Abstract
Anoplophora glabripennis (Motschulsky 1853) (Coleoptera: Cerambycidae), the Asian Longhorned Beetle, is native to temperate and subtropical areas of China and the Korean peninsula. Due to its wide range of host plants, it is considered among the most economically important invasive plant pests. The morphological identification of A. glabripennis larvae can be confirmed by DNA barcoding, but obtaining the specimens from infested trees can be a demanding and challenging task. Therefore, non-invasive diagnostic tools based on DNA extracted from frass samples can be of key importance in phytosanitary surveys. In this study, an in silico generated real-time quantitative PCR test was developed for the detection of A. glabripennis DNA from frass material, which is naturally extruded from larval tunnels through cracks in the bark. Specificity was confirmed against a wide range of other wood-boring insect species frequently encountered during phytosanitary surveys and inclusivity was demonstrated for different populations of A. glabripennis from all main European outbreak areas. The test proved sensitive and reliable in detecting A. glabripennis DNA extracted from woody frass material of Acer saccharinum and Aesculus hippocastanum at least up to the 100-fold dilution. Furthermore, the test allowed the molecular identification of any life stage of the insect, including eggs and young larvae, whose morphological identification is impossible or very challenging. This study provides a reliable and sensitive molecular tool to detect A. glabripennis DNA in woody frass material, thus allowing a non-invasive sampling approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Asian Longhorned Beetle Anoplophora glabripennis (Motschulsky, 1853) is considered one of the most economically damaging invasive plant pests worldwide. Native to China and the Korean peninsula (Cavey et al. 1998; Lingafelter and Hoebeke 2002), this xylophagous insect was introduced to North America (USA and Canada) (Haack et al. 1996) and to Europe (Austria, Belgium, Finland, France, Germany, Italy, Montenegro, the Netherlands, Switzerland and the United Kingdom) (Javal et al. 2019).
An official confirmation via responsible authorities requires phytosanitary measures according to harmonized European Union (EU) legislation. In line to Implementing Decision (EU) 2015/893 (European Union 2015) areas have to be demarcated, where the pest is exposed to eradication measures. It further lays down measures to prevent the introduction into and the spread of A. glabripennis within the EU. To date, some of these outbreaks have been successfully eradicated in Austria, France, Germany, Italy, Montenegro, the Netherlands, Switzerland and the United Kingdom. Nevertheless, single areas are still infested (EPPO 2021) and the constant risk of reintroduction to pest-free areas is a severe danger. Especially, because the beetle is known to attack a wide range of deciduous tree species. Acer, Aesculus, Betula, Populus, Salix, and Ulmus represent the significant majority from all infested tree genera in Europe (Faccoli and Favaro 2016; Tomiczek and Hoyer-Tomiczek 2007).
The European and Mediterranean Plant Protection Organization (EPPO) has registered A. glabripennis in the EPPO A1 list of pests recommended for regulation as quarantine pests continuously for more than twenty years. Furthermore, the European Commission included A. glabripennis in Annex II of the Implementing Regulation (EU) 2019/2072 (European Union) among the Union quarantine pests. The species was also selected as a priority pest for the EU and included in the list of 16 insect species whose potential economic, environmental or social impact is the most severe in respect of the Union territory (Annex of Commission Delegated Regulation (EU) 2019/1702).
In addition, another consequence of the afore-mentioned Implementing Decision (EU) 2015/893 (European Union 2015) is annual surveys to confirm the presence or absence of A. glabripennis on the Member States’ territory.
Since A. glabripennis activity typically occurs above the height of 1.5 m of the attacked trees, symptoms of infestation and specimens at different development stages should be looked for in the upper part of the trunk and the main branches of the tree canopy (Haack et al. 2010; EFSA et al. 2019). Visual examination of trees at the crown level is the key element for tracking down an A. glabripennis suspected case, focusing both on the pest itself at various life stages or symptoms on infested trees.
Ground monitoring for crown inspections by means of binoculars is of crucial importance for the first detection of infested trees, enabling to locate signs of the beetle activity on a tree in the upper part of the canopy, including oviposition sites, frass material and exit holes (EPPO 2013). Nevertheless, detection from the ground is not always accurate or feasible (Meng et al. 2015; Milius 1999; Smith et al. 2009; USDA 2013), making it necessary to use tree climbers or hydraulic lifts inspections. Visual inspections directly in the crown are more expensive and time-consuming than regular ground observations (Faccoli and Gatto 2016), but are more efficient, allowing the collection of frass and wood shavings produced by the larvae during their feeding and exit hole-boring activity and extruded from larval tunnels through cracks in the bark. Moreover, the presence of infestation signs on a tree can fail to be a specific indicator of A. glabripennis even for a well trained and experienced inspector, because several other native or introduced wood-boring insects produce similar signs of activity (Strangi et al. 2013). Tracking dogs trained for the detection of A. glabripennis have also been successfully used in Europe and USA, and controlled laboratory trials showed an accuracy of detection of 80–90% (Errico et al. 2012; Hoyer-Tomiczek and Sauseng 2013).
Visual examination alone is not, from a regulatory point of view, sufficient for the unambiguous identification of A. glabripennis infestation. In this perspective, a 100% reliable detection of a tree infested with A. glabripennis can only be based on the identification of juvenile specimens (eggs, larvae or pupae) that could be collected, usually invasively, from the suspected plant and on which to carry out morphological or molecular analyses. Pennacchio and colleagues provide a useful key for the morphological identification of A. glabripennis and its separation from sibling species including A. chinensis, the Citrus Longhorned Beetle (Pennacchio et al. 2012). In addition, EPPO standard PM 7/129 (EPPO 2016) provides protocols for the molecular diagnostics of arthropods including A. chinensis and A. glabripennis. However, obtaining such specimens from infested plants can be a demanding and difficult task, usually possible only after felling the tree. On the contrary, frass material is naturally ejected by the insect from the plant or can be collected from the galleries by removing a small bark portion. Therefore, a molecular diagnostic tool to confirm A. glabripennis infestation through the analysis of frass material collected on the outside of the plant can be an effective, non-invasive complement to traditional detection methods. Using the DNA barcoding primers LCO1490 and HCO2198 (Folmer et al. 1994), recommended in PM 7/129 (EPPO 2016), for frass material leads to unspecific co-amplification of eukaryotic genomic DNA from the wood matrix (Becker et al. 2020). Although this is not surprising as primers were originally designed for barcoding as many eukaryotes as possible, it turned out to be misleading while working on samples containing different DNA sources, such as A. glabripennis frass. These problems highlight the need for species-specific primers.
The tool presented in this study should allow both the extraction of sufficient amounts of DNA from insect tissue as well as frass containing inhibitory substances as phenolic compounds and a reliable identification of A. glabripennis. The requirements for a reliable diagnostic tool are specificity and sensitivity. The challenge is to create a specific tool that allows to detect A. glabripennis specimens comprising different populations from different geographic origin with slight genetic variations (inclusivity), and to distinguish them from closely related species like A. chinensis (exclusivity). Based on a comprehensive in silico analysis, cross-reaction with other wood-inhabiting insects should be avoided by covering all recent populations of the pathogen in vitro at once.
The interest in this kind of tools is testified by several studies published in the past few years, aiming at providing molecular tests for the detection of regulated pests from environmental matrices. Strangi proposed a protocol of molecular analyses based on end point polymerase chain reaction (PCR) amplification of DNA samples extracted from A. chinensis frass collected from potentially infested host trees (Strangi et al. 2013). Rizzo and colleagues provided diagnostic tests for the detection of Aromia bungii from frass samples based on real-time quantitative PCR (qPCR) (Rizzo et al. 2020a) and Loop-mediated Isothermal Amplification (LAMP) (Rizzo et al. 2021). A diagnostic protocol based on LAMP technology enabling the rapid detection of A. glabripennis DNA from frass samples was developed and validated (Rizzo et al. 2020b). A LAMP protocol for the detection of Agrilus planipennis in frass from different Fraxinus species and for the molecular identification of pooled specimens captured in traps was recently made available by Kyei-Poku (Kyei-Poku et al. 2020). The provision of multiple protocols in plant pest diagnosis is crucial, as different laboratories use different equipment.
In this study, a sensitive, specific and accurate real-time PCR test has been developed for the detection of A. glabripennis DNA from frass material providing a non-invasive sampling approach for phytosanitary surveys. In addition, the test allows the molecular identification of any life stage of the pest, including eggs, for which no morphological diagnostic characters are available, and larvae, whose morphological identification, although possible, is difficult and reserved for experienced entomologists. The diagnostic protocol presented in this article is supposed to facilitate detection of A. glabripennis in infested areas and to help plant protection services to eradicate this pest with a minimum amount of collateral damage.
Materials and methods
Specimens and frass samples
Most of the specimens tested were collected during the official survey and management (tree felling) activities of the Plant Protection Organizations in Italy, Germany and France, carried out in the A. glabripennis demarcated areas on their territory. All target specimens were identified morphologically according to Pennacchio (Pennacchio et al. 2012). Identification was confirmed through Sanger sequencing of the partial mitochondrial cytochrome oxidase subunit I (COI) gene, according to EPPO (EPPO 2016). Non-target species consisted of xylophagous insects sharing the same ecological niche as A. glabripennis and belonging to the orders Coleoptera (Cerambycidae: Cerambycinae, Lamiinae, Lepturinae) and Lepidoptera (Cossidae: Cossinae, Zeuzerinae; Sesiidae: Sesiinae). The non-target specimens were identified either morphologically or through DNA barcoding (EPPO 2016). All specimens have been stored in 96% ethanol prior to DNA extraction. In order to obtain frass material produced unequivocally by A. glabripennis, some portions of trunk (Acer saccharinum and Aesculus hippocastanum) resulting from felling cuts in a demarcated area and showing evident symptoms of A. glabripennis infestation (Fig. 1), have been preserved inside a double mesh cage (iron 5 mm mesh, aluminum 2 mm mesh). The extruded frass was collected at regular intervals until the adults emerged from the trunk, confirming the actual production by A. glabripennis. Other frass samples were obtained from wood samples in which frass was clearly associated with A. glabripennis larvae. All frass samples were dried and stored at room temperature until DNA extraction. Freezing the freshly collected frass samples at −20 °C is an appropriate alternative to drying them, but the higher fraction of water in the starting material needs to be considered according to the manufacturer’s DNA extraction protocol.
DNA extraction from insect and frass samples
DNA was isolated from insect tissue either by means of Maxwell RSC 48 automatic instrument (Promega, Madison, USA), using a CTAB based protocol (Maxwell RSC PureFood GMO and Authentication kit from Promega), or DNeasy Blood & Tissue Kit (Qiagen, Düren, Germany). DNA was eluted in 100 µL preheated elution buffer and either used for the real-time PCR reactions immediately or stored at −20 °C.
While DNA extraction of insects is well established meanwhile, for comparison different approaches were used to extract the DNA from frass. Accordingly, DNA was isolated from frass samples using a combination of a CTAB + PVP procedure (Calderón-Cortés et al. 2010; Doyle and Doyle 1990) and the Zymo Quick-DNA Fecal/Soil Microbe Miniprep Kit D6010 (Zymo Research Corporation, Irvine, USA). A second approach consisted in the InnuPREP TCM DNA Extraction Kit (Analytik Jena, Jena, Germany) tested according to the manufacturer’s protocol. Dry frass was homogenized for three minutes at 30.0 frequency in a 10-mL stainless steel grinding jar by means of Retsch MM400 homogenizer (Retsch, Haan, Germany) after freezing in liquid nitrogen. DNA extraction was performed on subsamples of 50 mg of the finely ground and homogenized material thus obtained. A second homogenization protocol consisting in the grinding of 50 mg of frass material for 3 min at maximum speed by means of MP FastPrep-24 5G (BM Biomedical, Irvine, USA) was also tested. Before proceeding with the Zymo kit protocol, the following purification steps were added: addition of 1 mL CTAB solution to the tubes (2% CTAB, 0.1 M TrisHCl pH 8, 1.4 M NaCl, 0.02 M EDTA pH 8, 4% PVP 40,000), vortexing, incubation for 30 min at 65 °C, centrifugation at maximum speed for 10 min, collection of 500 µL of the solution, leaving the debris at the bottom of the tube, transferring the solution into a new 1.5 µL tube, addition of 500 µL chloroform/ isoamyl alcohol 24:1 (under airflow cabinet) and centrifugation at maximum speed for 5 min. The supernatant (400 µL) was transferred to the Zymo column and the DNA isolation was performed according to manufacturer’s protocol. DNA was eluted in 100 µL elution buffer and either used for the qPCR reactions immediately or stored at – 20 °C.
DNA quality check
The concentration and 260/280 nm ratio of the DNA extracted from insect samples were checked using a NanoDrop™ ND2000 (Thermo Scientific, Waltham, USA). A universal primer pair (18S uni F/-R) and a YakimaYellow-BHQ1 dual labeled probe (18S uni-P) were used on a selected set of samples to target a region of the 18S ribosomal rDNA that is highly conserved throughout a wide range of eukaryotic organisms (Ioos et al. 2009). These primers and probe were selected in order to check the quality of the DNA extracted and to detect false-negative results that could potentially be caused by inhibition due to compounds present in the wood.
Primers and probe design
Primers and probe for qPCR were designed to target a fragment of the mitochondrial cytochrome c oxidase subunit I gene (COI) using the OligoArchitect™ Primers and Probe Online software (Sigma-Aldrich, St. Louis, USA) with the following specifications: a product size of 80 to 200 bp, a Tm (melting temperature) of 55 to 65° C, primer length of 18 to 22 bp and no secondary structure when possible. An in silico test of the oligonucleotides was then performed by means of BLAST® software (Basic Local Alignment Search Tool: http://www.ncbi.nlm.nih.gov/BLAST) to evaluate their specificity with respect to the target organism. Target sequences were available for A. glabripennis populations from Canada, China, Italy, Germany, South Korea and USA. Primers and probe were aligned on a set of sequences from target and non-target, closely related, organisms using the MAFFT software package (Katoh and Standley 2013) implemented within the Geneious® 10.2.6 software (Biomatters, Auckland, New Zealand). The primers and probe were synthesized by Eurofins Genomics (Ebersberg, Germany). The reporting dyes for A. glabripennis and 18S uni probes were selected to have distinctive fluorescence emission wavelengths in order to avoid any overlapping fluorescence spectra, which make duplexing possible, even if their simultaneous use in a single qPCR tube was not tested in this study.
The qPCR amplification products for one A. glabripennis larva and three frass samples were sent for Sanger sequencing in order to confirm that the primers successfully targeted the expected A. glabripennis COI fragment (GATC, Eurofins Genomics, Ebersberg, Germany). Purification of the amplification products was included in the service.
qPCR protocol
The qPCR was performed in a StepOnePlus Real-Time PCR System (Applied Biosystems (Thermo Fisher Scientific), Waltham, USA). The 20 µL reaction mixture contained 10 µL of 2X PerfeCTa qPCR ToughMix (QuantaBio, Beverly, MA) reaction buffer, 500 nM of each primer and 250 nM of TaqMan probe. The reaction buffer contained an optimized concentration of molecular-grade MgCl2 and dNTPs. 1 µL of total genomic DNA was added to the reaction mixture. The following qPCR program was used: initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 58 °C for 15 s and elongation at 68 °C for 30 s. The threshold was set automatically. Optimal annealing temperature was tested and set through gradient qPCR.
Performance of the qPCR test
To evaluate the performance of the qPCR test and the usability in routine analysis, the following performance criteria were determined: analytical specificity (inclusivity and exclusivity), analytical sensitivity, selectivity, repeatability and reproducibility. Table 1 shows the specimens used to determine the different performance characteristics. In addition, diagnostic specificity, diagnostic sensitivity and accuracy were calculated as follows:
where A = Obtained positives/expected positives (true positives), B = Obtained negatives/expected positives (false-negatives), C = False positives, and D = True negatives.
Validation was performed according to EPPO PM7/98 (EPPO 2019) and definitions of the performance criteria adopted from EPPO PM7/76 (EPPO 2017).
Analytical specificity, sensitivity and selectivity
Analytical specificity was evaluated on a sample set of target (inclusivity) and non-target (exclusivity) specimens from different outbreak areas in Europe (See Table 1). The set of target specimens comprised 18 A. glabripennis specimens from Italy, France and Germany. The non-target specimens belonged to 20 xylophagous species which share the same ecological niche as A. glabripennis and were collected from host plants belonging to genera such as Acer, Populus, Ulmus, Prunus, Betula and Malus. The amount of DNA obtained from each insect specimen was determined using a NanoDrop™ ND2000 (Thermo Scientific, Waltham, USA) and the DNA concentration adjusted with proper dilutions in the range of 10–20 ng µL−1. All the DNA samples were tested in triplicate on a StepOnePlus instrument (Applied Biosystems (Thermo Fisher Scientific), Waltham, USA).
Analytical sensitivity was tested as follows: serial tenfold dilutions of DNA ranging from 1.0 – 2.0 ng µL−1 to 1.0 – 2.0 fg µL−1 were prepared and tested for DNA extracted from eight A. glabripennis specimens (seven larvae and one adult). Each dilution was tested in triplicate in the same run and the testing was repeated three times (three runs), for a total of nine technical replicates per sample. The dilution for which amplification was observed for all the nine technical replicates was considered as the limit of detection of the test, i.e., the minimal concentration of target DNA that can be reliably detected. In order to evaluate the limit of detection in frass material, serial dilutions (with tenfold dilution steps) of DNA extracted from 50 mg of frass samples were also prepared. The frass samples were taken from A. glabripennis-infested Acer saccharinum and Aesculus hippocastanum. Aliquots of each frass sample were homogenized according to two different protocols (see DNA extraction from insect and frass samples). It was not possible to measure the initial concentration of the target organism’s DNA in frass samples because the solution contained also plant DNA and, potentially, DNA from other organisms that can colonize the wood shavings, like bacteria, fungi, nematodes and other arthropods. Each DNA subsample extracted from aliquots was subjected to a tenfold dilution series, and each dilution step was tested in duplicate.
To determine any effect of the matrix composition of the starting material for DNA extraction, selectivity was determined by testing different life stages (egg, larva, adult) of the target organisms and frass material produced by the trophic activity of the insect in different host plants (Acer spp., A. hippocastanum, Betula spp. and Malus domestica).
Repeatability and reproducibility
The same samples used for the evaluation of the analytical sensitivity were used to assess repeatability. Eight DNA samples at a concentration of 1.0 to 2.0 pg µL−1 were tested in triplicate and analysis repeated three times under the same conditions (operator, reagents, thermal cyclers and other laboratory equipment) on the same day. The level of agreement between the results obtained in the three qPCR runs was considered for the repeatability of the protocol. Reproducibility was determined by analyzing DNA subsamples from 11 samples (8 A. glabripennis frass samples and 1 larva, 2 A. chinensis larvae) under different conditions, i.e., location, technician and thermal cycler. Primers and probes were from the same batches, and qPCR runs were carried out with the same reagents. Three equal subsamples were prepared from DNA solutions of A. glabripennis larva and frass and A. chinensis larvae material and stored at –20 °C until use. Three different technicians in three different laboratories analyzed the entire subsample set in triplicate on three different thermal cyclers: Roche LightCycler 480 Instrument II, Bio-Rad CFX 96 Real-Time-System, and Analytik Jena qTower3.
Results
Primers and probe design
The newly designed primers target a 163 bp-long fragment of A. glabripennis mitochondrial COI region (accession number HM062991.1) (See Table 2). This region, although located outside the COI barcoding region from Folmer (Folmer et al. 1994), was chosen because it resulted highly conserved among A. glabripennis available sequences but suitable to distinguish this species from other species included in the same genus. Geographical origin of target sequences is indicated in the legend of Fig. 2. An A. glabripennis-specific primer pair was selected as a valid in silico candidate particularly because of the presence of five mismatches on the reverse primer, when compared to available A. chinensis sequences. Two of these mismatches were located near the 3’-end of the primer, conferring specificity to the assay because DNA polymerase extension during the polymerase reaction highly depends on a good stability of the 3’-end of the primer (Hyndman and Mitsuhashi 2003). Sanger sequencing results confirmed that the primers successfully amplified the expected A. glabripennis COI fragment in DNA from both larval (1) and frass (3) samples (98.8–100% identical to 75 A. glabripennis NCBI accessions, query coverage 99%). Similarity to available accessions of A. chinensis and A. macularia reached a maximum of 93.6% and 92.3%, respectively (Fig. 2).
Alignments of COI sequences of A. glabripennis and non-target species available from GenBank, showing in silico specificity of the test. The forward and reverse primers and the TaqMan probe designed in this study are reported on the reference sequence of Anoplophora glabripennis EU914811.1. Geographical origins of A. glabripennis accessions: GU003923, China, Jingyuan, Gansu; GU003924, USA, Manhattan, New York; GU003926, USA, Jersey City, New Jersey; GU003927, USA, Massapequa, New York; EU914464, USA, Carteret, New Jersey; EU914564, USA, Carteret, New Jersey; GU003922, USA, New Jersey; EU914673, Italy; EU914615, Canada: Toronto; EU914665, Canada: Toronto; EU914685, Germany; EU914484, USA: Sacramento, California; EU914738, China, Hebei Province; GU003919, China, Xi'an, Shanxi; GU003921, China, Jingyuan, Gansu; GU003920, China, Nengmenggu; HM062991, not available; EU914733, South Korea; EU914717, China, Anhui Province
Performance of the test
Analytical specificity
Amplification curves were obtained for all the target samples (true positives), with a mean Cq value of 18.6 ± 0.9 (4 specimens, 12 technical replicates, DNA concentration range 10–20 ng µL−1, Cq threshold = 0.12). No amplification was observed for any of the non-target samples, meaning that no false positive results for the non-target samples were obtained. Both target and non-target specimens DNA could be amplified using the universal 18S test, with mean Cq value of 20.1 ± 0.2 and 20.6 ± 0.1 for target and non-target samples, respectively (Cq threshold = 0.10). This confirms that the absence of signal for the non-target samples with the A. glabripennis specific test was truly due to the specificity of the test and not to reaction-inhibiting compounds possibly present in the DNA solution. The values of diagnostic sensitivity, diagnostic specificity and accuracy were 100% because neither false positive nor false-negative results were observed.
Analytical sensitivity
A concentration of 1.0 to 2.0 pg µL−1 of A. glabripennis DNA could be detected in all the replicates (nine technical replicates) for each of the eight specimens, with the exception of one specimen (eight replicates out of nine, Table 3). Mean Cq values were 33.2 ± 2.4 (Cq threshold = 0.027). For some of the DNA extracts, a lower concentration, in the range of 0.1—0.2 pg µL−1, was also detected in all of the nine replicates (see Table 3).
The test showed to have a coefficient of determination (R2) in the range of 98.2–99.9% as evaluated on serial dilution of DNA from three insect specimens (Fig. 3). The reaction efficiency ranged from 90.2 to 90.3% for samples S10 and S12 (larvae) to 97.1% for sample S16 (egg).
A-C. Three standard curves showing the relationship between Cq values (y-axis) and quantity of genomic DNA (tenfold dilution starting from a concentration of 10–20 ng µL−1, 3 technical replicates) of three A. glabripennis specimens (S10, S12, S16). Coefficient of determination (R2) resulted to be in a range of 98.2 to 99.9%. D. Corresponding amplification curves of S10 (amplification successful until 100,000-fold dilution, corresponding to 0.1—0.2 pg µL−1)
Anoplophora glabripennis DNA from frass samples could be detected in all replicates until the 100-fold dilution of the initial DNA solution, and in some replicates at the 1000-fold dilution with the only exception of the DNA extracted with the CTAB + Zymo Quick-DNA Fecal/Soil Microbe Miniprep Kit approach from A. hippocastanum and homogenized by means of MP FastPrep − 24 5G where no DNA could be detected at all. Especially at lower dilutions, non-negligible shift to higher Cq values could be observed for the MP FastPrep-24-homogenized samples compared to samples homogenized with Retsch MM400 homogenizer after liquid nitrogen freezing. For DNA from A. saccharinum frass, Cq mean value for undiluted to 100-fold dilution was 1.8 ± 0.2 higher for FastPrep than liquid nitrogen homogenization in the case of CTAB + Zymo extraction protocol; 1.3 ± 0.5 higher in the case of Analytik Jena extraction kit. Both DNA extraction approaches gave comparable results for each dilution step (Fig. 4).
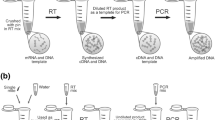
A. Performed sample homogenization and DNA extraction procedures resulting in four different sample-processing pathways. B, C. Curves showing the relationship between Cq values (y-axis) and quantity of genomic DNA (tenfold dilution series) for the differently processed Anoplophora glabripennis samples extracted from Acer saccharinum (B) and Aesculus hippocastanum (C) frass material
Selectivity
DNA extracted from all life stages of the target species (single egg, larva, pupa and adult) was successfully amplified, as well as DNA extracted from frass samples produced by the target species from different host plants (Acer sp., A. hippocastanum, Betula sp. and M. domestica), suggesting that the qPCR test is not affected by the type of matrix.
Repeatability and reproducibility
The same qualitative results were obtained in the three qPCR runs for all tested samples under the same conditions, with the exception of one specimen (eight replicates out of nine, Table 4) resulting in an overall repeatability of 99%. It has to be noted that the DNA samples were used at a concentration of 1.0 to 2.0 pg µL−1. Similarly, consistent results were obtained by different technicians on different thermal cyclers, yielding a reproducibility of 100% (Fig. 5).
Test of reproducibility: average Cq values for three technical replicates of 2 A. glabripennis larval and 8 A. glabripennis frass samples. Comparison of different thermal cyclers (Roche, Bio-Rad, Analytik Jena) used by different technicians in different laboratories. Standard deviation between technical replicates on the same cycler is shown by error bars; standard deviation between machines (together with average Cq) is shown as numbers above bars; the average standard deviation between replicates on the same machine is depicted in the insert in the upper right corner
The averaged standard deviation (SD) of the Cq values of ten A. glabripennis samples (two DNA extracts from one larval sample and eight frass samples) indicates the reproducibility of the results on different thermal cyclers. The averaged SD for the three machines is as follows: 0.24 cycles for Roche, 0.47 cycles for Bio-Rad and 0.48 cycles for Analytik Jena (Fig. 5). The averaged SD between the cyclers, being 0.58, is not much higher suggesting that the three tested cyclers provide almost the same results for all ten samples. Using the Roche thermal cycler, average Cq values were slightly (on average 1.1 °C) higher than average Cq values from the two other thermal cyclers (Fig. 5), which is, however, a neglectable difference.
Discussion
The Asian Longhorned Beetle Anoplophora glabripennis is considered among the most destructive invasive xylophagous insects worldwide. Effective eradication programs from non-native regions are very costly and rely on a prompt and rapid detection capability by National Plant Protection Organizations.
The newly designed test proved to be specific for A. glabripennis and no cross-reaction was observed for the closely related congeneric A. chinensis (both chinensis and malasiaca form, taxonomy according to Lingafelter and Hoebeke Lingafelter and Hoebeke (2002)) and nineteen other European or introduced wood-boring insect species that can be encountered during survey activities on the same A. glabripennis host plants. The availability of specimens or frass material from Italy, France, Germany and Finland and from different collection sites within their territories, allowed to test the inclusivity of the test with respect to the main invasive populations from outbreak areas currently reported in Europe. In addition, in silico evaluation of specificity included populations from Canada, China, Italy, Germany, South Korea and USA. The test allowed the identification of all A. glabripennis life stages. A DNA concentration of 1.0 to 2.0 pg µL−1 could be detected in all the samples analyzed, even though for some of them a concentration in the range of 0.1—0.2 pg µL−1 was also reliably detected. DNA from frass material obtained from rearing cages and from field was purified and successfully amplified. Both DNA extraction approaches and both homogenization procedures tested on frass material provided comparable results, pointing to robust sample processing. Further, statistically sound testing should be conducted to confirm possible effects of the homogenization protocol on the DNA quality (liquid nitrogen freezing vs. room temperature homogenization). Quality of the frass material is very important. We proved that DNA extracted from 50 mg freshly produced, pure frass material from Acer saccharinum and Aesculus hippocastanum can be reliably detected at least until a 100-fold dilution. However, under field conditions, frass material can easily undergo degradation processes due to exposure to environmental conditions. Exposure to sun light and weather conditions can affect the quality of the DNA present in the frass. Colonization by microorganisms, favored by high levels of atmospheric humidity, may contribute to a reduction in the quality of the DNA present in the frass, especially if exposed to unfavorable environmental conditions for a long time. Experience shows that recovering fecal DNA suitable for qPCR is more likely from freshly expelled frass material or from frass collected inside the wood (Strangi et al. 2013; authors’ personal observations). Alternatively, the removal of a small bark portion to collect material from inside the gallery could reduce the risk of false-negative results due to DNA degradation. It is worth noting that in this study it was possible to successfully amplify frass samples from 2012 and 2015, which were stored at room temperature in well-dried condition, but without any other special precautions.
The linearity of the qPCR standard curve, drawn from dilution series of DNA from three specimens, was highly significant, reaching a coefficient of determination value (R2) of 0.989, considering that the linear regression fitted to the standard curve data should have a R2 ≥ 0.98 for qPCR (Kralik and Ricchi 2017). The reaction efficiency was also satisfactory, varying from 90.2 (larval specimen) to 97.1% (egg), based on the three specimens used.
The qPCR test proved to be repeatable and reproducible when used under different laboratory conditions. Reproducibility data showed that results were not influenced by the different thermal cyclers used. To enhance amplification reactions in light of the potential presence of inhibitory molecules within the DNA solution extracted from woody material like frass, a ready-to-use reaction cocktail for qPCR amplification has been used which is particularly suitable for overcoming problems of PCR inhibitors that might be present in environmental or plant samples. Other master mixes and polymerases were not thoroughly tested in this study, but it is not excluded that they may be equally effective.
Very recently, a rapid diagnostic protocol based on loop-mediated isothermal amplification (LAMP) for the detection of A. glabripennis from frass material was published (Rizzo et al. 2020b) In this study, a second molecular tool is provided to extend the diagnostic possibilities for laboratories and to favor those that are more familiar with qPCR. Despite increasingly used in molecular diagnostics as an alternative to PCR based methods due to its high amplification efficiency, specificity, and relatively cheap costs, LAMP technique can sometimes be prone to cross-contamination and false positive results (Chou et al. 2011; Hardinge and Murray 2019; Karthik et al. 2014). Moreover, multiplexing is generally less recommended for LAMP assays (Karami et al. 2011), making it a less versatile technique. It is the opinion of the authors that these two diagnostic tests can be valid alternatives or complements to support phytosanitary surveillance activities for A. glabripennis so that applied eradication and containment measures can be effective more timely. Indeed, provided that the sampling of frass material is done in an accurate and representative way, these molecular tests allow to confirm or not the trees suspected of being infested by A. glabripennis before felling them, thus saving money for the National Plant Protection Organizations. The availability of different diagnostic tests opens the way for further comparative performance studies.
References
Becker M, König S, Hoppe B (2020) A simple PCR-based approach for rapid detection of Ips typographus and Ips duplicatus in the presence of (associated) symbionts and parasites. J Plant Dis Prot. https://doi.org/10.1007/s41348-020-00388-w
Calderón-Cortés N, Quesada M, Cano-Camacho H, Zavala-Páramo G (2010) A simple and rapid method for DNA isolation from xylophagous insects. Int J Mol Sci 11:5056–5064. https://doi.org/10.3390/ijms11125056
Cavey JF, Hoebeke ER, Passoa S, Lingafelter SW (1998) A new exotic threat to North American hardwood forests: an Asian Longhorned Beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae). I. Larval description and diagnosis. P Entomol Soc Wash 100:373–381
Chou P-H, Lin Y-C, Teng P-H, Chen C-L, Lee P-Y (2011) Real-time target-specific detection of loop-mediated isothermal amplification for white spot syndrome virus using fluorescence energy transfer-based probes. J Virol Methods 173:67–74. https://doi.org/10.1016/j.jviromet.2011.01.009
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:39–40
EPPO (2017) PM 7/76 (4) Use of EPPO diagnostic protocols. EPPO Bull 47:7–9. https://doi.org/10.1111/epp.12365
EPPO (2019) PM 7/98 (4) Specific requirements for laboratories preparing accreditation for a plant pest diagnostic activity. EPPO Bull 49:530–563. https://doi.org/10.1111/epp.12629
EPPO (2021) EPPO Reporting Service. https://www.eppo.int/RESOURCES/eppo_publications/eppo_reporting_service. Accessed 2 March 2021
EPPO (2013) PM 9/15 (1) Anoplophora glabripennis: procedures for official control. EPPO Bull 43:510–517. https://doi.org/10.1111/epp.12064
EPPO (2016) PM 7/129 (1) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull 46:501–537. https://doi.org/10.1111/epp.12344
Errico F, Napolitano F, Nisticò R, Usiello A (2012) New insights on the role of free D-aspartate in the mammalian brain. Amino Acids 43:1861–1871. https://doi.org/10.1007/s00726-012-1356-1
European Union Commission Implementing Regulation (EU) 2019/2072 of 28 November (2019) establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019: OJ L 319. Official J Eur Union 2019:pp 1–279
European Union Commission Implementing Decision (EU) 2015/893 of 9 June (2015) as regards measures to prevent the introduction into and the spread within the Union of Anoplophora glabripennis (Motschulsky) (notified under document C(2015) 3772: C/2015/3772. Official J Eur Union 2015:pp 16–28
European Food Safety Authority (EFSA), Hoppe B, Schrader G, Kinkar M, Vos S (2019) Pest survey card on Anoplophora glabripennis. EFS3 16:1750E. https://doi.org/10.2903/sp.efsa.2019.EN-1750
Faccoli M, Favaro R (2016) Host preference and host colonization of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera Cerambycidae), in Southern Europe. Bull Entomol Res 106:359–367. https://doi.org/10.1017/S0007485315001157
Faccoli M, Gatto P (2016) Analysis of costs and benefits of Asian longhorned beetle eradication in Italy. Forestry (lond) 89:301–309. https://doi.org/10.1093/forestry/cpv041
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Haack RA, Cavey JF, Hoebeke ER, Law K (1996) Anoplophora glabripennis: A new tree-infesting exotic cerambycid invades New York. Mich Entomol Soc Newsl 41:1–3
Haack RA, Hérard F, Sun J, Turgeon JJ (2010) Managing invasive populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: a worldwide perspective. Annu Rev Entomol 55:521–546. https://doi.org/10.1146/annurev-ento-112408-085427
Hardinge P, Murray JAH (2019) Reduced false positives and improved reporting of loop-mediated isothermal amplification using quenched fluorescent primers. Sci Rep 9:7400. https://doi.org/10.1038/s41598-019-43817-z
Hoyer-Tomiczek U, Sauseng G (2013) Sniffer dogs to find Anoplophora spp. infested plants. J Entomol Acarol Res 45:10–12
Hyndman DL, Mitsuhashi M (2003) PCR primer design. In: Bartlett JMS, Stirling D (eds) PCR protocols. Humana Press, New Jersey pp 81–88
Ioos R, Fourrier C, Iancu G, Gordon TR (2009) Sensitive detection of Fusarium circinatum in pine seed by combining an enrichment procedure with a real-time polymerase chain reaction using dual-labeled probe chemistry. Phytopathology 99:582–590. https://doi.org/10.1094/PHYTO-99-5-0582
Javal M, Lombaert E, Tsykun T, Courtin C, Kerdelhué C, Prospero S, Roques A, Roux G (2019) Deciphering the worldwide invasion of the Asian longhorned beetle: a recurrent invasion process from the native area together with a bridgehead effect. Mol Ecol 28:951–967. https://doi.org/10.1111/mec.15030
Karami A, Gill P, Motamedi MH, Saghafinia M (2011) A review of the current isothermal amplification techniques: applications, advantages and disadvantages. J Global Infect Dis 3(3):293–302
Karthik K, Rathore R, Thomas P, Arun TR, Viswas KN, Dhama K, Agarwal RK (2014) New closed tube loop mediated isothermal amplification assay for prevention of product cross-contamination. MethodsX 1:137–143. https://doi.org/10.1016/j.mex.2014.08.009
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kralik P, Ricchi M (2017) A basic guide to real time PCR in microbial diagnostics: definitions, parameters, and everything. Front Microbiol 8:108. https://doi.org/10.3389/fmicb.2017.00108
Kyei-Poku G, Gauthier D, Quan G (2020) Development of a loop-mediated isothermal amplification assay as an early-warning tool for detecting emerald ash borer (Coleoptera: Buprestidae) incursions. J Econ Entomol 113:2480–2494. https://doi.org/10.1093/jee/toaa135
Lethmayer C (2013) First data on the dispersal and potential spread of Anoplophora spp. J Entomol Acarol Res 45:7
Lingafelter SW, Hoebeke ER (2002) Revision of the genus Anoplophora (Coleoptera: Cerambycidae), 595. 7648 L5. Entomological Society of Washington , Washington DC
Meng PS, Hoover K, Keena MA (2015) Asian Longhorned beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. J Integr Pest Manag 6:4. https://doi.org/10.1093/jipm/pmv003
Milius S (1999) Son of Longhorned beetles. Sci News 155:380. https://doi.org/10.2307/4011462
Pennacchio F, Sabbatini Peverieri G, Jucker C, Allegro G, Roversi PF (2012) A key for the identification of larvae of Anoplophora chinensis, Anoplophora glabripennis and Psacothea hilaris (Coleoptera Cerambycidae Lamiinae) in Europe. Redia 95:57–65
Rizzo D, Luchi N, Da Lio D, Bartolini L, Nugnes F, Cappellini G, Bruscoli T, Salemi C, Griffo RV, Garonna AP, Rossi E (2021) Development of a loop-mediated isothermal amplification (LAMP) assay for the identification of the invasive wood borer Aromia bungii (Coleoptera: Cerambycidae) from frass. 3 Biotech. https://doi.org/10.1007/s13205-020-02602-w
Rizzo D, Taddei A, Da Lio D, Bruscoli T, Cappellini G, Bartolini L, Salemi C, Luchi N, Pennacchio F, Rossi E (2020b) Molecular identification of Anoplophora glabripennis (Coleoptera: Cerambycidae) from frass by Loop-Mediated Isothermal Amplification. J Econ Entomol 113:2911–2919. https://doi.org/10.1093/jee/toaa206
Rizzo D, Taddei A, Da Lio D, Nugnes F, Barra E, Stefani L, Bartolini L, Griffo RV, Spigno P, Cozzolino L, Rossi E, Garonna AP (2020a) Identification of the Red-Necked Longhorn Beetle Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) with real-time PCR on frass. Sustainability 12:6041. https://doi.org/10.3390/su12156041
Smith MJ, Turgeon JJ, de Groot P, Gasman B (2009) Asian Longhorned beetle Anoplophora glabripennis (Motschulsky): lessons learned and opportunities to improve the process of eradication and management. Am Entomol 55:21
Strangi A, Sabbatini Peverieri G, Roversi PF (2013) Managing outbreaks of the citrus Longhorned beetle Anoplophora chinensis (Forster) in Europe: molecular diagnosis of plant infestation. Pest Manag Sci 69:627–634. https://doi.org/10.1002/ps.3416
Tomiczek C, Hoyer-Tomiczek U (2007) Asian Longhorned beetle (Anoplophora glabripennis) and citrus Longhorned beetle (Anoplophora chinensis) in Europe—actual situation. Forstschutz Aktuell pp 2–5
USDA (2013) Asian Longhorned beetle cooperative eradication program in Clermont County, Ohio. https://www.aphis.usda.gov/plant_health/ea/downloads/2013/OHClermontCountyRevised_EA_May_final.pdf. Accessed 2 March 2021
Acknowledgements
The authors would like to thank Regione Lombardia Planth Health Service, ERSAF (Ente Regionale per i Servizi all’Agricoltura e alle Foreste, Milano, Italy) and the Regione Lombardia Plant Health Laboratory c/o Fondazione Minoprio (Vertemate con Minoprio, Italy) for starting this project and launching the cooperation among laboratories. A special thanks goes to B. Cavagna, M. Ciampitti, A. Bianchi, M. Zugno and to all the phytosanitary inspectors, agents and technicians involved in the survey and application of official measures for A. glabripennis and A. chinensis in Lombardia who provided valuable biological material. We are grateful to C. Lethmayer from the Institute for Sustainable Plant Production of AGES (Austrian Agency for Health and Food Safety, Vienna, Austria) and V. Welling from the Finnish Food Authority (Helsinki, Finland) for supplying A. glabripennis frass material; to M.C. Bon, N. Ramualde, G. Desurmont from the European Biological Control Laboratory of USDA (US Department of Agriculture, Montpellier, France) for providing pure A. glabripennis and A. chinensis frass material from rearing facilities to be used in follow-up studies and as reference material for routine analysis.
The authors would like to thank Timo Hillebrand for the continuous developing and provision of the Analytik Jena DNA extraction kit used in this study. The authors are sincerely grateful to the reviewers for the time devoted to review the manuscript and the suggestions for its improvement.
Funding
Open Access funding enabled and organized by Projekt DEAL. Partial financial support was received from FNR Project “ANOPLOdiag—Diagnosis of wood-inhabiting regulated harmful organisms exemplified on the Asian Longhorned Beetle (Anoplophora glabripennis, Cerambicidae) based on frass and gnaw shavings”; Grant number 22010316.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Björn Hoppe and Domenico Rizzo These authors are joint senior authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taddei, A., Becker, M., Berger, B. et al. Molecular identification of Anoplophora glabripennis (Coleoptera: Cerambycidae) and detection from frass samples based on real-time quantitative PCR. J Plant Dis Prot 128, 1587–1601 (2021). https://doi.org/10.1007/s41348-021-00501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-021-00501-7