Abstract
Septoria tritici blotch (STB; Zymoseptoria tritici) is the most important leaf disease of wheat in Northern and Western Europe. The problem of fungicide resistance in Z. tritici populations is challenging future control options. In order to investigate differences in azole performances against STB, 55 field trials were carried out during four seasons (2015–2018). These trials were undertaken in ten different countries across Europe covering a diversity of climatic zones and agricultural practices. During all four seasons, four single azoles (epoxiconazole, prothioconazole, tebuconazole and metconazole) were tested. Increasing variability in the performances of these azoles against STB was observed across Europe. The efficacy of the tested azoles varied considerably across the continent and between countries. The shifts in disease control from these commonly used azoles were confirmed by increasing EC50 values for epoxiconazole, prothioconazole-desthio and metconazole. The sensitivity towards tebuconazole remained relatively low across the four years. The frequencies of CYP51 mutations varied substantially across Europe, with a clear pattern of significantly decreasing frequencies of D134G, V136A and S524T in the local Z. tritici populations from west to east. In contrast, no major differences were seen for CYP51 mutations V136C, A379 and I381V. The four azoles showed different levels of cross-resistance, which again depended on specific CYP51 mutations. Across the four seasons, the single azoles increased the yields between 9 and 11% on average.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septoria tritici blotch (STB) caused by Zymoseptoria tritici is the most significant yield-reducing disease in Western Europe (Jørgensen et al. 2014). Fungicides are intensively used to reduce losses from this disease, since only partly resistant cultivars are available and other cultural means of reducing disease severity, like delayed sowing, give only moderate reductions in disease epidemics (Gladders et al. 2001; Jørgensen et al. 2014). The ability of the pathogen to produce large quantities of inoculum that can be spread rapidly between fields and also between regions makes it challenging to use traditional agronomic practices, like altered crop rotations, to minimise this pathogen (McDonald and Mundt 2016).
Depending on the season and region, winter wheat crops in Europe are typically treated with fungicides between one and four times per season. The critical fungicide applications for control of STB target the two upper leaves, which are known to be most important for retaining yields (Lupton 1972). This high reliance on fungicides is controversial and problematic, in light of the general public's wish for less dependence on pesticides. Notably, the expected restrictions on the number of pesticides available in the EU highlight the precarious current situation (Jess et al. 2014). Additionally, the increasing problems with fungicide resistance in Z. tritici populations to several active ingredients (AHDB 2018; Gisi et al. 2005; Torriani et al. 2015) present a challenge to wheat production in Europe in the future.
Due to the reasons mentioned above, European growers have few fungicide groups at hand for the control of STB. Currently, these include multi-site inhibitors such as folpet, chlorothalonil, sulphur and the single site azoles (e.g. prothioconazole, epoxiconazole, tebuconazole) and succinate dehydrogenase inhibitors (SDHIs) (e.g. bixafen, fluxapyroxad, fluopyram). In several studies, it has been shown that European Z. tritici populations have acquired different degrees of resistance to both azoles and SDHIs, which increases the problem of managing the disease (Blake et al. 2018; Cools and Fraaije 2013; Dooley et al. 2016; Garnault et al. 2019; Heick et al. 2017a). The quinone outside inhibitors (QoIs) no longer provide reliable control of STB in Western Europe, although they might still be effective in some areas in Eastern Europe (Jørgensen et al. 2017; Mäe et al. 2020). The most effective and most widely used multi-site inhibitor, chlorothalonil, will disappear as a control option in the EU after 2020, leaving a major gap in options for adding efficient multi-site inhibitors.
Azole resistance in the Z. tritici population is well documented and linked to multiple molecular mechanisms. Alterations in the target site, overexpression of the target site and overexpression of efflux pumps are among the predominate mechanisms (Cools and Fraaije 2013). Alterations associated with reductions in azole sensitivity have been reported in European Z. tritici populations since the early 1990s (Zhan et al. 2008). Mutations leading to alterations D134G, V136A/C/G, A379G, I381V, S524T and deletions at amino acid position 459–460 are claimed to have the highest effect on the sensitivity to azoles (Cools et al. 2011). These typically occur in combination, and the presence or absence of a single mutation can impact the isolates' level of resistance substantially, with over 30 different CYP51 haplotypes reported (Cools and Fraaije 2013; Huf et al. 2018).
The changes in field efficacy of azoles against STB have, to a great extent, been associated with these CYP51 mutations and elevated EC50 values for several azoles (Blake et al. 2018; Huf et al. 2018). The level of resistance is found to be highly influenced by the intensity of fungicide input in the control programme, which reflects the on-site risk of STB (Heick et al. 2017b; Jørgensen et al. 2017). Major resistance shifts occurring in field populations of Z. tritici initially had little or no effect on the field performance of epoxiconazole and prothioconazole. However, the efficacy of these compounds has been declining in recent years (Blake et al. 2018; Kildea et al. 2019; Jørgensen et al. 2017).
The overall goal of the current study was to generate updated efficacy profiles of azoles, which have been commonly used for STB control in wheat across Europe over a long period. More specifically, the study aims were (1) to investigate the field performances of major single azoles against the current Z. tritici populations across Europe, (2) to elucidate the interrelation of azole field performances, in-vitro sensitivity of Z. tritici populations and CYP51 mutation frequencies across Europe, (3) to discuss this interrelationship between EC50 values, specific CYP51 mutations and field performances.
The Eurowheat azole project is a follow-up study to a previous collaboration in the EuroWheat group—initiated by activities in the European Network of excellence—ENDURE (Jørgensen et al. 2014). The data of the first two seasons of this network (2015 and 2016) have been published previously and highlighted a high level of diversity in efficacy across Europe (Jørgensen et al. 2018). The addition of data from two additional seasons (2017 and 2018) has enabled a further examination of this variability, with a focus on how efficacy, sensitivity and CYP51 mutations have changed across the four seasons and further exploration of the potential links between these parameters.
Material and methods
Field trials
The current study was carried out over the field seasons of 2015, 2016, 2017 and 2018 at locations across Europe, covering a variety of different climate zones and agricultural practices. A total of 66 trials were carried out following similar protocols, where specific treatments were tested at all seasons and sites (Table 1). Of those 66 trials, 55 provided usable data on STB, of which 19 trials were conducted in 2015, 12 trials in 2016, 11 trials in 2017 and 13 trials in 2018 (Tables 2, 3). The trials were carried out in Denmark (DNK), Scotland (SCT), England (ENG), Poland (POL), France (FRA), Germany (DEU), Ireland (IRL), Belgium (BEL), Latvia (LVA) and Hungary (HUN) by local scientific organisations (Table 3). All experiments were carried out using standard EPPO procedures (Oepp/Eppo 2014a) and a randomised plot design with a minimum plot size of 10 m2 and three to four replicates. Winter wheat varieties with moderate to high susceptibility to STB were chosen for all trials. Equipment used for fungicide applications varied from knapsack sprayers to self-propelled sprayers. Fungicides were applied at a pressure of 1.8–4 bar and water volumes of 196–300 L/ha. The four azoles were applied at flag leaf emergence at growth stage (GS) 37–39 BBCH (Lancashire et al. 1991). In several cases, the trials were treated with cover sprays to reduce early infections of powdery mildew (Blumeria graminis), yellow rust (Puccinia striiformis) and STB as required. In order to prevent early outbreaks of STB, powdery mildew and yellow rust, chlorothalonil, metrafenon and pyraclostrobin were applied respectively, no later than GS 32.
The assessments were done following the EPPO guideline “Foliar and ear diseases on cereals” (1/26 (4)) (OEPP/EPPO 2014a), which provides two acceptable methods using either individual leaves assessments from 10 to 20 tillers per plot or a crop stand assessment using an average scoring per plot. Severity was assessed as percent symptomatic area. The emphasis was on the assessments carried out at 30–45 days after application (DAA), GS 73–83 on flag leaf (FL) and flag leaf minus one (F-1). All trials were carried through to harvest. Grain yields were measured for every plot and adjusted to 85% dry matter. Fungicides were supplied by BASF SE (Limburgerhof, Germany) and applied at full recommended label rates (Table 1).
CYP51 mutation frequencies and EC50 values
Leaf samples (2 × 10 leaves per replicate) with STB symptoms from 46 trials were collected at GS 65–75 from untreated plots. One of the two samples per plot was forwarded to BASF SE (Limburgerhof, Germany) for mutation analysis and the other one to EpiLogic (Freising, Germany) for sensitivity analysis. DNA was extracted based on a bulk sample of leaves (40 leaves) merged from the four replicates. The following five CYP51 mutations were tested using pyrosequencing: D134G, V136A/C, A379G and I381V, while S524T was detected using qPCR (Stammler et al. 2012; Sierotzki et al. 2019). These mutations were selected as they have previously been associated with decreases in azole sensitivity throughout Europe (Stammler et al. 2008; Leroux and Walker 2011; Cools and Fraaije 2013).
With some exceptions, ten single pycnidium isolates were tested per site for their sensitivity to epoxiconazole (ECA), prothioconazole-desthio (PTH-D), metconazole (MCA) and tebuconazole (TCA) using a microtitre plate assay following the 'SEPTTR microtitre monitoring method BASF 2009 v. 1’ (FRAC 2009). Seven days after inoculation, the growth is measured in a photometer at 405 nm. The values are corrected by comparison with the blanks. EC50 values are calculated by probit analysis. In total, 403 isolates were tested across the four seasons (Table 4).
Statistical analysis
Out of the 66 trials, which all included the testing of the four azoles, 55 trials provided some useable data, either disease data (dataset 1), sensitivity data (dataset 2) or mutation data (dataset 3) (Tables 2, 4). In dataset 1, 43 of these 55 trials were included as minimum 5% STB severity in the untreated control and significant yield increases were used as the criteria for selection. The minimum disease severity was based on common efficacy practise, where lower disease severity is seen as too unreliable for efficacy evaluation as also stated in EPPO guidelines (OEPP/EPPO PP 1/226 2014b). Several trials were affected by desiccation during the dry season of 2018, and thus, significant yield increases were used as standardised way of selecting reliable trials. In total, 46 trials were included in datasets 2 and 3. In specific trials, no data on EC50 values and/or mutations could be obtained. In some cases, it was not possible to isolate single strains from leaf samples, which resulted in a complete lack of sensitivity data.
Based on observed patterns of field efficacy, EC50 values and CYP51 mutations, these data were subdivided into geographical regions: East: Poland, Latvia, Hungary. North: Denmark, Northern Germany. South: France, Belgium, Southern Germany. West: England, Scotland, Ireland.
Statistical analyses of CYP51 mutation frequencies, EC50 values, field efficacy and yield data were carried out using RStudio version 1.2.5019 (RStudio Team 2019) with α = 0.05 for all tests. In certain cases, outliers were removed from specific trials. Nevertheless, proper residuals could not be obtained for these variables. Therefore, the non-parametric kruskal.test with post-hoc dunnTest of the FSA R packages (R Core Team 2017) was used for distinguishing significant differences between levels of the factors "year" and "region" and their interactions.
The correlations between the sensitivity of the isolate collections and the four azoles were illustrated by pair-wise scatter plots, and correlation coefficients (Pearson's r) were calculated. P values for the correlations were tested for with Holm's correction for multiple tests using corr.test of the psych R package.
Furthermore, correlations between mutations, EC50 values and field effects were explored using principal components analysis (PCA). Two outliers were identified visually with scoreplot and qqPlot of the pls and car packages, respectively. PCA biplots of the first two principal components were created from standardised data using the ggbiplots package. Ellipses corresponding to 95% confidence intervals of regions were also added using this function.
Results
Control of STB across Europe
Forty-three trials had levels of STB severity which allowed evaluation of treatment effects. The data were organised into four groups based on the geographical regions as well as the split between years (Fig. 1). STB severities were moderate to high in most trials. On flag leaves, severities varied between 5 and 50% and on F-1 between 10 and 80%. STB severities were generally lower in the Eastern region, but apparent variation was also observed between years. Disease severity in 2018 was particularly low as a result of dry conditions in Northern and Western Europe. These data were not statistically analysed as the STB severity data depend on many factors, of which climate, cultivar susceptibility and day of assessment after application are regarded as the most important.
Average septoria leaf blotch severity (% symptomatic leaf area) in untreated plots in specific years (2015–2018) and regions of Europe on FL (left) and F-1 (right) at GS 67–85, 64–50 DAA. A total of 43 trials were included. East: Poland, Latvia, Hungary. North: Denmark, Northern Germany. South: France, Belgium, Southern Germany. West: England, Scotland, Ireland
Field trial data from 2015 to 2018 showed that the variation in efficacy of ECA, PTH and MCA increased during this period, as seen in Fig. 2. The results from analyses of main effects of region and interaction effects (year × region) are also presented in Fig. 2. The control levels of ECA varied between 9 and 95%, of PTH between 12 and 95%, of MCA between 0 and 87% and of TCA between 0 and 88%. Efficacy levels of PTH were significantly higher in Eastern than in the other regions (not considering the interaction with years), while ECA was significantly more effective in Eastern and Northern Europe than the other regions. MCA was significantly more effective in Eastern than in Northern and Southern Europe, whereas the effects of this azole in Western Europe could not be statistically distinguished from those in the other regions. The efficacy of TCA also varied between years, but no apparent and significant differences were seen between the four regions. On average, ECA, PTH, TCA and MCA gave 61%, 64%, 59% and 55% control, respectively, indicating very similar control against STB across Europe overall. However, the ranking varied substantially between localities, as seen in the supplementary table (S-1). When analysing the main effects of years, significant differences between obtained efficacies were only seen for PTH and MCA, whereas differences were not significant for ECA and TCA (Table 5). Indications of declining efficacies mainly occurred in the Western and Northern regions (Fig. 2). Analysis of the interactions between years and regions is produced on a few significant differences.
Efficacy of four azoles across four regions in Europe and in 4 years (2015–2018) assessed on FL and F-1 at GS 67–85, 64–50 DAA. East: Poland, Latvia, Hungary. North: Denmark, Northern Germany. South: France, Belgium, Southern Germany. West: England, Scotland, Ireland. Different letters represent statistically significant differences between regions (above boxplots) and their interaction with years (below boxplots) (P = 0.05). Epoxiconazole = ECA, prothioconazole = PTH, metconazole = MCA and tebuconazole = TCA
EC50 values for azoles
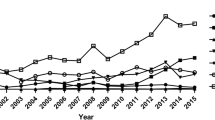
The number of isolates of Z. tritici varied between years and regions but generally fell between 70 and 149 (Table 4). ECA had EC50 values between 0.05 and 1.40 ppm, PTH-D between 0.01 and 0.42 ppm, MCA between 0.05 and 0.94 ppm and TCA between 0.37 and 8.00 ppm. EC50 values for the four azoles showed significant differences across the different regions. Figure 3 shows an overall increase of EC50 values for three of the four azoles from 2015 to 2018. The increase was apparent for ECA, PTH-D and MCA with Western Europe representing the highest values. The pattern of increasing EC50 values was steep and similar for ECA and PTH-D, but slightly more moderate for MCA. The pattern of EC50 values for TCA differed compared to the other three azoles, where no significant increases were seen across years or regions. Southern Europe did, however, show a Z. tritici population that was significantly more sensitive to TCA compared with all other regions when considering the main effects of region (Fig. 3). Considering the main effects of years; the sensitivity of Z. tritici isolates changed significantly to all four azoles between 2015 and 2018 (Table 5).
EC50 values for the four azoles across Europe from 2015 to 2018. East: Poland, Latvia, Hungary. North: Denmark, Northern Germany. South: France, Belgium, Southern Germany. West: England, Scotland, Ireland. Different letters represent statistically significant differences between regions (above boxplots) and their interaction with years (below boxplots) (P = 0.05). Epoxiconazole = ECA, prothioconazole-desthio = PTH-D, metconazole = MCA and tebuconazole = TCA
The correlations between the EC50 values of the four azoles are shown in Fig. 4, and the statistical correlations are given in Table 5. PTH-D showed a high degree of cross-resistance to ECA (r = 0.89) and to MCA (r = 0.46). EC50 values of TCA and ECA were not significantly correlated (r = 0.08), while those of TCA and PTH-D were negatively correlated to a small degree (r = − 0.19). More pronounced was the cross-resistance between TCA and MCA (r = 0.65) (Table 6).
Correlations between log-transformed EC50 values of the azoles ECA, MCA, TCA and PTH-D across Europe. Data points below the diagonal are colour-coded by frequencies of the CYP51 mutation V136A (%) and above the diagonal by European regions (East: Poland, Latvia, Hungary; North: Denmark, Northern Germany; South: France, Belgium, Southern Germany; West: England, Scotland, Ireland). Epoxiconazole = ECA, prothioconazole-desthio = PTH-D, metconazole = MCA and tebuconazole = TCA
When the EC50 values are marked depending on region (Fig. 4, upper part), a clear grouping can be seen. In Eastern Europe, EC50 values for ECA and MCA were clustering together in the lower, and TCA in the higher end of the scale, than in Western Europe in particular. When taking V136A frequencies into account (Fig. 4, lower part), similar groupings of the data are seen. This mutation was found at high frequencies in the population of Western Europe and low frequencies in Eastern Europe, indicating that TCA shows a higher degree of cross-resistance with both PTH-D, ECA and MCA, when the frequency of V136A increases.
CYP51 mutations
Analyses of DNA from bulked leaf samples revealed a variable distribution of the six CYP51 mutations tested (Fig. 5). S524T varied significantly between the regions, and the overall frequency increased during the trial period in the Southern and Western regions (Fig. 5). In the UK and Ireland (grouped as 'west'), the frequency of S524T increased most during the four seasons from 35 to 60%. However, no significant differences were found between years for any of the regions. Overall, I381V was the most common mutation, at an average frequency of 94% of all investigated populations collected from trial sites in all countries. Very similar frequencies of this mutation were seen in all regions, but those of the Western region were significantly higher. The level of V136C was relatively low and quite similar across all years and regions with an average of ca. 20%. The A379G mutation was detected at similar frequencies, approximately 20%, during the four seasons, and no significant differences were measured between regions or seasons. The two mutations D134G and V136A were detected at comparable frequencies in the medium range at most localities. The exceptions were Poland, Hungary and Latvia ('east') with around 10%. The frequencies of D134G and V136A varied significantly between 'east' compared to 'south' and 'west'. There was a trend towards increasing frequencies of V136A from 2015 to 2018, although yearly differences were not significant.
Frequencies of the CYP51 mutation S524T, I381V, V136C, A379G, V136A, D134G (%) across Europe from 2015 to 2018. East: Poland, Latvia, Hungary. North: Denmark, Northern Germany. South: France, Belgium, Southern Germany. West: England, Scotland, Ireland. Different letters represent statistically significant differences between regions (above figures) and their interaction with years (inside figures) (P = 0.05). NS, no significant differences
Link between parameters
A principal component analysis was performed to identify putative relationships between field efficacy of specific azoles on STB, their EC50 values, as well as frequencies of CYP51 mutations identified in the local populations (Fig. 6). An apparent link between specific CYP51 mutations and EC50 values was identified, particularly for ECA, PTH-D and MCA and mutations D134G, V136A, V136C, I381V and S524T. PTH-D and S524T were highly positively correlated. TCA correlated with A379G as the only one of the four azoles.
Principal component analysis including the variables: Field effects of azoles against septoria tritici blotch (ECA STB, PTH STB, TCA STB and MCA STB), EC50 of ECA, TCA, MCA and PTH-D and CYP51 mutation frequencies (D134G, V136A, V136C, A379G, I381V and S524T). Observations are shown as points and variables as vectors in the PC1-PC2 plane. PC axes are in s.d. units (explained variance in %). Ellipses: 95% confidence intervals of regions. Symbols: years 2015–2018. Epoxiconazole = ECA, prothioconazole-desthio = PTH-D, metconazole = MCA and tebuconazole = TCA
The PCA showed low correlation between EC50 values and field performances for control of STB, except for TCA, for which field performance and EC50 values were highly negatively correlated. Again, the specific points showed an obvious grouping identifying differences between the regional populations, which were most marked for the Western and Eastern populations.
Yield responses from azole treatments
Yield data of 43 trials are summarised in Fig. 7, only including trials with considerable STB severity and statistically significant yield increases. The overall yield level in control plots was ca. 80 dt/ha with a range from 25 to 108 dt/ha. As for efficacy, the average yield responses for the four azoles were significant and very similar, although again showing between year variations. Average yearly yield increases ranged from 5.4 to 10.9 dt/ha equivalent to 9–11%. Overall, no significant differences were found between the increases from the four azoles when testing for year as the main effect.
Yield of 43 trials from 2015 to 2018. Different letters represent statistically significant differences between treatments (above boxplots) and their interaction with years (below boxplots) (P = 0.05). Untreated = Untr, Epoxiconazole = ECA, prothioconazole-desthio = PTH-D, metconazole = MCA and tebuconazole = TCA
Discussion
The azole fungicide group has been used for control of fungal leaf diseases in wheat for more than four decades. Today, it is still considered one of the core groups in control strategies, with one to four of the fungicide applications applied to winter wheat including an azole component. Despite problems with resistance in the European population of Z. tritici, the azole fungicides are still widely used for control of STB as well as rust diseases in European winter wheat crops. The most common azoles are ECA, PTH, TCA and MCA used either as solo products, or more often as mixing partners with SDHI fungicides.
In this study, data from 43 field trials located across Europe confirmed that the efficacy of ECA, PTH, TCA and MCA against STB overall has become more variable and declined from 2015 to 2017. The data from 2018 with low disease levels generally did not show any further decrease in efficacy. The data provided in this paper are in line with the previous findings showing that the efficacy of individual azoles has declined (Kildea 2016; Blake et al. 2018; Heick et al. 2020) and varies substantially across Europe with reduced effects from east to west (Jørgensen et al. 2018). On average, ECA, PTH, TCA and MCA gave very similar control of around 60% against STB. However, control levels varied considerably between localities and years. In approximately 30% of the trials, the azoles gave below 50% control of STB. Such levels of control are markedly inferior to other options for STB control. In a few cases, control levels were close to minimal efficacy, e.g. specific trials in England, France and Germany where disease reduction was below 30% (Table S-1). The field data also indicate that TCA does not behave as the other azoles and its efficacy has not significantly changed or decreased during the study period across regions. In specific trials from certain sites, e.g. Ireland and Belgium, TCA has even proved to be the best performing azole (Fig. 2, Table S-1). These results confirm previous studies, which indicated that the performance of TCA has stabilised, or even improved in some regions (Heick et al. 2020). This in stark contrast to the initial drop in efficacy for TCA, seen around 2004–2006 in several regions in Western Europe (Clark 2006; Jørgensen et al. 2016). The data in this study indicated that various regions might have had distinct and different changes of efficacy from 2015 to 2018. In particular, Northern and Southern Europe seemed to have almost opposing changes in efficacy from 2016 to 2018 for ECA, PTH and MCA. Nevertheless, the interaction between years and regions produced only a few significant differences. This could be due to the weakness of the non-parametric test used for the statistical analysis, which does not reflect the high degree of variation characterising the data. Even though a parametric test might have revealed more significant differences, this test would be unreliable since it was not possible to obtain normal distribution and homogeneous variance of the data.
Major differences in sensitivity have been detected in the in vitro testing, showing a clear gradient from east to west for ECA, PTH-D and MCA (Fig. 4), although not for TCA. The data suggest a clear link between the changes in the sensitivity measured in vitro and the reduced azole field performances against STB across Europe during the course of the study. The field performance of TCA showed a different pattern compared to the three other azoles, since no significant differences were found between efficacy in the different regions and years as main effects. The patterns of in vitro TCA sensitivity were generally similar across regions, but the Southern region had significantly higher sensitivity on average. The sensitivity of Z. tritici towards TCA decreased significantly in 2018 across regions. When comparing data from across regions, results from the Southern region indicated such a development, but only in the Northern and Western regions were these changes significant.
Overall, the data indicate a stepwise decrease in sensitivity, with less sensitive strains in the population replacing the more sensitive, to the point that the most sensitive strains in the 2015 collection from Western Europe were similar in sensitivity to the least sensitive in Northern, Southern and Eastern Europe in 2018. A recent study from Ireland, based on data from 2005 to 2015, also showed changes in sensitivity in Z. tritici (Kildea et al. 2019), and an accompanying decline in the field performance of azole fungicides against STB. A similar pattern in UK Z. tritici populations was described by Blake et al. (2018). Changes in pathogen sensitivity have also been observed in France (Garnault et al. 2019) and in Denmark from 2012 to 2018 with the performance of ECA and PTH particularly affected (Heick et al. 2017a; Heick et al. 2020).
As a result of the intensive use of azoles, fungicide resistance is gradually evolving in the European Z. tritici population, although at different rates. In areas with high disease pressure and intensive fungicide use, such as in Ireland and the UK, the adaptation of the local Z. tritici populations to the azoles has been faster. In the current study, the recorded disease level was quite similar in the Southern, Northern and Western part of Europe. Only the Eastern part of Europe had less severe intensity of STB. However, the collected data do confirm that a gradual erosion in efficacy has taken place and selection for resistance is ongoing in most parts of Europe, as previously highlighted (Heick et al. 2017a; Huf et al. 2018; Garnault et al. 2019; Mäe et al. 2020).
The current study also shows apparent differences in the CYP51 mutations across Europe for specific regions. Although in general, the frequencies of the CYP51 mutations, V136C, A379G and I381V have been quite stable across the four years, this has not been the case for other mutations. For example, the S524T mutation was more frequent in the Western European Z. tritici population—with approximately 50% appearance in 2018 compared with Eastern Europe, which had only trace levels in the populations. Other studies have shown that isolates with high EC50 values for ECA and PTH-D carried the S524T mutation in combination with mutations like D134G and V136A (Leroux and Walker 2011; Cools and Fraaije 2013; Kildea et al. 2019). The mutations D134G and V136A are often linked and have now been recorded across most of Europe. Huf et al. (2018) concluded that strains harbouring alterations D134G and V136A showed a decreased sensitivity to ECA and PTH-D. Those strains have likely been selected for by the widespread use of ECA and PTH in recent years. However, strains with these mutations tend to have remained sensitive to other azoles, such as difenoconazole and TCA (Leroux and Walker 2011; Buitrago et al. 2014). The A379G mutation was relatively stable across the four seasons and the four regions. The principal component analysis in this study showed that this mutation had an adverse effect on TCA efficacy, and in this respect might also represent a less shifted population, as seen in Eastern Europe. From 2007 to 2010, isolates with A379G in combination with I381V dominated the European Z. tritici population (Stammler et al. 2008; Stammler and Semar 2011; Buitrago et al. 2014).
Over the past 15 years, a considerable number of mutations in the CYP51 gene of Z. tritici have emerged and been documented (Leroux and Walker 2011; Cools and Fraaije 2013). These mutations often occur in combinations giving rise to numerous haplotypes (Huf et al. 2018). The populations described in this paper reflect the overall dominance of the most important specific mutations based on STB symptoms from leaf samples and do not represent specific haplotypes. Full description of haplotypes requires the isolation of a significant numbers of Z. tritici isolates per locality and full sequencing. Several of the specific haplotypes of Z. tritici have variable impacts on particular azoles. In a recent study, a new nomenclature described 33 different CYP51 haplotypes based on a collection of 331 isolates across Europe. Nine of these haplotypes were found to represent 85% of all isolates, suggesting a very heterogeneous distribution across Europe (Huf et al. 2018). The most dominant haplotype 'E4’ carrying D134G, V136A and I381V was the most widespread across Europe (Huf et al. 2018).
In the present study, it should also be noted that the sensitivity profile and mutation frequencies are from isolates from the untreated control plots. The PCA analysis could look somewhat different if the sensitivity and mutation frequencies were from the various fungicide treatments. The main result from the PCA is that with a very diverse set of trials with extremely diverse sensitivity profiles the most common theme (PC1, accounting for 1/3 of the variation in the data) among them all was that increased mutations or decreased sensitivity was inversely related to efficacy. In this study, we focused on development of European azole resistance in the Z. tritici population, but recent reports from the USA and Australia have also shown a delayed but still significant adaptation of the local Z. tritici populations towards azole fungicide (Sykes et al. 2018; McDonald et al. 2019).
There are currently two established theories on how azole resistance in Z. tritici might have spread within Europe. Brunner et al. (2008) proposed that resistance inducing CYP51 mutations emerged at a few places, perhaps in the UK or Denmark, from where it spread eastward due to the prevailing wind direction from west to east. The gradient in mutation frequencies across Europe identified in the presented study could support this theory. However, an alternative theory is that the variable profiles of CYP51 mutations in the Z. tritici populations have emerged and been selected for locally. This theory cannot be ruled out and, indeed, was shown to explain the development of STB resistance in Australia (McDonald et al.2019). The variation in frequencies results from differences in disease pressure and intensity of fungicide use. Spread of resistance in Z. tritici populations could, however, be a combination of both theories, where both the spread of ascospores across vast distances in a prevailing wind direction in combination with the intensive use of fungicides drive the selection. Studies have shown that selection of CYP51 mutations can take place locally, as demonstrated in specifically designed field trials, where individual azoles and azole-combinations have been applied (Heick et al. 2017b).
While EC50 values showed clear cross-resistance patterns (Fig. 4), field performance results were less clear. Field testing represents a more uncertain environment, where many factors can influence the final efficacy. These factors include among others: disease levels, the timing of application, the timing of last assessments and the impact of other leaf diseases. Similar examples of weak links between field efficacy, mutation frequencies and in vitro sensitivity of local Z. tritici populations have been reported in other studies (Stammler et al. 2008). Even so, in the study based on the first two years of data (Jørgensen et al. 2018), the best correlations between field efficacies were found for ECA, PTH and MCA and the lowest correlations between TCA and ECA and PTH. Interestingly MCA correlates relatively well with all the other azoles. The PCA analysis presented in this paper (Fig. 6) again confirms a closer link between ECA, PTH and MCA, while TCA shows less cross-resistance.
Although a significant reduction in azole sensitivity has been observed in the European Z. tritici population, the azole fungicides still play a critical role in the control of STB across the continent. This role concerns both direct control of STB, and as a partner for the SDHIs as part of a broader anti-resistance strategy (Dooley et al. 2016). It has been shown that selection for azole resistance can be reduced using spray strategies composed of azoles with a different cross-resistance pattern compared to spray strategies composed of azoles representing only one cross-resistance group (Heick et al. 2017b). Jørgensen et al. (2018) demonstrated that higher STB control levels could be achieved from azole mixtures compared to the use of a single azole. Although all azoles are affected by sensitivity shifting and mutations, Mullins et al. (2011) have demonstrated that the effects of specific azoles are differently impacted by mutations occurring in the azole-binding pocket, providing variable affinity of the various azoles. However, the intensive use of azole mixtures has increased the risk for development of more complex CYP51 variants. This was, for example, the case for mixtures of prochloraz (selecting for V136A) and TCA (selecting for I381V), which resulted in selection for variants with both V136A and I381V (Lucas et al. 2015).
Other strategies to reduce the emergence of azole resistance, such as diversifying the spraying strategy by including multi-site inhibitors or single-site inhibitors with other modes of action (MoA) in spray programs, can reduce azole selection pressure (van den Bosch et al. 2014a). Several other strategies, e.g. limiting the number of applications, adjusting dose rates and applying alternation or mixing of fungicides with different MoA, have been described as effective tools in fungicide resistance management in a number of studies (van den Berg et al. 2013; van den Bosch et al. 2014b; Lucas et al. 2015).
In 2020, a new azole mefentrifluconazole (Bryson et al. 2018) was introduced to the European market. This new active is expected to replace some of the currently available azoles due to its high intrinsic activity on STB, as shown in several trials across Europe (Jørgensen et al. 2020a). Although mefentrifluconazole outperformed the four tested azoles in the field for control of STB, in vitro studies have shown cross-resistance with TCA and difenoconazole (Heick et al. 2020). The expected introduction of the Quinone inside Inhibitor (QiI) fenpicoxamid (Owen et al. 2017) and a new generation of QoI fungicides like metyltetraprole (Suemoto et al. 2019) may introduce options for more diverse and successful control of STB and reduce the overall pressure on using azoles.
In a previous yield response analysis, the benefit from the azoles was linked to the dominating disease, where the control of yellow rust gave rise to the biggest yield increases (Jørgensen et al. 2018). Yield data from trials in this project verify that despite the reduced efficacy of the azoles, they can still provide a yield benefit of around 10% when used as single products. Although, from a farmer's viewpoint, this is still a beneficial increase, the yield benefit is inferior to other more efficient fungicides, such as SDHI/azole mixtures and the new azole, mefentrifluconazole (Jørgensen et al. 2020b). The drop in efficacy of azoles highlights the needs to include other IPM-related means of managing STB like resistant varieties, adjustments of sowing dates, etc.
Conclusion
Data from four season’s azole testing across Europe showed variable, but still beneficial levels of STB control from the four included azoles at the majority of sites tested. The field performances have become significantly more variable in terms of achieved control of STB, and at 30% of the sites, the control was below 50%. The in vitro sensitivity of local Z. tritici populations to all four azoles has also decreased significantly during the four seasons, with a drift in sensitivity from east to west in Europe. The decrease in sensitivity is closely linked to key CYP51 mutations. The three azoles ECA, PTH and MCA showed higher levels of cross-resistance than TCA.
References
AHDB (2018) https://cereals.ahdb.org.uk/crop-management/disease-management/fungicide-performance.aspx. Accessed 4 Apr 2018
Blake JJ, Gosling P, Fraaije BA, Burnett FJ, Knight SM, Kildea S, Paveley ND (2018) Changes in field dose–response curves for demethylation inhibitor (DMI) and quinone outside inhibitor (QoI) fungicides against Zymoseptoria tritici, related to laboratory sensitivity phenotyping and genotyping assays. Pest Manag Sci 74:302–313. https://doi.org/10.1002/ps.4725
Brunner PC, Stefanato FL, McDonald BA (2008) Evolution of the CYP51 gene in Mycosphaerella graminicola: evidence for intragenic recombination and selective replacement. Mol Plant Pathol 9:305–316. https://doi.org/10.1111/j.1364-3703.2007.00464.x
Bryson RJ, Stammler G, Hu A, Strobel D, Meyer L, Moronval MH (2018) Mefentrifluconazole - the first Isopropanol-azole fungicide for the control of Zymospetoria tritici including field isolates with known complex CYP51 haplotypes. In 12e Conference Int. sur les Mal. des Plantes, 11 12 decembre 2018, Tours, Fr. Vegephyl, pp 222–231
Buitrago C, Frey R, Wullschleger J, Sierotzki H (2014) An update on the genetic changes in the CYP51 gene of Mycosphaerella graminicola and their relationship to DMI fungicides sensitivity. In: Dehne DH, Deising HB, Fraaije B et al (eds) Proceedings of the modern fungicides and antifungal compounds 2014. Phytomedizinische Gesellschaft, Reinhardsbrunn, pp 103–110
Clark B (2006) Fungicide resistance: Are we winning the battle but losing the war? Asp Appl Biol 78:127–132
Cools HJ, Fraaije BA (2013) Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag Sci 69:150–155. https://doi.org/10.1002/ps.3348
Cools HJ, Mullins JG, Fraaije BA, Parker JE, Kelly DE, Lucas JA et al (2011) Impact of recently emerged sterol 14a-demethylase (CYP51) variants of Mycosphaerella graminicola on azole fungicide sensitivity. Appl Environ Microbiol 77:3830–3837. https://doi.org/10.1128/aem.00027-11
Dooley H, Shaw MW, Spink J, Kildea S (2016) Effect of azole fungicide mixtures, alternations and dose on azole sensitivity in the wheat pathogen Zymoseptoria tritici. Plant Pathol 65:124–136. https://doi.org/10.1111/ppa.12395
FRAC (2009) SEPTTR microtiter monitoring method BASF 2009 v. 1. https://www.frac.info/docs/default-source/monitoring-methods/approved-methods/septtr-microtiter-monitoring-method-basf-2009-v1.pdf?sfvrsn=ad99419a_4. Accessed 6 Mar 2018
Garnault M, Duplaix C, Leroux P, Couleaud G, Carpentier F, David O, Walker A-S (2019) Spatiotemporal dynamics of fungicide resistance in the wheat pathogen Zymoseptoria tritici in France. Pest Manag Sci 75:1794–1807. https://doi.org/10.1002/ps.5360
Gisi U, Pavic L, Stanger C, Hugelshofer U, Sierotzki H (2005) Dynamics of Mycosphaerella graminicola populations in response to selection by different fungicides. In: Dehne HW, Gisi U, Kuck KH, Russell PE, Lyr H (eds) Modern fungicides and antifungal compounds IV. 14th international Reinhardsbrunn symposium. Friedrichroda, Thuringia, Germany, April 25–29, 2004. British Crop Protection Council, UK, pp 89–101
Gladders P, Paveley N, Barrie I, Hardwick N, Hims M, Langton S, Taylor M (2001) Agronomic and meteorologic factors affecting the severity of leaf blotch caused by Mycosphaerella graminicola in commercial wheat crops in England. Ann Appl Biol 138:301–311. https://doi.org/10.1111/j.1744-7348.2001.tb00115.x
Heick TM, Justesen AF, Jørgensen LN (2017a) Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic-Baltic region—a status. Eur J Plant Pathol 149:669–682. https://doi.org/10.1007/s10658-017-1216-7
Heick TM, Justesen AF, Jørgensen LN (2017b) Anti-resistance strategies for fungicides against wheat pathogen Zymoseptoria tritici with focus on DMI fungicides. Crop Prot 99:108–117. https://doi.org/10.1016/j.cropro.2017.05.009
Heick TM, Matzen N, Jørgensen LN (2020) Reduced field efficacy and sensitivity of demethylation inhibitors in the Danish and Swedish Zymoseptoria tritici populations. Eur J Plant Pathol 157:625–636. https://doi.org/10.1007/s10658-020-02029-2
Huf A, Rehfus A, Lorenz KH, Bryson R, Voegele RT, Stammler G (2018) Proposal for a new nomenclature for CYP51 haplotypes in Zymoseptoria tritici and analysis of their distribution in Europe. Plant Pathol 67:1706–1712. https://doi.org/10.1111/ppa.12891
Jess S, Kildea S, Moody A, Rennick G, Murchie AK, Cooke LR (2014) European Union policy on pesticides: implications for agriculture in Ireland. Pest Manag Sci 70:1646–1654. https://doi.org/10.1002/ps.3801
Jørgensen LN, Hovmøller MS, Hansen JG, Lassen P, Clark B, Bayles R, Rodemann B, Flath K, Jahn M, Goral T, Czembor J, Cheyron P, Maumene C, Pope C, Ban R, Nielsen GC, Berg G (2014) IPM strategies and their dilemmas including an introduction to www.eurowheat.org. J Integr Agric 13:265–281. https://doi.org/10.1016/S2095-3119(13)60646-2
Jørgensen LN, Matzen N, Kristjansen HS, Jensen KP, Almskou-Dahlgaard A (2016) Disease control in cereals. In: Applied crop protection 2015, vol 074. DCA rapport Markbrug, pp 18–66
Jørgensen LN, van den Bosch F, Oliver RP, Heick TM, Paveley ND (2017) Targeting fungicides inputs according to need. Annu Rev Phytopathol 55:181–203. https://doi.org/10.1146/annurev-phyto-080516-035357
Jørgensen LN, Matzen N, Hansen JG, Semaskiene R, Korbas M, Danielewicz J, Glazek M, Maumene C, Rodemann B, Weigand S, Hess M, Blake J, Clark B, Kildea S, Batailles C, Ban R, Havis N, Treikale O (2018) Four azoles’ profile in the control of Septoria, yellow rust and brown rust in wheat across Europe. Crop Prot 105:16–27. https://doi.org/10.1016/j.cropro.2017.10.018
Jørgensen LN, Heick TM, Matzen N, Madsen HP, Kirkegaard S, Kristjansen HS, Almskou-Dahlgaard A (2020a) Disease control in cereals. In: Applied crop protection 2019, vol 167. DCA report, pp 17–57
Jørgensen LN, Matzen N, Havis N, Holdgate S, Clark B, Blake J, Glazek M, Korbas M, Danielewicz J, Maumene C, Rodemann B, Weigand S, Kildea S, Bataille C, Brauna-Morževska E, Gulbis K, Ban R, Berg G (2020b) Efficacy of common azoles and mefentrifluconazole against septoria, brown rust and yellow rust in wheat across Europe. In: Deising HB; Fraaije B; Mehl A; Oerke EC; Sierotzki H; Stammler G (eds) Modern fungicides and antifungal compounds, vol IX pxx. ©2019 Deutsche. Phytomedizinische Gesellschaft, Braunschweig (in press)
Kildea S (2016) Wheat disease control and resistance issues. In: National tillage conference 2016. Teagasc crops environment and land use programme, Oak Park Crops Research, Carlow, pp 39–46
Kildea S, Heick TM, Grant J, Mehenni-Ciz J, Dooley H (2019) A combination of target-site alterations, overexpression and enhanced efflux activity contribute to reduced azole sensitivity present in the Irish Zymoseptoria tritici population. Eur J Plant Pathol 154:529–540. https://doi.org/10.1007/s10658-019-01676-4
Lancashire PD, Bleiholder H, Langeluddecke P, Stauss R, van den Boom T, Weber E, Witzen-Berger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601. https://doi.org/10.1111/j.1744-7348.1991.tb04895x
Leroux P, Walker A-S (2011) Multiple mechanisms account for resistance to sterol 14α-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag Sci 67:44–59. https://doi.org/10.1002/ps.2028
Lucas JA, Hawkins NJ, Fraaije BA (2015) The evolution of fungicide resistance. Adv Appl Microbiol 90:29–92
Lupton FGH (1972) Further experiments of photosynthesis and translocation in wheat. Ann Appl Biol 71:69–79
Mäe A, Fillinger S, Sooväli P, Heick TM (2020) Fungicide sensitivity shifting of Zymoseptoria tritici in the Finnish-Baltic region and a novel insertion in the MFS1 promoter. Front Plant Sci 11:385. https://doi.org/10.3389/fpls.2020.00385
McDonald BA, Mundt CC (2016) How knowledge of pathogen population biology informs management of septoria tritici blotch. Phytopathology 106:948–955. https://doi.org/10.1094/PHYTO-03-16-0131-RVW
McDonald MC, Renkin M, Spackman M, Orchard B, Croll D, Solomon CPS, Milgate A (2019) Rapid parallel evolution of azole fungicide resistance in Australian populations of the wheat pathogen Zymoseptoria tritici. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01908-18
Mullins JG, Parker JE, Cools HJ, Togawa RC, Lucas JA, Fraaije BA, Kelly DE, Kelly SL (2011) Molecular modelling of the emergence of azole resistance in Mycosphaerella graminicola. PLoS ONE. https://doi.org/10.1371/journal.pone.0020973
Oepp/Eppo (2014a) Foliar and ear diseases on cereals (1/26 (4)). EPPO Bull 44
Oepp/Eppo (2014b) PP 1/226 (3) Number of efficacy trials. Bulletin OEPP/EPPO Bulletin (2019) 49(1):21–24
Owen WJ, Yao C, Myung K, Kemmitt G, Leader A, Meyer KG, Bowling AJ, Slanec T, Kramer VJ (2017) Biological characterisation of fenpicoxamid, a new fungicide with utility in cereals and other crops. Pest Manag Sci 73:2005–2016. https://doi.org/10.1002/ps.4588
R Core Team (2017) R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria. https://www.R-project.org/. Accessed 20 Apr 2018
RStudio Team (2019) RStudio: integrated development for R. RStudio, Inc., Boston, MA. https://www.rstudio.com/. Accessed 20 Sep 2019
Sierotzki H, Mehl A, Stammler G (2019) Molecular detection methods for fungicide resistance. In: Stevenson KL, McGrath MT, Wyenandt CA (eds) Fungicide resistance North America, 2nd edn. APS, St. Paul, pp 175–193
Stammler G, Semar M (2011) Sensitivity of Mycosphaerella graminicola (anamorph: Septoria tritici) to DMIs across Europe and impact on field performance. EPPO Bull 41:149–155
Stammler G, Carstensen M, Koch A, Semar M, Strobel D, Schlehuber S (2008) Frequency of different CYP51-haplotypes of Mycosphaerella graminicola and their impact on epoxiconazole-sensitivity and -field efficacy. Crop Prot 27:1448–1456. https://doi.org/10.1016/j.cropro.2008.07.007
Stammler G, Taher K, Koch A, Haber J, Liebmann B, Bouagila A, Yahyaoui A, Nasraoui B (2012) Sensitivity of Mycosphaerella graminicola isolates from Tunisia to epoxiconazole and pyraclostrobin. Crop Prot 34:32–36
Suemoto H, Matsuzaki Y, Iwahashi F (2019) Metyltetraprole, a novel putative complex III inhibitor, targets known QoI-resistant strains of Zymoseptoria tritici and Pyrenophora teres. Pest Manag Sci 75:1181–1189
Sykes EM, Sackett KE, Severns PM, Mundt CC (2018) Sensitivity variation and cross-resistance of Zymoseptoria tritici to azole fungicides in North America. Eur J Plant Pathol 151:269–274. https://doi.org/10.1007/s10658-017-1370-y
Torriani SF, Melichar JP, Mills C, Pain N, Sierotzki H, Courbot M (2015) Zymoseptoria tritici: A major threat to wheat production, integrated approaches to control. Fungal Genet Biol 79:8–12. https://doi.org/10.1016/j.fgb.2015.04.010
van den Berg F, van den Bosch F, Paveley ND (2013) Optimal fungicide application timings for disease control are also an effective antiresistance strategy: a case study for Zymoseptoria tritici (Mycosphaerella graminicola) on wheat. Phytopathology 103:1209–1219. https://doi.org/10.1094/PHYTO-03-13-0061-R
van den Bosch F, Paveley N, van den Berg F, Hobbelen P, Oliver R (2014a) Mixtures as a fungicide resistance management tactic. Phytopathology 104:1264–1273. https://doi.org/10.1094/PHYTO-04-14-0121-RVW
van den Bosch F, Oliver R, van den Berg F, Paveley N (2014b) Governing principles can guide fungicide-resistance management tactics. Annu Rev Phytopathol 52:175–195. https://doi.org/10.1146/annurev-phyto-102313-050158
Zhan J, Stefanato FL, McDonald BA (2008) Selection for increased cyproconazole tolerance in Mycosphaerella graminicola through local adaptation and in response to host resistance. Mol Plant Pathol 7:259–268. https://doi.org/10.1111/j.1364-3703.2006.00336.x
Acknowledgements
Special thanks to all the technicians, who have been involved in the trial work, and to BASF SE, who financed the project (Grant No. 22498) and for collaboration with Dr. Rosie Bryson, Dr. Jens Bruns and Mr. Dieter Strobel, all three from BASF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jørgensen, L.N., Matzen, N., Heick, T.M. et al. Decreasing azole sensitivity of Z. tritici in Europe contributes to reduced and varying field efficacy. J Plant Dis Prot 128, 287–301 (2021). https://doi.org/10.1007/s41348-020-00372-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00372-4