected by the infection factor (Scl) (Table 2). In sunflower, the germination rate was higher in –Scl variants than in + Scl variants and ranged from 34.72% (+ Scl ‘Control’) to 83.33% (− Scl ‘GWB + Comp’). While the substrate did not influence the germination rate in –Scl plants, the germination rate was higher in the + Scl variants with compost than in the substrates without compost—the substrate with compost and GWB showed the best germination rate (Table 3). In soybean, the germination rate ranged between 56.94% (+ Scl ‘Control’) and 79.17% (–Scl ‘WB + Comp’ and ‘GWB + Comp’) and was lower in the presence of the pathogen (+ Scl) (Table 3).
Disease incidence assessment
The incidence of Sclerotinia disease in sunflower and soybean plants was evaluated 20 d after seeding. The only treatment that reduce disease incidence, compared with the compost-free substrates, was ‘GWB + Comp.’ At 36.11%, the disease incidence in ‘GWB + Comp’ was approximately half that in the control treatment (69.44%). A comparable but less pronounced scenario was observed for soybean. Here again, the highest disease suppression was provided by ‘GWB + Comp,’ followed by ‘WB + Comp’ (Table 4).
In vitro mycelial growth in root exudates
Mycelial growth of S. sclerotiorum was recorded in root exudates from pathogen-free plants and plants inoculated with the pathogen. Acetate buffer, which was also used for root exudate collection, served as the reference medium. The results showed significantly higher mycelial growth in sunflower root exudates from –Scl plants than in + Scl sunflower plants. On the other hand, the substrate did not influence mycelial growth. In soybean, the reverse was found—crop inoculation with S. sclerotiorum did not affect the mycelial growth pattern in root exudates, while the substrate affected mycelial growth and showed significant interactions with Scl. In root exudates from –Scl soybean plants, ‘WB + Comp’ resulted in the lowest mycelial growth, whereas in + Scl plants, ‘GWB + Comp’ was the lowest (Table 5).
Discussion
Organic amendments, such as compost and biochar, are widely used in agriculture. The material used and the crop can affect the performance of the organic amendments. In the present study, we used compost, green waste biochar, and wood biochar in soybean and sunflower plants in the presence and absence of S. sclerotiorum. We found little difference between the two plant species studied with regard to disease incidence, but there were differences in the other parameters studied. Sunflower is known for myceliogenic germination of S. sclerotiorum (Huang and Dueck 1980). The main Sclerotinia wilt infection in sunflower occurs at or below the soil line, characterized by root rot and further extending into the hypocotyl axis. In contrast, soybean is known for the carpogenic germination of the pathogen (Cline and Jacobsen 1983). It has been reported that the primary infection court of germinated ascospores in soybean plants is the shoots or branches of the shoots and leaf axils. Therefore, S. sclerotiorum is expected to have a more negative impact on the root system of sunflower than that of soybean plants when infected by the mycelium, as in our study. Interestingly, although both plant species reacted differently in terms of their growth characteristics, the disease incidence was similar. Only the combination of compost and green waste biochar reduced disease incidence in both plant species compared with the standard substrate. Sunflower and soybean showed a similarly high disease incidence in the control variant on the one hand, and in the GWB variant on the other. The data show that myceliogenic infection can also lead to high disease incidence in soybean, at the level of carpogenic infection (Safaei Asadabadi et al. 2021). However, we found differences in seed germination rates upon pathogen inoculation. In sunflowers, germination rates were significantly increased in some substrates; the data suggest that the addition of compost partially offsets the negative effects of the pathogen. Nonetheless, in the non-inoculated variants, the germination rate was largely uniform. In comparison, in soybean, the seed germination rate was significantly reduced by inoculation with S. sclerotiorum, without effect of the kind of substrate.
The feedstock, as well as the properties of the final compost, determines the outcome of the effect in a certain pathosystem, which is not the same for every pathogen. For example, SerraWittling et al. (1996) showed that the application of compost from municipal solid waste significantly reduced Fusarium wilt in flax. Termorshuizen et al. (2006) compared 18 commercial composts made from different feedstocks and tested them in pathosystems, including Fusarium, Verticillium, Phytophthora, Cylindrocladium, and Rhizoctonia species. They achieved a disease reduction of 54%, an increase of 3%, and no effects in the other pathosystems. Comparable data for compost are available from Scheurell et al. (2005) for Pythium and Rhizoctonia species. For biochar, disease-reducing effects are also known for the pathogens mentioned, as well as for Botrytis, Colletotrichum, Leveillula, and Podosphaera species (Bonanomi et al. 2015; Akther et al. 2015). However, our data showed that although the organic amendments did not result in significant differences with regard to disease incidence (except for the GWB and compost combination), individual substrate combinations can provide better protection of the seedling against S. sclerotiorum depending on the host plant. Numerous studies reported that both compost (Zhang et al. 1998; Pharand et al. 2002; Paplomatas et al. 2005; Coventry et al. 2005; Noble and Coventry 2005) and biochar (reviewed in Graber et al. 2014) can induce systemic resistance in host plants. Differences in soil microbial activity depending on plant species and associated changes in metabolic structures could be suggested as another explanation (Pérez-Piqueres 2006). In our study, however, the comparison of plants inoculated with S. sclerotiorum with those not inoculated indicates that this effect may be due to growth stimulation rather than a direct effect or induced resistance.
In our study, sunflowers responded to compost-containing soil amendments, in contrast to soybean, in terms of plant growth. In soybeans, the addition of compost did not result in a general growth-promoting effect. Compost alone did not show better shoot dry matter than the control treatment; here, biochar addition was more effective in increasing shoot biomass. The pathogen inoculated sunflowers performed worse than the pathogen-free plants; in soybean, there was no difference in this respect. The positive effect of compost on sunflower shoot growth might be due to the presence and availability of various constituent nutrients of compost in the soil. The addition of compost enhanced the nitrogen content, which may have promoted the growth of sunflower shoots. In soybean, one could assume that the nitrogen effect is masked by nitrogen-fixing rhizobia. In our study, however, we did not detect any nodulation. Furthermore, the modulation of soil pH by adding compost and biochar might have affected nutrient availability. Sunflowers are known to show the best growth performance on well-drained soil with an almost neutral pH (6.5–7.5) (Gonzalez-Perez and Vereijken 2007) and thus might be favored by compost addition. The frequently mentioned nutrient effect of biochar (Glaser et al. 2002; Lehmann et al. 2009; Sohi et al. 2010) did not seem to be present in sunflower. The best nutrient availability and biological nitrogen fixation in soybeans are between 6.3 and 6.5 pH (Staton 2012). Interestingly, although a high pH value characterized both biochars in our study, the amendment of biochar alone or in combination with compost resulted in increased shoot biomass in soybean. The question arises as to why compost had a better effect on shoot growth in sunflower, whereas biochar led to better results in soybean. One explanation could be found in the lower nitrogen requirement at this early growth stage of soybean (George and Singleton 1992). The importance of host plant nutritional status for disease development of S. sclerotiorum in sunflowers or soybeans has not been sufficiently studied. On the one hand, increasing nitrogen has been attributed to an increased disease incidence in mustard (Shukla 2005). On the other hand, low nitrogen supply has been associated with disease reduction in carrot (Couper 2001), while studies in oilseed rape (Söchting and Verreet 2004) indicate the opposite. Our data showed no consistent growth effects of the ‘GWB and compost’ variants, which are characterized by the lowest disease incidence. The nutrient effect is therefore likely to play only a minor role in disease development. A recent study (Xu et al. 2015) has shown that pH has a significant influence on the growth, reproduction, pathogenicity and virulence of S. sclerotiorum. This fact could explain the lower disease incidence of the combination ‘GWB and Compost’ in our study, as it can be assumed that the addition of these two components caused the greatest pH change in the substrate. The pH differences could also be responsible for the tendency of GWB to have a stronger impact compared to WB. Furthermore, this could also explain the effects on the germination rate of the + Scl variants in sunflower and at least tendentially in soybean.
In the present study, the root dry matter was similar in all uninoculated sunflowers but higher in inoculated plants treated with compost (alone and in combination with biochar). Again, the patterns in soybean differed. Notably, substrates amended with compost alone had the lowest root dry matter in both –Scl and + Scl soybean plants. The reason for this remains unclear. In general, nutrients such as potassium, phosphorus, calcium, zinc, and organic matter are described as determining factors for the growth of soybean roots (Müller et al. 2021). However, soybean and sunflower seeds differ significantly in their composition. Soybean comprises approximately 36% protein and 19% oil (Liu 1997), while sunflower seeds contain approximately 20% protein and 35–42% oil (Guo et al. 2017). During germination and seedling development, changes in nutrient catabolism and degradation of carbohydrates, proteins, and lipids occur, accompanied by an increase in free amino acids and organic acids (Aguilera et al. 2013). The seed constituents can largely meet the nutrient requirements in the early development phase, but the different seed compositions might explain the different responses of both plant species.
Root exudates from sunflower plants are known to promote the myceliogenic germination of sclerotia (Saharan and Mehta 2008). Our study was related to mycelial development. Mycelial growth increased by adding soybean exudates compared with control. We measured the highest mycelial growth in the root exudates of non-inoculated sunflowers and inoculation with the pathogen increases mycelial growth by sunflower exudates, whereas the different substrates had no effect. We therefore assume that inoculation of sunflowers with S. sclerotiorum changes the composition of the exudate. Metabolic changes associated with S. sclerotiorum infection in sunflowers are well documented. In cotyledons of sunflower, mainly carbohydrates such as glucose, fructose, sucrose and glutamate were detected. Infection with S. sclerotiorum leads to a decrease in sugars and amino acids, especially sucrose and fructose (Jobic et al. 2007). The sunflower capitulum changes in metabolites, such as major and minor sugars and sugar alcohols, organic acids, amino acids, fatty acids and others, were found upon S. sclerotiorum infection (Peluffo et al. 2010). In particular, changes in the composition of sugars and organic acids in root exudates due to pathogen infestation are known from other pathosystems, such as F. oxysporum and tomato (Hage-Ahmed et al. 2013). However, root exudates from the two plants examined in our study resulted in different mycelial growths of S. sclerotiorum. Root exudates of soybean have been attributed to the stimulation of apothecia formation (Chaves et al. 1996); however, this could not be confirmed by our experimental approach. Nevertheless, considering uninoculated plants, our work indicates a different exudation pattern of the two plants. The effects of infection with the pathogen on root exudation appear to be host plant specific in the case of S. sclerotiorum, which is in contrast to the results of studies on other fungi. In the arbuscular mycorrhizal fungus–plant system, the change in root exudation after fungal inoculation is hardly determined by the host plant (Scheffknecht et al. 2007).
The application of compost and biochar can alter the quality and composition of root exudates in terms of their effects on soil-borne fungi (Akther et al. 2015). In our study, the picture differed depending on the plant species used. In contrast to sunflower, some substrate combinations had a decisive influence on mycelial growth in soybean exudates, while inoculation with the pathogen had no effect. Root exudation and growth are determined by nutrient availability and other biotic and abiotic factors (Badri and Vivanco 2009). In particular, compost is considered a source of nutrients for microorganisms and therefore may favor the development of microflora (Bailey and Lazarovits 2003). The differences in nutrient supply could have led to different changes in the exudation of the plants, depending on the substrate composition. Our work is contradictory in this respect. The combination of compost and biochar resulted in the lowest mycelial growth in soybean; in particular, exudates from uninoculated plants showed the lowest value in WB and GWB in inoculated plants. A detailed analysis of the chemical composition of root exudates can provide more precise information.
In conclusion, our study has clarified some effective components of organic materials for control S. sclerotiorum. Soil amendments such as the addition of biochar and compost can not only improve some plant growth parameters but also have a favorable effect against S. sclerotiorum infection. However, our study indicates that not only the pathogen, but also the host plant is a decisive factor for the mode of action of the supplemented organic material.
References
Abawi GS, Grogan RG (1975) Source of primary inoculum and effects of temperature and moisture on infection of beans by Whetzelinia sclerotiorum. Phytopathology 65:300–309. https://doi.org/10.1094/Phyto-65-300
Agegnehu G, Srivastava AK, Bird MI (2017) The role of biochar and biochar-compost in improving soil quality and crop performance: a review. Appl Soil Ecol 119:156–170
Aguilera Y, Díaz MF, Jiménez T, Jiménez T, Benítez V, Herrera T, Cuadrado C, Martín-Pedrosa M, Martín-Cabrejas MA (2013) Changes in nonnutritional factors and antioxidant activity during germination of nonconventional legumes. Jagr Food Chem 61:8120–8125. https://doi.org/10.1021/jf4022652
Akhter A, Hage-Ahmed K, Soja G, Steinkellner S (2015) Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp lycopersici. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00529
Antal MJ, Gronli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42:1619–1640. https://doi.org/10.1021/ie0207919
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98. https://doi.org/10.1016/j.tplants.2013.11.006
Bailey KL, Lazarovits G (2003) Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res 72:169–180. https://doi.org/10.1016/s0167-1987(03)00086-2
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Can J Plant Pathol 16:93–108. https://doi.org/10.1080/07060669409500766
Bonanomi G, Ippolito F, Scala F (2015) A “black” future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J Plant Pathol 97:223–234. https://doi.org/10.4454/jpp.v97i2.3381
Butler TJ, Han KJ, Muir JP, Weindorf DC, Lastly L (2008) Dairy manure compost effects on corn silage production and soil properties. Agron J 100:1541–1545. https://doi.org/10.1080/1065657X.2009.10702395
Chan KY, Xu ZH, Lehmann J, Joseph S (2009) Biochar: nutrient properties and their enhancement. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 67–84
Chaves MS, Martinelli JA, Loch LC (1996) Effect of root exudates of soybeans on carpogenic germination of Whetzelinia sclerotiorum sclerotia. Summa Phytopathol 22:256–258
Cline MN, Jacobsen BJ (1983) Methods for evaluating soybean cultivars for resistance to Sclerotinia sclerotiorum. Plant Dis 67:784–786. https://doi.org/10.1094/pd-67-784
Couper G (2001) The biology, epidemiology and control of Sclerotinia sclerotiorum on carrots in North East Scotland. University of Aberdeen, Aberdeen, Scotland
Coventry E, Noble R, Mead A, Whipps JM (2005) Suppression of Allium white rot (Sclerotium cepivorum) in different soils using vegetable wastes. Eur J Plant Pathol 111:101–112. https://doi.org/10.1007/s10658-004-1420-0
Curtis MJ, Claassen VP (2005) Compost incorporation increases plant available water in a drastically disturbed serpentine soil. Soil Sci 170:939–953. https://doi.org/10.1097/01.ss.0000187352.16740.8e
Elad Y, David DR, Harel YM, Borenshtein M, Ben Kalifa H, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 100:913–921. https://doi.org/10.1094/phyto-100-9-0913
Epstein E (1997) Science of composting. Technomic Publishing Company, Lancaster U.S.A, p 487
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51(6):2061–2069. https://doi.org/10.13031/2013.25409
George T, Singleton PW (1992) Nitrogen assimilation traits and dinitrogen fixation in soybean and common bean. Agron J 84:1020–1028. https://doi.org/10.2134/agronj1992.00021962008400060022x
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - a review. Biol Fertil Soil 35:219–230. https://doi.org/10.1007/s00374-002-0466-4
Gonzalez-Perez S, Vereijken JM (2007) Sunflower proteins: overview of their physicochemical, structural and functional properties. J Sci Food Agr 87:2173–2191. https://doi.org/10.1002/jsfa.2971
Graber ER, Frenkel O, Jaiswal AK, Elad Y (2014) How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag 5:169–183. https://doi.org/10.1080/17583004.2014.913360
Grau CR, Radke VL, Gillespie FL (1982) Resistance of soybean cultivars to Sclerotinia sclerotiorum. Plant Dise 66:506–508. https://doi.org/10.1094/pd-66-506
Guo S, Ge Y, Na Jom KA (2017) Review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem Cent J 11:95. https://doi.org/10.1186/s13065-017-0328-7
Hage-Ahmed K, Moyses A, Voglgruber A, Hadacek F, Steinkellner S (2013) Alterations in root exudation of intercropped tomato mediated by the arbuscular mycorrhizal fungus Glomus mosseae and the soilborne pathogen Fusarium oxysporum f. sp lycopersici. J Phytopath 161:763–773. https://doi.org/10.1111/jph.12130
Hancock JG (1972) Changes in cell-membrane permeability in sunflower hypocotyls infected with Sclerotinia sclerotiorum. Plant Physiol 49:358–364. https://doi.org/10.1104/pp.49.3.358
Hassan AK (2017) Induction of systemic resistance of eggplant against Sclerotinia sclerotiorum infection using biochar and bio-health. Pakistan J Biotechnol 14:653–661
Huang HC, Dueck J (1980) Wilt of sunflower from infection by mycelial germinating sclerotia of Sclerotinia sclerotiorum. Can J Plant Pathol 2:47–52. https://doi.org/10.1080/07060668009501437
Jobic C, Boisson AM, Gout E, Rascle C, Fevre M, Cotton P, Bligny R (2007) Metabolic processes and carbon nutrient exchanges between host and pathogen sustain the disease development during sunflower infection by Sclerotinia sclerotiorum. Planta 226:251–265. https://doi.org/10.1007/s00425-006-0470-2
Lehmann J, Joseph S (2009) Biochar for environmental management: Science and Technology. Routledge, London, pp 1–12
Lehmann J, da Silva JP, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357. https://doi.org/10.1023/a:1022833116184
Liu KS (1997) Soybeans: Chemistry, technology, and utilization. Chapman and Hall, London
Mehta CM, Palni U, Franke-Whittle IH, Sharma AK (2014) Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manage 34:607–622. https://doi.org/10.1016/j.wasman.2013.11.01
Monteiro FP, Pacheco LP, Lorenzetti ER, Armesto C, de Souza PE, de Abreu MS (2012) Exudates of cover crops in the development of Sclerotinia sclerotiorum. Biosci J 28(1):87–93
Müller M, Schneider JR, Klein VA et al (2021) Soybean root growth in response to chemical, physical, and biological soil variations. Front Plant Sci 12:602569. https://doi.org/10.3389/fpls.2021.602569
Noble R, Coventry E (2005) Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci Tech 15:3–20. https://doi.org/10.1080/09583150400015904
Paplomatas EJ, Tjamos SE, Malandrakis AA, Kafka AL, Zouvelou SV (2005) Evaluation of compost amendments for suppressiveness against Verticillium wilt of eggplant and study of mode of action using a novel Arabidopsis pathosystem. Europ J Plant Pathol 112:183–189. https://doi.org/10.1007/s10658-005-3502-z
Paulin B, O'Malley P (2008) Compost production and use in horticulture. Department of Primary Industries and Regional Development, Western Australia, Perth. Bulletin 4746
Pedra F, Plaza C, Fernández JM, García-Gil JC, Polo A (2008) Effects of municipal solid waste compost and sewage sludge on chemical and spectroscopic properties of humic acids from a sandy Haplic Podzol and a clay loam Calcic Vertisol in Portugal. Waste Manage 28:2183–2191. https://doi.org/10.1016/j.wasman.2007.09.031
Peluffo L, Lia V, Troglia C, Maringolo C, Norma P, Escande A, Hopp HE, Lytovchenko A, Fernie AR, Heinz R, Carrari F (2010) Metabolic profiles of sunflower genotypes with contrasting response to Sclerotinia sclerotiorum infection. Phytochemistry 71:70–80. https://doi.org/10.1016/phytochem.2009.09.018
Perez-Piqueres A, Edel-Hermann W, Alabouvette C, Steinberg C (2006) Response of soil microbial communities to compost amendments. Soil Biol Biochem 38:460–470. https://doi.org/10.1016/j.soilbio.2005.05.025
Pharand B, Carisse O, Benhamou N (2002) Cytological aspects of compost-mediated induced resistance against Fusarium crown and root rot in tomato. Phytopathology 92:424–438. https://doi.org/10.1094/phyto.2002.92.4.424
Safaei Asadabadi R, Hage-Ahmed K, Steinkellner S (2021) Biochar, compost and arbuscular mycorrhizal fungi: a tripartite approach to combat Sclerotinia sclerotiorum in soybean. J Plant Dis Prot 128:1433–1445. https://doi.org/10.1007/s41348-021-00495-2
Saharan GS, Mehta N (2008) Sclerotinia diseases of crop plants: biology, ecology and disease management. Springer, Dortrecht, London
Scheffknecht S, St-Arnaud M, Khaosaad T, Steinkellner S, Vierheilig H (2007) An altered root exudation pattern through mycorrhization affecting microconidia germination of the highly specialized tomato pathogen Fusarium oxysporum f. sp.lycopersici (Fol) is not tomato specific but also occurs in Fol nonhost plants. Can J Bot 85:347–351. https://doi.org/10.1139/B07-015
Scheuerell SJ, Sullivan DM, Mahaffee WF (2005) Suppression of seedling damping-off caused by Pythium ultimum, P. irregulare, and Rhizoctonia solani in container media amended with a diverse range of Pacific Northwest compost sources. Phytopathology 95:306–315. https://doi.org/10.1094/Phyto-95-0306
SerraWittling C, Houot S, Alabouvette C (1996) Increased soil suppressiveness to Fusarium wilt of flax after addition of municipal solid waste compost. Soil Biol Biochem 28:1207–1214. https://doi.org/10.1016/0038-0717(96)00126-5
Shukla AK (2005) Sclerotinia rot-its prevalence in Indian mustard at different levels of nitrogen. Indian Phytopathol 58:493–494
Söchting HP, Verreet JA (2004) Effects of different cultivation systems (soil management, nitrogen fertilization) on the epidemics of fungal diseases in oilseed rape (Brassica napus L. var. napus). J Plant Dis Prot 111:1–29. https://doi.org/10.1007/BF03356129
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82. https://doi.org/10.1016/s0065-2113(10)05002-9
Staton M (2012) Managing soil pH for optimal soybean production. Michigan State University Extension. https://www.canr.msu.edu/news/managing_soil_ph_for_optimal_soybean_production
Steiner C, Glaser B, Teixeira WG, Lehmann J, Blum WEH, Zech W (2008) Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J Plant Nutr Soil Sci 171:893–899. https://doi.org/10.1002/jpln.20062519
Steinkellner S, Mammerler R (2007) Effect of flavonoids on the development of Fusarium oxysporum f. sp lycopersici. J Plant Interact 2(1):17–23. https://doi.org/10.1080/17429140701409352
Steinkellner S, Mammerler R, Vierheilig H (2005) Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J Plant Interact 1(1):23–30. https://doi.org/10.1080/17429140500134334
Termorshuizen AJ, van Rijn E, van der Gaag DJ, Alabouvette C, Chen Y, Lagerlof J, Malandrakis AA, Paplomatas EJ, Ramert B, Ryckeboer J, Steinberg C, Zmora-Nahum S (2006) Suppressiveness of 18 composts against 7 pathosystems: variability in pathogen response. Soil Biol Biochem 38:2461–2477. https://doi.org/10.1016/j.soilbio.2006.03.002
Thies JE, Rillig M (2009) Characteristics of biochar: biological properties. In: Lehmann J, Joseph S (eds) Biochar for Environmental Management: Science and Technology. Routledge, London, pp 85–105
Xu L, Xiang M, White D, Chen W (2015) pH dependency of sclerotial development and pathogenicity revealed by using genetically defined oxalate-minus mutants of Sclerotinia sclerotiorum. Environ Microbiol 17:2896–2909
Zhang W, Han DY, Dick WA, Davis KR, Hoitink HAJ (1998) Compost and compost water extract-induced systemic acquired resistance in cucumber and Arapidopsis. Phytopathology 88:450–455. https://doi.org/10.1094/phyto.1998.88.5.450
Acknowledgements
We are grateful to Karin Baumgartner for her excellent technical assistance.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). This research did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asadabadi, R.S., Hage-Ahmed, K. & Steinkellner, S. Response of sunflower and soybean to infection with Sclerotinia sclerotiorum with addition of organic amendments. J Plant Dis Prot 129, 1367–1376 (2022). https://doi.org/10.1007/s41348-022-00643-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00643-2