Abstract
Manipulating phytophagous insects with light-based repelling techniques has shown its potential to be a useful tool in integrated pest management systems in the future. Underlying optical mechanisms can be applied in field and in protected cultivation, with reflecting materials or emitting light sources, such as LEDs. Many pest insects are characterised by their cryptic lifestyle to avoid intervening pest protection measurements. In addition, there is a high degree of resistance mechanisms against insecticides in certain species. The idea of most light-repelling techniques is to reduce the immigration and the settlement of pest species on hostplants before population growth even starts. We conducted experiments with narrow-banded blue LEDs arranged around the plants and emitting radiation towards the sky. For compact rosette Lactuca sativa and upright-branched Euphorbia pulcherrima, we tested the suitability of the measure on settlement of Trialeurodes vaporariorum in 2 choice experiments. In further choice experiments with reduced number of untreated plants, T. vaporariorum and Nasonovia ribisnigri were evaluated for the effect on hostplant settlement of the light barrier on lettuce plants under more practical conditions. The light barrier shows high repellent impact on hostplant settlement by greenhouse whitefly, independent of different plant architectures. The modified choice experiment showed strong decrease in hostplant settlement for greenhouse whitefly. For currant-lettuce aphid, tendencies are shown, but no statistical effect could be demonstrated. Possible applications and differences between the insect species used for the experiments are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preventing the infestation of plants by phytophagous insects by physical measures is one of the key factors in minimising chemical use in integrated pest management (IPM) systems (IOBC-WPRS, IBMA, PAN-Europe 2019). The now existing availability of light-emitting diodes (LEDs) with narrow-banded wavelengths and high intensities (HP-LEDs) gives the possibility to adapt specifically tailored light barriers for concepts of integrated plant protection.
Whiteflies are important pests in vegetables and ornamental plants (Byrne and Bellows 1991). Trialeurodes vaporariorum Westwood, the greenhouse whitefly (Hemiptera: Aleyrodidae), is highly polyphagous and infests more than 82 plant families including about 800 species (Mound and Halsey 1978). Trialeurodes vaporariorum is a serious pest in protected cultivation systems in Europe but also in field crops in warmer climates. Due to the hidden and protected lifestyle on the underside of host leaves, chemical control is often only effective using systemic insecticides or special application techniques. However, reports of insecticide resistances are numerous (Gorman et al. 2002, 2007; Luo et al. 2010).
Aphids are one of the economically most important pest of cultivated plants. The currant-lettuce aphid Nasonovia ribisnigri Mosley (Hemiptera: Aphididae) is known to infest more than 160 plant species from 15 different families (Holman 2009), but it is most important as a pest in lettuce (Lactuca sativa var. (Linnaeus)) cultivation (Hommes et al. 2003; Reinink and Dieleman 1993). It is difficult to control with chemicals as well as biological methods, especially due to its preferred accumulation on inner leaves of lettuce heads. Therefore, when growing lettuces, pest control at an advanced stage of lettuce development can only be carried out destructively, since combating the aphids on the heartleaves is very difficult (Diaz et al. 2007). Moreover, many populations already developed high resistance to various insecticides (Kift et al. 2004; Rufingier et al. 1997).
For both pest groups whiteflies and aphids, the control of initial infestation is essential to avoid the establishment of destructive populations. Due to the exponential and fast population growth, these pests quickly develop from single immigrated individuals to large populations.
Colours and colour contrasts have a strong influence on the landing and take-off behaviour of aphids and whiteflies as well. Green and yellow colour reflections often trigger a landing reflex (Moericke 1955, 1969; Shimoda and Honda 2013; Vaishampayan et al. 1975b; Zhang et al. 2020), additionally also controlled by light intensity (brightness), whereas UV components of radiation are most important for controlling above crop flight activity (Döring and Chittka 2007; Stukenberg and Poehling 2019). However, exceptions prove the rule here and are probably host plant dependent (Döring and Chittka 2007; Farnier et al. 2014; Straw et al. 2011).
Regarding visual reception in general, trichromacy was proposed for the green peach aphid M. persicae by Kirchner et al. (2005) after ERG (electroretinogram) measurement of alate female summer migrants. Measurements of the spectral sensitivity of aphids and whiteflies showed the same sensitivity peaks at 550 nm for behavioural sensitivities (Döring et al. 2011; Hardie 1989; Mellor et al. 1997; Nottingham et al. 1991) and around 520–530 nm for physiological sensitivities (Döring et al. 2011; Mellor et al. 1997). The visual behaviour of the greenhouse whitefly was studied more in detail by Stukenberg and Poehling (2019), Stukenberg (2018) and Stukenberg et al. (2015). They showed recently that the preference for yellow colours is based on a colour opponent mechanism of green and blue, and that target attractiveness can be much enhanced if green without inhibiting influence of blue is offered, vice versa additional blue showed a repellent effect. Stukenberg and Poehling (2019) also confirmed Coombe (1981) who showed that T. vaporariorum responds differently depending on the light intensity as well as on its wavelength. Like Legarrea et al. (2012a), we assume that comparable reactions in the host finding and alighting process in whiteflies and aphids occur, as the similarities in colour vision are quite evident (Hardie 1989; Prokopy and Owens 1983).
UV-light is relevant for aphid and whitefly orientation, especially in space. They use the dorsal-light reaction (Goodman 1965) for orientation between ground and sky. A shift in the ratio of ambient UV-light above crop stands by UV-blocking film tunnels, or greenhouse cover glasses can strongly reduce immigration. Manipulation of UV reflection from the ground by reflecting mulches on the other hand leads to increased take-off behaviour (Antignus et al. 2001; Doukas and Payne 2007), host-seeking (Antignus 2000; Legarrea et al. 2012a), dispersal (Dáder et al. 2017; Mutwiwa et al. 2005) and prevention of virus-spread (Antignus et al. 2001; Antignus and Ben-Yakir 2004; Kumar and Poehling 2006; Legarrea et al. 2012b; Stukenberg and Poehling) by aphids, whiteflies and other light sensitive pest species (Díaz et al. 2006; Johansen et al. 2011a). Moreover, the behavioural reaction of insects to light is not only depending on the light source, but as well on the physiological status of the insect and abiotic factors (Döring and Chittka 2007; Kim et al. 2019; Moericke 1962; Shimoda 2018). For T. vaporariorum, Stukenberg and Poehling (2019) showed that UV-light has a migratory effect.
In a previous greenhouse study, it was described that T. vaporariorum showed reduced infestation of lettuce plants (Lactuca sativa L. var. capitata) when they are grown on white foil with high reflection in the blue range between 400 and 490 nm. (Niemann et al. 2021). That leads to the hypothesis that a shift in the ratio of blue light from the sky and/or the reflecting ground could show the same avoiding effect as shown for UV-deficient ambient. Studies using artificial light sources to shift the UV-ratio are rare. Mutwiwa and Tantau (2005) showed reduced number of T. vaporariorum in UV deficient ambient compartments, in choice tests using UV-emitting fluorescent lamps (340–380 nm) to create UV rich ambient. Stukenberg et al. (2015), and Stukenberg and Poehling (2019) used UV-A and near-UV narrow-banded LEDs (light-emitting diode) for their research on T. vaporariorum. However, we could not find any publication (except the patent publication) where the UV-ratio is shifted by emitting light sources from the ground to repel insect pest species. Usually, UV-light is used as an additional trigger which is added to other wavelength of the spectra to enhance trap attractiveness (Johansen et al. 2011b; Zhang et al. 2020) or as an attractant on its own (Park and Lee 2017).
To confirm the blue–green opponency described above for T. vaporariorum and to examine it for N. ribisnigri, as it was shown for aphids in general (Döring and Chittka 2007; Hardie 1989), investigations were carried out with a patented prototype (Rakoski and Stukenberg 2018) which was kindly provided by the patent owners. Aim was to test the suitability of the patented system for practical plant protection.
Materials and methods
Experimental plants and insects
Lettuce plants (Lactuca sativa L. var. crispa, Rijk Zwaan, cultivar Diamantinas) were used as experimental host plant for both experiments. Plants were grown in 12 cm PET pots (Teku®). Growing conditions were 21° ± 1 °C during the day (6 a.m. to 10 p.m.) and 17 ± 1 °C at night (10 p.m. to 6:00 a.m.) in a climate chamber. Lettuce plants in the phenological BBCH (Biologische Bundesanstalt (now Julius-Kühn Institut), Bundessortenamt, Chemische Industrie)-scale stage 16 to 18, representing seedlings with 6–8 fully developed leaves (Feller et al. 1995), were used for the experiments. Height was 9 ± 1 cm.
Poinsettia plants (Euphorbia pulcherrima Willd. ex Klotzsch) served as secondary experimental host plant for the first experiment. They were potted as cuttings and grown under the same conditions as the lettuce plants. Plants were used at phenological BBCH stage 18–20 for the experiments. Poinsettia plants had about the same leaf area as the lettuce seedlings and a height of 24 ± 2 cm.
Adult T. vaporariorum and alate N. ribisnigri were obtained from separate rearing stocks. Trialeurodes vaporariorum was reared on tobacco (Nicotiana tabacum L.), N. ribisnigri on ice-lettuce (same cultivar as for experiments). Acceptance and suitability for reproduction of used lettuce cultivar by T. vaporariorum were confirmed before starting the experiments. Since no eggs were found in the aphid rearing, we concluded that the alate aphids used in the experiments were summer migrants. Both cultures were held at 21 °C and 16 h light exposure (Son-t Agro 400 W) in gauze-covered wooden cages with clear plastic tops (acrylic glass, 3 mm). For experiments, insects were gently removed from rearing stocks with an aspirator (aphids with a fine brush) and transferred into a glass tube (height: 10 cm, diameter: 2.9 cm) approx. 20 min. before the experiment started and kept in the experimental greenhouse to adapt on light situation. Vitality was checked visually before use.
Location and experimental setup
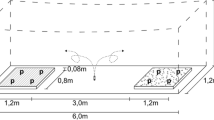
Experiments were carried out in a shaded greenhouse environment at 21 ± 2 °C in the Institute of Horticultural Production Systems, Herrenhäuserstr. 2, 30419 Hannover, Germany. Size of the greenhouse cabinet was 10 m × 10 m. Choice experiments were performed in flight cages (size: 1.2 m × 6.0 m × 2.0 m—see Fig. 1) made of white mesh, arranged in the middle of the greenhouse. Cages were placed in a distance of 2 m to each other on the long side and more than 2 m in each direction to the heating system. Flight cages were used to enable removing of unsettled insects from the experimental arena for sequential repetitions of the experiment. Additional light (Son-t Agro 400 W) was applied during experiments from 6 am to 10 pm.
Experiment setup phase I/II schematic. Flight trials in gauze tents (6 m × 1.2 m × 2 m) shown as scattered line, = ⊗take-off point. Three LED panels (blue bars, control treatment without). Plant ratio 4:4, plant spacing 32 cm × 32 cm. Experimental plants: Lactuca sativa L. (iceberg lettuce), BBCH 16–18; Euphorbia pulcherrima (poinsettia), cuttings (8–10 leaves) in a pot (Ø 12 cm; H = 12 cm)
Flight cages were divided into three compartments (Fig. 1): the whiteflies release area in the middle and one settlement plot on each site of the cage. These plots consisted of plants, placed in a square (32 cm × 32 cm planting distance). One of the treatment plots contained a modified light-based insect repellent device based on a patented concept (Rakoski and Stukenberg 2018) around the plants. The device consisted of LED Panels, made from 2 mm steel sheets (450 mm × 50 mm × 120 mm). Each panel had three high power (HP) LEDs (blue, 470 nm peak, 2.5 W, 3.5 V, 700 mA, constant current, Art.nr. 77800045, World trading Net GmbH, Bleicherode, Germany) fixed with thermal conductive double-sided adhesive tape (Ak-tt12-80, Akasa Ltd., Greenford, UK) one in the middle of the panel, the other two also centrally in 20 cm distance to the first. Constant current was realised with LED drivers (DC Mini Jolly 123400, TCI, Saronno Italy). The control cage remained without a device (blue bars in Figs. 1 and 2).
Experiment setup phase II/II schematic. Flight trials in gauze tents (6 m × 1.2 m × 2 m) shown as scattered line, = ⊗take-off point. Six LED panels (blue bars, control treatment without). Plant ratio 10:2, plant spacing 32 cm × 32 cm. Experimental plants: Lactuca sativa L. (iceberg lettuce), BBCH 16–18
Experimental design
Phase I/II: Test on suitability of the light emitting device with different plant architectures
We conducted two different types of choice experiments to gain different kinds of information. The first experiment was a 2-choice experiment. Aim of the experiment was to “calibrate” the device (Is it working as described in the patent publication?) and to check if plant height had an influence on the effectiveness of the device, or if the height of the repelling device had to be adjusted to plant height. On each side of the flight cages, four plants were arranged in a square as described before. Three LED panels were placed between the “rows”. First experiment was done with T. vaporariorum only, but with two different plant species (Poinsettia and Lettuce) and types of plant architecture (upright branched and compact rosette) blockwise in two chronologically separated trials. To exclude possible influence of odours by the plants, experiments with E. pulcherrima were separated from those with L. sativa. Each trial (experiments per day) consisted of a control and two experimental variants. The control showed no LED panels regardless the side of the flight cage. The two variants only differ in the direction, where the panels were placed. One cage with LEDs was in direction north south, the other with LEDs south north. Three trials per day were conducted in three equal flight cages as described before, which were changed in position randomly (L. sativa n = 27, E. pulcherrima n = 12). In every trial and repetition, 25 insects were released. 24 h later settled insects on plants were counted.
Phase II/II: Testing the stability of the preliminary results with a changed plant ratio
Since no effect was shown by orientation or plant architecture on the performance of the device, the second type of experiment was designed to test the effect of the light-based device under more practical conditions. The number of plants on one side of the flight cage, surrounded by LEDs, was set up to 10; the number of panels was six. On the other side of the cage, 2 trap plants were placed. Nasonovia ribisnigri and T. vaporariorum were used in the experiments. To exclude influences of the insect species on each other, trials with N. ribisnigri were block wise chronologically separated from those with T. vaporariorum. For all trials (and repetitions) in the second experiment, L. sativa was used as experimental plant (T. vaporariorum n = 16, N. ribisnigri n = 12). In every trial, one cage contained LEDs and a control cage remained with no LED panels (blue bars in Fig. 2). Orientation of cages was changed daily.
Statistics
Statistic was done using the software R (R Core Team 2017). Since the observations were based on the proportions of recaptured insects on plants with LED illumination or without, we use this proportion as response variable for model building and graphical representation. Generalised linear models with quasibinomial distribution were fitted. Explanatory factors were the treatment (LED, No LED) and the date of the consecutive trials (block factor). Based on this model, ANOVA was run and pairwise mean comparisons of the effects of the LED treatments were done using the emmeans package (Lenth 2018). All tests were run with α = 0.05. Graphics were made using R Studio (RStudio Team 2015) and the ggplot2 (Wickham 2016) package.
Results
The proportion of recaptured T. vaporariorum (insects on LED plants/insects on LED and No LED plants) 24 h after release on treated (LED) and untreated (No LED) Lactuca sativa plants is shown in Fig. 3a. The proportion of T. vaporariorum settled on treated plants LED was 0.44 at maximum; minimum was 0.0 (six times) with a mean value of 0.12. The control plants (No LED) showed a mean proportion of 0.53 with a maximal value of 0.7 and a minimum of 0.4. The corresponding graph for the proportion of recaptured T. vaporariorum 24 h after release on treated (LED) and untreated (No LED) Euphorbia pulcherrima plants is shown in Fig. 3b. The treated plants (LED) showed a mean proportion of 0.10 with a maximal value of 0.25 and a minimum of 0.0 (four times). The proportion of T. vaporariorum on untreated plants was 0.63 at maximum; minimum was 0.46 with a mean value of 0.54. The treatment with the narrow-banded blue 470 nm LEDs showed significant reduction in the number of whiteflies settling on both plant species during the experiment, compared to the non-treated plants.
Proportion of Trialeurodes vaporariorum on Lactuca sativa (a) or Euphorbia pulcherrima (b) plants with (LED) or without (No LED) LED-repelling device (n = 27 (a), n = 12 (b), Plant ratio 4:4). Mean values are marked with crosses, 0.5 proportion is indicated by dashed line. Pairwise mean comparisons significance codes: ***ρ < 0.001, **ρ < 0.01, *ρ < 0.05
Figure 4a shows the proportion of recaptured N. ribisnigri (insects on LED plants/insects on LED and No LED plants) 24 h after release on treated (LED) and untreated (No LED) Lactuca sativa plants. The treated plants showed a mean proportion of 0.35 with a maximal value of 0.63 and a minimum of 0.14. The proportion of N. ribisnigri on untreated plants was 0.6 at maximum; minimum was 0.38 with a mean value of 0.47. It is obvious from the graph that only a slight but not significant reduction in settling aphids could be determined.
A corresponding graph for T. vaporariorum is given in Fig. 4b. The proportion of recaptured T. vaporariorum on treated plants (LED) was 0.33 at maximum; mean was 0.21 with a minimum of 0.0. For untreated plants (No LED), the proportion was 0.65 at maximum; mean was 0.60 with a minimum of 0.55. The proportion of recaptured T. vaporariorum was higher than in the first experiment (Fig. 3) but still significantly reduced, compared to the non-treated plants.
Discussion
The results showed that the light repelling device is especially suitable for reducing the alighting and settlement of T. vaporariorum. As we used LEDs with 470 nm (blue), the experiment is a proof for the proposed blue–green opponency (Stukenberg and Poehling 2019) of this species.
For N. ribisnigri, there seems to be only a slight repellent effect, not statistically significant. The recapture rate for N. ribisnigri was lower than of T. vaporariorum, which was probably caused by the strong motivation for long distance dispersal of the summer migrants only available for the studies (Kennedy et al. 1961; Moericke 1955). For various species, it has been shown that winged aphids (Johnson 1958; Kennedy and Booth 1963) and other insects (Graham 1959) are attracted to light before their maiden flight and that this dispersal impulse is suppressed by flight, or even long walking distances. Since aphids are not able to settle and reproduce on a host when in the dark (Johnson 1958, 1959) flight seems to be a positive trigger for alighting, settlement and larviposition in addition to, but not because of its effect on light responses (Kennedy and Booth 1963).
Another reason for the different reaction of both species may be the morphology of the visual system. Trialeurodes vaporariorum eyes are divided in a dorsal region with 54 to 55 ommatidia and a ventral region with 29 to 31 ommatidia per eye (Mellor et al. 1997). For N. ribisnigri, there are no available data about number of ommatidia, but a study by Döring and Spaethe (2009) showed a median number of 165 ommatidia for 14 aphid species. Since the lowest number of ommatidia in the mentioned study was 127 (Rhopalosiphum padi L.) per eye, we assume a better visual acuity and resolution for N. ribisnigris eyes than for those of T. vaporariorum based on the different number of ommatidia. This could enable N. ribisnigri to distinguish the lettuce from the background despite the disturbing blue illumination from below. However, influences of divided eyes (whiteflies) and spherical eyes (aphids) on field of vision and resolution are unknown.
Other materials, besides the repellent LEDs, showed the spectral peak at 470 nm mentioned before (blue foils, white foils, silver/reflective foils) but do not consistently show the expected repellent effect (Greer and Dole 2003). This indicates that the triggering or prevention of the alighting approach is not only determined by the individual wavelength, but that the mechanism is subject to a complex interaction of various light parameters. According to Antignus (2000; Vaishampayan et al. 1975a), to be able to distinguish a potential host from its surroundings, these parameters are primarily the dominant emitted hue or the maximum wavelength (1), the colour saturation (2) and the brightness (light intensity) compared to the surroundings (3). In the present study, with the orientation of the LEDs from the ground upwards, the radiated hue was enriched with a dominant wavelength of 470 nm. This added blue hue (470 nm) was narrow-banded and therefore very pure in hue. The results showed that the blue–green opponency for T. vaporariorum described by Stukenberg and Poehling (2019) is also valid for illumination from the ground in a practical application with LEDs.
The results in the present study showed a strong effect of blue LED illumination from the ground on the settlement of T. vaporariorum initiated by the narrow-band blue light emission at 470 nm. Since the number of alighting whiteflies could not be reduced to zero, for practical use a combination of the light repelling device with secondary measures will be necessary, depending on respective threshold value of the cultivated plant. Especially in fast growing crops with low thresholds for insect damage (e.g. lettuce, seeding nurseries), a combination of optical manipulation and strict monitoring could reduce the number of necessary insecticide applications or other intervening measures. This method could be applied in organic growing as in conventional practice.
Practical application could be promising for greenhouse cultures with high output (e.g. nurseries, fast growing herbs) or high plant values (e.g. medicinal plants). For outdoor use in field cultures, the device would have to be adapted, but LED systems for field use are not implementable economically. To use the repellent effect of the light in the blue range under field conditions, an optical system could be involving reflecting mulch foils with high intensive but narrow-banded reflection, or foil tunnels with high transmission, in the blue range (420–500 nm). However, it is not possible to estimate how stable this effect would be in the field with appropriate foil materials under changed light conditions (compared to the greenhouse), as films also showing reflection effects (e.g. light polarisation, soil effect). Such materials are not commercially available now.
Since only a few model insects can be used for comparisons, further investigation of these optical mechanisms would be desirable. Further studies, both on contrast behaviour and on colour orientation by insects, are necessary to understand the mechanisms in more detail. The use of optical manipulation for plant protection is offering many tools for application, most of them are compatible with other integrated measures in organic farming.
It has always to be kept in mind that the plant production has to stay in focus, and possible side-effects on growth and quality of the product have to be avoided (Paul et al. 2005, 2012). In addition, for use in practice a light-based repellent device has to be tested with natural occurring and/or introduced natural enemies of the targeted pest species, to exclude negative effects on their performance. They have to be adjusted according to plant species, production system and other integrated pest management measurements. The results in this first study about optical manipulation of sucking insects by light-emitting devices from the ground are a very promising basis for further studies under more practical conditions.
References
Antignus Y (2000) Manipulation of wavelength-dependent behaviour of insects: an IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res 71:213–220. https://doi.org/10.1016/S0168-1702(00)00199-4
Antignus Y, Ben-Yakir D (2004) Ultraviolet-absorbing barriers, an efficient integrated pest management tool to protect greenhouses from insects and virus diseases. In: Horowitz AR, Ishaaya I (eds) Insect pest management: field and protected crops. Springer, Berlin, pp 319–335
Antignus Y, Nestel D, Cohen S, Lapidot M (2001) Ultraviolet-deficient greenhouse environment affects whitefly attraction and flight-behavior. Environ Entomol 30:394–399. https://doi.org/10.1603/0046-225X-30.2.394
Byrne DN, Bellows TS (1991) Whitefly biology. Annu Rev Entomol 36:431–457. https://doi.org/10.1146/annurev.en.36.010191.002243
Coombe PE (1981) Wavelength specific behaviour of the whitefly Trialewodes vaporariorum (Homoptera: Aleyrodidae). J Comp Physiol 144:83–90. https://doi.org/10.1007/BF00612801
Dáder B, Moreno A, Gwynn-Jones D, Winters A, Fereres A (2017) Aphid orientation and performance in glasshouses under different UV-A/UV-B radiation regimes. Entomol Exp Appl 163:344–353. https://doi.org/10.1111/eea.12583
Díaz BM, Biurrún R, Moreno A, Nebreda M, Fereres A (2006) Impact of ultraviolet-blocking plastic films on insect vectors of virus diseases infesting crisp lettuce. HortScience 41:711–716. https://doi.org/10.21273/HORTSCI.41.3.711
Diaz BM, Muñiz M, Barrios L, Fereres A (2007) Temperature thresholds and thermal requirements for development of Nasonovia ribisnigri (Hemiptera: Aphididae). Environ Entomol 36:681–688. https://doi.org/10.1603/0046-225x(2007)36[681:ttatrf]2.0.co;2
Döring TF, Chittka L (2007) Visual ecology of aphids—a critical review on the role of colours in host finding. Arthropod Plant Interact 1:3–16. https://doi.org/10.1007/s11829-006-9000-1
Döring TF, Spaethe J (2009) Messungen der Augengröße und Sehschärfe bei Blattläusen (Hemiptera: Aphididae). Entomologia 32:77–84. https://doi.org/10.1127/entom.gen/32/2009/77
Döring TF, Kirchner SM, Skorupsky P, Hardie J (2011) Spectral sensitivity of the green photoreceptor of winged pea aphids. Physiol Entomol 36:392–396. https://doi.org/10.1111/j.1365-3032.2011.00805.x
Doukas D, Payne CC (2007) Greenhouse whitefly (Homoptera: Aleyrodidae) dispersal under different UV-light environments. J Econ Entomol 100:389–397. https://doi.org/10.1093/jee/100.2.389
Farnier K, Dyer AG, Steinbauer MJ (2014) Related but not alike: not all Hemiptera are attracted to yellow. Front Ecol Evol 2:263. https://doi.org/10.3389/fevo.2014.00067
Goodman LJ (1965) The role of certain optomotor reactions in regulating stability in the rolling plane during flight in the desert locust, Schistocerca Gregaria. J Exp Biol 42:385–407
Gorman K, Hewitt F, Denholm I, Devine GJ (2002) New developments in insecticide resistance in the glasshouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest Manag Sci 58:123–130. https://doi.org/10.1002/ps.427
Gorman K, Devine G, Bennison J, Coussons P, Punchard N, Denholm I (2007) Report of resistance to the neonicotinoid insecticide imidacloprid in Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Pest Manag Sci 63:555–558. https://doi.org/10.1002/ps.1364
Graham K (1959) Release by flight exercise of a chemotropic response from photopositive domination in a Scolytid Beetle. Nature 184:283–284. https://doi.org/10.1038/184283b0
Greer L, Dole JM (2003) Aluminum foil, aluminium-painted, plastic, and degradable mulches increase yields and decrease insectvectored viral diseases of vegetables. HortTechnology 13:276–284. https://doi.org/10.21273/HORTTECH.13.2.0276
Hardie J (1989) Spectral specificity for targeted flight in the black bean aphid, Aphis fabae. J Insect Physiol 35:619–626. https://doi.org/10.1016/0022-1910(89)90124-8
Holman J (2009) Host plant catalog of aphids: Palaearctic Region. Springer, Dordrecht, p 468
IOBC-WPRS, IBMA, PAN-Europe (2019) Integrated pest management: working with nature. https://www.iobc-wprs.org/index_news_Resouces_on_IPM.html. Accessed 11 Feb 2020
Johansen NS, Vänninen I, Pinto DM, Nissinen AI, Shipp L (2011a) In the light of new greenhouse technologies: 2. Direct effects of artificial lighting on arthropods and integrated pest management in greenhouse crops. Ann Appl Biol 159:1–27. https://doi.org/10.1111/j.1744-7348.2011.00483.x
Johansen NS, Eriksen AS, Mortensen L (2011b) Light quality influences trap catches of Frankliniella occidentalis (Pergande) and Trialeurodes vaporariorum (Westwood). IOBC/WPRS Bull 68:89–92
Johnson B (1958) Factors affecting the locomotor and settling responses of alate aphids. Anim Behav 6:9–26. https://doi.org/10.1016/0003-3472(58)90004-6
Johnson B (1959) Studies on the degeneration of the flight muscles of alate aphids—II. Histology and control of muscle breakdown. J Insect Physiol 3:367–377. https://doi.org/10.1016/0022-1910(59)90039-3
Kennedy JS, Booth CO (1963) Free flight of aphids in the laboratory. J Exp Biol 40:67–85. https://doi.org/10.1242/jeb.40.1.67
Kennedy JS, Booth CO, Kershaw WJ (1961) Host finding by aphids in the field. Ann Appl Biol 49:1–21. https://doi.org/10.1111/j.1744-7348.1961.tb03587.x
Kift NB, Mead A, Reynolds K, Sime S, Barber MD, Denholm I, Tatchell GM (2004) The impact of insecticide resistance in the currant-lettuce aphid, Nasonovia ribisnigri, on pest management in lettuce. Agric for Entomol 6:295–309. https://doi.org/10.1111/j.1461-9555.2004.00226.x
Kim K-N, Huang Q-Y, Lei C-L (2019) Advances in insect phototaxis and application to pest management: a review. Pest Manag Sci 75:3135–3143. https://doi.org/10.1002/ps.5536
Kirchner SM, Döring TF, Saucke H (2005) Evidence for trichromacy in the green peach aphid, Myzus persicae (Sulz.) (Hemiptera: Aphididae). J Insect Physiol 51:1255–1260. https://doi.org/10.1016/j.jinsphys.2005.07.002
Kumar P, Poehling H-M (2006) Uv-blocking plastic films and nets influence vectors and virus transmission on greenhouse tomatoes in the humid tropics. Environ Entomol 35:1069–1082. https://doi.org/10.1603/0046-225X-35.4.1069
Legarrea S, Weintraub PG, Plaza M, Viñuela E, Fereres A (2012a) Dispersal of aphids, whiteflies and their natural enemies under photoselective nets. Biocontrol 57:523–532. https://doi.org/10.1007/s10526-011-9430-2
Legarrea S, Betancourt M, Plaza M, Fraile A, García-Arenal F, Fereres A (2012b) Dynamics of nonpersistent aphid-borne viruses in lettuce crops covered with UV-absorbing nets. Virus Res 165:1–8. https://doi.org/10.1016/j.virusres.2011.12.012
Lenth R (2018) Emmeans: estimated marginal means, aka least-squares means. R package version 1.2.3. https://CRAN.R-project.org/package=emmeans. Accessed 14 July 2020
Luo C, Jones CM, Devine G, Zhang F, Denholm I, Gorman K (2010) Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot 29:429–434. https://doi.org/10.1016/j.cropro.2009.10.001
Mellor HE, Bellingham J, Anderson M (1997) Spectral efficiency of the glasshouse whitefly Trialeurodes vaporariorum and Encarsia formosa its hymenopteran parasitoid. Entomol Exp Appl 83:11–20. https://doi.org/10.1046/j.1570-7458.1997.00152.x
Moericke V (1955) Über die Lebensgewohnheiten der geflügelten Blattläuse (Aphidina) unter besonderer Berücksichtigung des Verhaltens beim Landen1. Zeitschrift Für Angewandte Entomologie 37:29–91. https://doi.org/10.1111/j.1439-0418.1955.tb00775.x
Moericke V (1962) Über die optische Orientierung von Blattläusen. Zeitschrift Für Angewandte Entomologie 50:70–74. https://doi.org/10.1111/j.1439-0418.1962.tb04410.x
Moericke V (1969) Hostplant specific colour behaviour by Hyalopterus pruni (Aphididae). Entomol Exp Appl 12:524–534. https://doi.org/10.1111/j.1570-7458.1969.tb02550.x
Mound LA, Halsey SH (1978) Whitefly of the world: a systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. British Museum (Natural History) and Wiley, London
Mutwiwa UN, Borgemeister C, Bv E, Tantau H-J (2005) Effects of UV-absorbing plastic films on greenhouse whitefly (Homoptera: Aleyrodidae). J Econ Entomol 98:1221–1228. https://doi.org/10.1603/0022-0493-98.4.1221
Mutwiwa UN, Tantau HJ (2005) Suitability of a UV lamp for trapping the greenhouse whitefly Trialeurodes vaporariorum Westwood (Hom: Aleyrodidae). Commission of Agricultural Engineering (CIGR, Commission Internationale du Genie Rural) E-Journal 2005
Niemann J-U, Menssen M, Poehling H-M (2021) Manipulation of landing behaviour of two whitefly species by reflective foils. J Plant Dis Prot 128:97–108. https://doi.org/10.1007/s41348-020-00394-y
Nottingham SF, Hardie J, Tatchell GM (1991) Flight behaviour of the bird cherry aphid, Rhopalosiphum padi. Physiol Entomol 16:223–229. https://doi.org/10.1111/j.1365-3032.1991.tb00559.x
Park J-H, Lee H-S (2017) Phototactic behavioral response of agricultural insects and stored-product insects to light-emitting diodes (LEDs). Appl Biol Chem 60:137–144. https://doi.org/10.1007/s13765-017-0263-2
Paul ND, Jacobson RJ, Taylor A, Wargent JJ, Moore JP (2005) The use of wavelength-selective plastic cladding materials in horticulture: understanding of crop and fungal responses through the assessment of biological spectral weighting functions. Photochem Photobiol 81:1052–1060. https://doi.org/10.1562/2004-12-06-RA-392
Paul ND, Moore JP, McPherson M, Lambourne C, Croft P, Heaton JC, Wargent JJ (2012) Ecological responses to UV radiation: interactions between the biological effects of UV on plants and on associated organisms. Physiol Plant 145:565–581. https://doi.org/10.1111/j.1399-3054.2011.01553.x
Prokopy RJ, Owens ED (1983) Visual detection of plants by herbivorous insects. Annu Rev Entomol 28:337–364. https://doi.org/10.1146/annurev.en.28.010183.002005
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rakoski M, Stukenberg N (2018) “Vorrichtung und Verfahren zum Schutz von grünen Pflanzen vor herbivoren Insekten“ DE 10 2018 208 424 B3: Vorrichtung und Verfahren zum Schutz von grünen Pflanzen vor herbivoren Insekten(DE 10 2018 208 424 B3)
Hommes M, Siekmann G, Piepenbrock O, Baur U, Fricke A, Thieme T (2003) Reduzierung des Blattlausbefalls an ausgewählten Gemüsekulturen durch Mulchen mit verschiedenen Materialien und Farben. Bundesanstalt für Landwirtschaft und Ernährung, Bonn, Geschäftsstelle Bundesprogramm Ökologischer Landbau. https://orgprints.org/16630/. Accessed 5 Feb 2020
Reinink K, Dieleman FL (1993) Survey of aphid species on lettuce. Bull OILB/SROP 16:56–68
RStudio Team (2015) RStudio: Integrated Development for R. RStudio Inc., Boston, MA. http://www.rstudio.com/.
Rufingier C, Schoen L, Martin C, Pasteur N (1997) Resistance of Nasonovia ribisnigri (Homoptera: Aphididae) to five insecticides. J Econ Entomol 90:1445–1449. https://doi.org/10.1093/jee/90.6.1445
Shimoda M, Honda K (2013) Insect reactions to light and its applications to pest management. Appl Entomol Zool 48:413–421. https://doi.org/10.1007/s13355-013-0219-x
Shimoda M (2018) Recent advances in the optical control of insect pests using light and color. In: Proceedings of the 2018 international symposium on proactive technologies for enhancement of integrated pest management of key crops, pp 87–102. http://210.69.150.18:8080/handle/345210000/9855. Accessed 12 Feb 2020
Straw NA, Williams DT, Green G (2011) Influence of sticky trap color and height above ground on capture of alate Elatobium abietinum (Hemiptera: Aphididae) in Sitka spruce plantations. Environ Entomol 40:120–125. https://doi.org/10.1603/EN09344
Stukenberg N, Poehling H-M (2019) Blue–green opponency and trichromatic vision in the greenhouse whitefly (Trialeurodes vaporariorum) explored using light emitting diodes. Ann Appl Biol 175:146–163. https://doi.org/10.1111/aab.12524
Stukenberg N, Gebauer K, Poehling H-M (2015) Light emitting diode(LED)-based trapping of the greenhouse whitefly (Trialeurodes vaporariorum). J Appl Entomol 139:268–279. https://doi.org/10.1111/jen.12172
Stukenberg N (2018) LED based trapping of whiteflies and fungus gnats: from visual ecology to application. PhD thesis
Vaishampayan SM, Waldbauer GP, Kogan M (1975a) Visual and olfactory responses in orientation to plants by the greenhous whitefly, Trialeurodes vaporariorum (Homoptera : Aleyrodidae). Entomol Exp Appl 18:412–422. https://doi.org/10.1111/j.1570-7458.1975.tb00418.x
Vaishampayan SM, Kogan M, Waldbauer GP, Wooley JT (1975b) Spectral specific responses in the visual behavior of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae). Entomol Exp Appl 18:344–356. https://doi.org/10.1111/j.1570-7458.1975.tb00407.x
Wickham H (2016) Ggplot2: elegant graphics for data analysis. Use R! Springer, Cham
Zhang J, Li H, Liu M, Zhang H, Sun H, Wang H, Miao L, Li M, Shu R, Qin Q (2020) A greenhouse test to explore and evaluate light-emitting diode (LED) insect traps in the monitoring and control of Trialeurodes vaporariorum. Insects. https://doi.org/10.3390/insects11020094
Acknowledgements
This work was funded by the Fachagentur Nachwachsende Rohstoffe (FNR), Germany, under the Grant No. 22008214/14NR082. We like to acknowledge Dr. Niklas Stukenberg and M.Sc. Mirko Rakoski for the possibility to use the patented “Vorrichtung und Verfahren zum Schutz von grünen Pflanzen vor herbivoren Insekten“(DE 10 2018 208 424 B3) as a model for our experimental setup.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Niemann, JU., Poehling, HM. Effect of narrow-banded blue LED device on host plant settlement by greenhouse whitefly and currant-lettuce aphid. J Plant Dis Prot 129, 1217–1225 (2022). https://doi.org/10.1007/s41348-022-00622-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00622-7