Abstract
Marine macroalgae contain a variety of bioactive substances that have many different uses. Ulvan, a sulfated hetero-polysaccharide isolated from Ulva lactuca, has been described and investigated for its antioxidant, antiviral, antibacterial, and cytotoxic action against the cell lines PC-3 and A-549. The yield of polysaccharides was 26.53 ± 0.32. (Algal dry weight) with 2.23% uronic acid. According to HPLC estimates, the polysaccharide isolated from Ulva lactuca contains the neutral sugars glucose, rhamnose and galactose, as well as fructose, and melibiose. The FT-IR spectrum of ulvan revealed bands at 1227.73 cm− 1 and 1333.97 cm− 1, respectively, indicative of sulfate esters and uronic acids. This study came to the conclusion that Ulva lactuca ulvan (ULU) contains beneficial elements that can stop the development of cancer cells. The lowest viability of PC-3 cells, which occur at the highest concentration of ULU (500 µg/ml), was 17.58% with an IC50 of 237.02 ± 6.84 µg/ml, while the lowest viability of lung carcinoma cells was 13.91% with an IC50 of 124.38 ± 4.78 µg/ml. This indicates that the cytotoxicity against the prostate cancer cell line was lower than that against the lung carcinoma cell line. Although ULU has no activity against adenovirus, it had antiviral efficacy against HAV-10. ULU has been shown to have antibacterial efficacy against pathogenic bacteria, including Staphylococcus aureus ATCC25923 Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC 29,212, Pseudomonas aeruginosa ATCC 9027, Klebsiella pneumonia ATCC 13,883, Pseudomonas fluorescent ATCC13525 and Streptococcus agaloctiae ATCC13813. The polysaccharide, meanwhile, has limited antioxidant action.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Since ancient times, our ancestors have exploited Mother Nature as an outstanding source of important natural substances for a wide range of uses, including medicinal, biological, nutritional, and cosmetic ones. (Salih et al. 2021). One of the dozens of natural product families, polysaccharides are macromolecular polymeric carbohydrate molecules consisting up of long chains of monosaccharide units. They are primarily used for structural or storage purposes and can be found in plants, animals, and microbes. Numerous studies have demonstrated the significant inhibitory effects of natural polysaccharides and their chemically modified derivatives against viral infections including HIV and herpes simplex virus (HSV) (Lee et al. 2017; Chen and Huang 2018).
Sulfated polysaccharides (SPs) are a form of negatively charged polysaccharide that have sulfate moieties in their carbohydrate backbone, either naturally occurring or artificially induced. The majority of SPs are found in the cell walls of marine algae or seaweeds; they are far less frequent in some animals, such as fish skins, and very uncommon in mangrove plants. Studying SPs is crucial for drug discovery efforts because they have important biological functions like antioxidant, anti-allergic, antiviral, anticancer, and anticoagulant characteristics (Chen and Huang 2018; Ngo and Kim 2013).
The percentage of sulfated polysaccharides in the seaweed cell wall is about 40%, which is higher than the norm in other sources. The most intriguing marine algal SPs are produced from brown macroalgae (fucoidan), red macroalgae (carrageenan), and green macroalgae (ulvan) (Chen and Huang 2018; Hans et al. 2021; Muthukumar et al. 2020). The antiviral mechanisms of these polysaccharides are influenced by a number of variables, including the degree of sulfation, molecular weight, content, and structure. Polysaccharides from marine algae offer a variety of antiviral actions, low production costs, low cytotoxicity, and widespread acceptability, which could result in promising antiviral medications (Geetha and Tuvikene 2021). In this regard, treatment of HSV infected Hep-2 cells with a highly sulfated (SO4− 2 = 22%) ulvan fraction from U. compressa resulted in 100% HSV inhibition at 100 µg ml− 1 and had an IC50 of 28.2 µg ml− 1, compared to 153 µg ml− 1 for native ulvan (SO4− 2 = 6%) (Lopes et al. 2017).
Ulvan and its oligosaccharides may have anticancer and immunomodulatory activities, according to several studies (Jiao et al. 2011; Liu et al. 2019). The cytotoxic effects of ulvan on three human cancer cell lines—hepatocellular carcinoma, human breast cancer, and cervical cancer were examined by Thanh et al. (2016), who found that it exhibits considerable cytotoxic activity. In mice with the H22 tumor and animals that had been rendered immunosuppressed by cyclophosphamide, Zhao et al. (2020) examined the anticancer and immunological regulatory actions of Ulva lactuca polysaccharide (ULP) and discovered promising outcomes. Ulvan from Ulva pertusa has been reported to have substantial antioxidant and antihyperlipidemic capabilities, although the specific group of heteropolysaccharides responsible for these actions is unclear (Li et al. 2018a). By assessing antiviral, antibacterial, anticancer, and antioxidant capabilities, this study aims to isolate, characterize, and validate the pharmacological, medical, and dietary applications of ULU. It is worth mentioning that this is the first attempt to assess ULU cytotoxicity against (PC-3 & A-549) and antiviral activity against (HAV-10 & Adenovirus) to benefit from this alga, which may produce green bloom and environmental pollution in some regions (Abu-Ahmed et al. 2021).
Material and Method
Collection and Processing of Algal Samples
Ulva lactuca Linnaeus, a member of the Ulvaceae family, was used in this study. U. lactuca was identified using the methods of Aleem (1978), Aleem (1993), Lipkin and Silva (2002), and confirmed using the Algae Base website (M.D. Guiry in Guiry 2020). In May 2020, samples were collected by hand from the rocky areas of Abu Qir bay in Alexandria, Egypt (latitudes 30° 16’ and 30° 28’ N and longitudes 31° 03’ and 31° 22’ E.). Following collection, all samples were washed several times in seawater to remove adhering debris, associated biota, and sand, and then in tap water to remove salts. The algal samples were air dried in a shaded area before being dried in an oven (Memmert, Germany) at 60 °C for about 3 h. The dried samples were ground into fine particles using a coffee grinder (Brown mill) and stored at room temperature in plastic bags for future experiments.
Extraction of Ulvan
A total of 30 g of dried algal powder was subjected to hot water extraction in an autoclave for 30 min at 120 oC using deionized water (DI) in a 1:20 (w/v) ratio. The hot aqueous extract was then filtered with nonwoven fabric and centrifuged for 15 min at 6708 g in a cooling centrifuge (Centrikon T-124, Italy). To remove small-size elements, the extract was dialyzed for 48 h against distilled water at 4 oC. Using a lyophilizer, the dialyzed aqueous extract was concentrated to 10–20% of its initial volume. At -20 oC, four volumes of absolute ethanol were added to the concentrated aqueous extract to precipitate ULU. After 48 h the precipitate was centrifuged at 6708 g for 15 min in a cooling centrifuge. Finally, the recovered precipitate was dried in a vacuum oven at 60 oC. For future experiments, the dried ULU sample was stored in sterilized falcon tubes (Reis et al. 2018). The percentage of ULU yield was calculated by dividing the final ulvan weight by the initial weight of the dried powder (Yaich et al. 2017). ULU yield (%) = (We/Wf)×100 Where We is the dry ulvan weight extracted and Wf is the macroalga dry weight.
Characterization of the Extracted ULU
Elemental Analysis, Protein, Water, and Ash Content
Combustion was used to quantify the elements carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) in ULU powder (Vario MICRO cube, elementar, Germany). The Lowry method was used to calculate the soluble protein content (Lowry et al. 1951), while the total sugar content was determine in trifluoroacetic acid (TFA) hydrolyzate by phenol-sulfuric acid assay (DuBois et al. 1956) in which a volume of 0.5 ml of 5% (v/v) phenol solution and 2.5 ml of concentrated sulfuric acid were added to 0.5 ml hydrolyzed sample. The mixture was shaken, heated in a boiling water-bath for 20 min and cooled to room temperature. The absorption of the solution at 490 nm was then determined by UV-VIS spectrophotometer. Total sugar concentration was calculated from a standard curve using different concentrations of glucose. The water content of the samples was determined after 24 h of ignition at 103 °C in an oven, it was determined as a percentage of the dry weight (Madany 2019). According to Lahaye and Jegou (1993), the ash content was determined gravimetrically, in a muffle furnace, 70 mg of dried polysaccharide samples were incinerated for 14 h at 550 °C. Meta-hydroxydiphenyl method was used in determination of uronic acid content (Filisetti-Cozzi and Carpita 1991) in which 0.4 ml of ulvan (1 mg\ml) in water was added to 2.4 ml of concentrated H2SO4 containing up to 120 mM sodium tetraborate, then the sample was heated to about 100 °C in a boiling water bath for 20 min in 6 ml tube capped with marbles to prevent condensation from contaminating the sample. The tube were chilled in an ice bath, up to 150µL of m-hydroxydiphenyl reagent was added, incubation of the tube was done at ambient temperature for 15 min to 1 h before measurement of absorbance scanned from 400 to 700 nm, and by using a standard curve we can determine the uronic acid concentration.
FTIR
The functional groups in the extracted ulvan were identified using an FTIR spectrometer (Bruker, ALPHA, Germany) equipped with the attenuated total reflectance (ATR) technique. The transmission spectra were recorded using KBr (Merck®) pellets containing 2.5 mg of ULU powder. The spectra were acquired in the 4000–400 cm− 1 range with a resolution of 4.0 cm− 1 over 128 scans after subtracting the atmospheric background interferences (Saleh et al. 2020).
ULU HPLC Analysis
With some modifications, a ULU sample was subjected to acid hydrolysis as described by Toskas et al. (2012). In the reaction tube, 5 ml of 4 M TFA was added to 13 mg of the sample. Hydrolysis was performed in an oven at 124 °C for 6 h. At 40 °C, the hydrolysate was evaporated. After drying, the hydrolysate was dissolved in 3 ml DI water and analyzed for monosaccharide composition using HPLC (Agielnt, USA), which was outfitted with a Binary HPLC pump with an injector, a refractive index detector (RI, 2410) at 35 °C, and a software monitor with the Breeze program. Supelco supplied the LC-NH2 column (SUPELCOILTM LC- NH2, 250 4.6 mm, 5 μm). The column effluent was monitored using a refractive index detector. The mobile phase acetonitrile/water (85: 15) solvent system at 1.5 ml/min flow rate. Ten µl samples were injected into the HPLC using acetonitrile/water as eluent at a flow rate of 1.5 ml\ min. Chromatographic peaks were identified by comparison with reference sugars (L-rhamnose, galactose, glucose, xylose, L-arabinose, melibiose and fructose), supplied by Sigma Aldrich, India, for monosaccharide determination, the software program Breeze identified and quantified the sugar profile in the sample.
Evaluation of ULU Cytotoxicity
Mammalian Cell Lines
The American Type Culture Collection (ATCC, Manassas, VA, USA) provided the Vero cells (derived from the kidney of an African green monkey), PC-3 and A-549 cell lines. The used cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM), which included 10% heat-inactivated foetal bovine serum (FBS), 1% L-glutamine, HEPES buffer, and 50 µg/ml gentamycin. All cells were cultured twice a week and kept at 37 °C in a humidified atmosphere with 5% CO2 (Vijayan et al. 2004).
Cytotoxicity Evaluation Using Viability Assay
Tumor cell lines were suspended in the medium at a concentration of 5 × 104 cell/well in Corning® 96-well tissue culture plates, and then incubated for 24 h. After that, ULU was added to 96-well plates in three replicates to achieve ten concentrations. As a control, six vehicle controls with media or 0.5% DMSO were run for each 96-well plate. After incubating for 24 h, the numbers of viable cells were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2 H-tetrazolium bromide test (MTT test) (MTT test). The viability percentage was calculated as [(ODt/ODc)]x100%, where ODt represents the mean optical density of wells treated with ULU and ODc represents the mean optical density of untreated cells. The survival curve of each tumor cell line after treatment with ULU is obtained by plotting the relationship between surviving cells and drug concentration. The 50% inhibitory concentration (IC50, the concentration required to cause toxic effects in 50% of intact cells) was calculated using Graphpad Prism software (San Diego, CA, USA) and graphic plots of the dose-response curve for each concentration (Mosmann 1983).
Evaluation of the Antiviral Activity of ULU
Mammalian Cell Line
The American Type Culture Collection (ATCC, Manassas, VA, USA) provided the Vero cells (derived from the kidney of an African green monkey). Vero cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), which included 10% heat-inactivated foetal bovine serum (FBS), 1% L-glutamine, HEPES buffer, and 50 µg/ml gentamycin. All cells were cultured twice a week and kept at 37 °C in a humidified atmosphere with 5% CO2 (Vijayan et al. 2004).
Virus Propagation and Antiviral Assay
In confluent Vero cells, the cytopathogenic HAV HM175 strain (ATCC VR-1402) of the Hepatitis A virus and Adenovirus strain 2 were propagated and tested. The Regional Center for Mycology and Biotechnology conducted the antiviral screening (RCMB, Al-Azhar University, Cairo, Egypt). This assay was chosen to demonstrate specific inhibition of a biological function, specifically a cytopathic effect in susceptible mammalian cells measured using the MTT method (Hu and Hsiung 1989; Al-Salahi et al. 2015; Randazzo et al. 2017). The Spearman-Karber method was used to count infectious viruses by determining the 50% tissue culture infectious dose (TCID50) with eight wells per dilution and 2 µl of inoculum per well (Pinto et al. 1994). In the cytotoxicity assay, the Vero cell lines were seeded in 96-well plates at a cell concentration of 2 × 105 cells per ml in 100 µl of growth medium. After 24 h of seeding, a fresh medium with varying concentrations of ULU was added. A multichannel pipette was used to add serial two-fold dilutions of ULU (ranging from 1 µg/ml to 500 µg/ml) to confluent cell monolayers dispensed into 96-well, flat-bottomed microtiter plates (Falcon, Jersey, NJ, USA). The microtiter plates were incubated for 48 h at 37 °C in a humidified incubator with 5% CO2. For each concentration of the tested sample, three wells were used. Control cells were incubated with or without test samples and DMSO. The small amount of DMSO present in the wells (maximum 0.1%) did not affect the experiment. Following the incubation period, the viable cell yield was determined using an MTT colorimetric method (Mosmann 1983) and the following equation: [(A − B)/(C − B)] × 100% where A, B, and C indicate the absorbance of the ULU with virus-infected cells, the absorbance of the virus control and the absorbance of the cell control, respectively.
The 50% cytotoxic concentration (CC50), or the concentration required to cause toxic effects in 50% of intact cells, was calculated using Graphpad Prism software from graphic plots of the dose-response curve for each concentration (San Diego, CA. USA). ULU’s maximum non-toxic concentration (MNTC) was also determined and will be used in future biological studies.
Data Analysis
Using the STATA modeling software, the dose that inhibited viral infection by 50% (EC50) in comparison to the virus control was calculated. The percentages of viral inhibition concerning each tested virus represent the mean standard error of three different experiments’ mean values. The EC50 values were calculated directly from the curve obtained by plotting the virus yield inhibition against the concentration of ULU. The selectivity index (SI) was calculated using the CC50/EC50 ratio to determine whether each compound had a sufficient antiviral activity that outweighed its toxicity (Al-Salahi et al. 2015). This index is known as a therapeutic index, and it was used to determine whether a compound was necessary. Compounds with an SI-value of 2 or higher were considered active (Al-Salahi et al. 2015).
Antibacterial Activity
Bacterial Strain Activation
Bacterial strains were inoculated into Mueller Hinton broth (Difco) and incubated at 37oC for 24 h. The cultures were subjected to three successive 24 h transfers before use. All cultures were adjusted to 106 CFU (cell forming unite) per ml before use.
In Vitro Microbial Sensitivity Tests
One gram of ULU was dissolved separately in 10 ml of distilled sterilized water to give a stock solution of 100 mg/ml). The stock solution was autoclaved and kept at 4º C until used. The tested sample was evaluated for its antibacterial activity against Staphylococcus aureus ATCC25923, Escherichia coli ATCC8739, Pseudomonas aeruginosa ATCC 9027, Bacillus subtilis ATCC6633, Enterococcus Faecalis ATCC 29,212, Klebsiella pneumonia ATCC13883, Pseudomonas fluorescent ATCC13525, Streptococcus agalactiae ATCC13813, and Klebsiella pneumoniae ATCC13883.
All tested microorganisms were cultured onto a Muller Hinton agar medium which was prepared by adding 38 g of agar powder to one liter of distilled water and the mixture was boiled. The solution was autoclaved at 121 °C for 20 min and cooled to 50 °C in a water bath. It was then conveyed into sterile petri dishes.
Bacterial and spore suspensions of the tested microorganisms were set to a concentration of 108 cells/ml. In the disc diffusion method, the aliquot was spread onto Muller Hinton agar by a sterilized cotton swab. Then the plated solid medium was allowed to dry at room temperature (Lopez et al. 2011). Sterile paper discs of 6 mm in diameter were saturated with 5 µL samples (about 0.5 mg/disc), left for one hour for dryness and placed on the agar surface of the inoculated plates. Each disc needs be pushed down to ensure complete contact with the agar surface. The discs must be distributed no closer than 25 mm from center to center of the 150 mm plate. Discs of tetracycline (30 µg) used as a positive control. The plates are inverted and placed in an incubator set to 30 °C within 15 min after the discs are applied (Kalemba and Kunicka 2003). After 48 h the diameters of the zones of complete inhibition are measured, including the diameter of the disc. All statistical analyses were performed using SPSS software (version 16 for Windows), then, they subjected to ANOVA one way and the means were compared using Student–Newman–Keuls (SNK) multiple range test at p ≤ 0.05.
Antioxidant Activity of ULU
The antioxidant activity of ULU was determined in triplicate at Al- Azhar University’s Regional Center for Mycology and Biotechnology (RCMB) using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays, with average values considered.
DPPH Radical Scavenging Activity
A freshly prepared (0.004%w/v) DPPH radical methanol solution was prepared and stored at 10 ℃ in the dark. ULU aqueous solution (2-1000 µg/ml) was prepared. To 3ml of DPPH solution, a 4 µl aliquot of ULU aqueous solution was added. A UV-visible spectrophotometer was used to immediately measure absorbance at 515 nm (Milton Roy, Spectronic 1201). The decrease in absorbance was measured continuously, with data recorded every 1 min until the absorbance stabilized (16 min). The absorbance of the DPPH radical (control) and the reference compound ascorbic acid were also measured. The DPPH radical’s percentage inhibition (PI) was calculated using the formula: PI = [{(AC- AT)/ AC} x 100] Where AC = Absorbance of the control at t = 0 min and AT = absorbance of ULU + DPPH at t = 16 min (Yen and Duh 1994). Graphpad Prism software was used to estimate the 50% inhibitory concentration (IC50), or the concentration required to achieve 50% DPPH radical scavenging activity (San Diego, CA. USA).
Ferric Reducing Antioxidant Power (FRAP)
The reducing power of ULU was determined using the methods proposed by Banerjee and Maulik in 2002 and Sutharsingh et al. in 2011. This method is based on the reduction of ferricyanide in the presence of various concentrations of ULU.1ml ULU aqueous solution was mixed with 2.5ml 0.2 M sodium phosphate buffer (pH 6.6) and 2.5ml potassium ferricyanide [K3Fe (CN)6] (1%, w/v). After 20 min at 50 °C, the reaction mixture was acidified with 2.5 ml of 10% w/v trichloroacetic acid. For 10 min, the reaction mixture was centrifuged at 1000 g. The supernatant solution was combined with 2.5 ml of deionized water and 0.5 ml of freshly prepared ferric chloride (0.1%, w/v). A spectrophotometer was used to measure the absorbance of the resulting solution at 700 nm versus a blank (Milton Roy, Spectronic 1201). The reference standard was ascorbic acid. According to Canabady-Rochelle et al. (2015), the reducing capability percentage (%) was calculated as follows.
where, A0: absorbance of the control solution. As: ULU absorbance.
Result and Discussion
Ulva lactuca Collection and Extraction Yield
In comparison to Hussein et al. (2015)’s estimate for U. lactuca (14.83 ± 0.78%), the ULU yield (dry weight) was 26.53 ± 0.32%, which is almost twice as much. The extraction yield is significantly influenced by the time of year that algae are collected. The algae were harvested in May because this is actively growing month, and the large production during this time may be caused by insufficient ulvan settling in freshly constructed walls (Robic et al. 2009; Madany 2019).
Chemical Analysis and Monosaccharide Composition of ULU
Table 1 and Fig. 1 show the proximate chemical analyses, monosaccharide content, and elemental analysis of ULU. 30 g (dry weight) of U. lactuca powder yields 7.96 g ulvan, which represents 26.53%, 13.3% ash, 41.96% water, 3.27% protein, and 35.06% total sugars. According to the elemental analysis, the percentages of C, H, N, and S in ULU are 29.23, 4.61, 2.04, and 7.08, respectively. The HPLC analysis of ULU monosaccharide revealed the presence of three neutral sugars, galactose, rhamnose and glucose, with concentrations of 5.11, 6.02 and 8.05 µg/g dry wt., with % dry weight 19.2, 23.3 and 35.21 respectively, and this is consistent with Hussein et al. (2015), who found four neutral sugars (rhamnose, glucose, galactose, and xylose) in Ulva fasciata ulvan. Detections of fructose, and melibiose at 4.22 and 6.14 µg/g dry wt., with % dry weight 9.2 and 12.59 respectively, were also recorded (Fig. 1), it is worth to say that rhamnose occur in small amount as collection time was in May and that goes in harmony with Madany 2019 who collected Ulva sp in May and October and recorded the lowest amount of rhamnose in May, the detected uronic acid content was 2.23% and this result also goes in harmony with Madany 2019 who detected small amount of uronic acid in Ulva sp collected in May. The seaweeds’ enhanced photosynthetic activity during the collecting season (May), which accelerates their growth rate and maturation, may be responsible for the high sugar content and decreased protein content (Rico and Fernández 1996).
Otherwise, the high ash content was mostly brought on by the high sulfate content of seaweed at the maximal growth period (Costa et al. 2012), which is consistent with other research findings (Peasura et al. 2015; Mao et al. 2006). The hydrophilic and hygroscopic nature of ulvan makes its water content relatively high 41.96% as this percentage is compatible with its function in the algae where it acts as osmo-protectant and forms stiff gel to increase the stiffness of the cell wall while maintain its flexibility (Alves et al. 2010).
FTIR Analysis of ULU
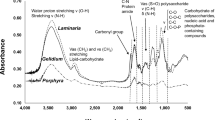
The FTIR analysis of the ULU show several distinct absorption peaks. The IR spectra of the sulfated polysaccharides obtained from other Ulva species are similar and equal (Hernández-Garibay et al. 2011; Peasura et al. 2015; Tian et al. 2015; Aguilar-Briseno et al. 2015; Olasehinde et al. 2019). Following that, the IR bands were allocated based on previously published sulfated polysaccharide data, which are summarized in (Fig. 2). The FTIR spectrum revealed a broad and strong absorption band at 3429.02 cm− 1, which related to the stretching vibration of hydroxyl (O-H) group. The peak at 2930 cm− 1 was recognized to the stretching vibration of the methyl group’s aliphatic C-H bond, which is typical of polysaccharides (Mao et al. 2006). The bands in the spectrum around 1714.48-1734.25 cm− 1 are suggestive of C = C stretching of phenyl compounds, while the signal at 1633.24 cm− 1 was allocated to the stretching vibration of the (C = O) group, and the asymmetric stretching vibration of the (COO-) group, and the signal at 1417.98-1457.52 cm− 1 was assigned to the symmetric stretching vibration of the COOH group’s bond (Colthup 1950; Mao et al. 2006; Robic et al. 2009; Tian et al. 2015). Bands of carboxylate groups of uronic acid with similar intensities are present in spectra range around 1633.24 and 1417.98 cm− 1 (Andrade et al. 2020) but due to the low content of uronic acid that was detected in the sample, the peaks appear weak. The sulfated nature of the polysaccharide was determined by the absorption band at 1227.73 cm− 1, which is related to the stretching vibration of the sulfate ester group (S = O), which agrees with Ibrahim et al. (2022) who used FTIR analysis to confirm the presence of sulfated groups attached to the carbohydrate backbone in Ulva lactuca ulvan. The stretching vibration of the C-O-C band was assigned the band at 1096.05 cm− 1 (Tian et al. 2015).
Cytotoxicity of ULU Against PC-3 & A-549 Cell Lines
Uncontrolled cell development that can invade, metastasize, and spread to other locations is known as cancer (El-Kassas and Attia 2014). The creation of novel biological therapies that target various aspects of the tumor has been made possible by the expanding understanding of cancer biology, which has occurred concurrently with the acquisition of new technologies (such as molecular profiling and chemical plasticity) (Zugazagoitia et al. 2018). ULU had an anti-cancer effect against lung carcinoma cell line at all concentrations except 1–15.6 µg/ml, where there was no effect, with IC50 = 124.38 ± 4.78 µg/ml (Table 2).
According to Mofeed et al. (2021), who found that algal extracts displayed strong dose-dependent anti-cancer action, the viability of carcinoma cells declines with increasing ULU extract concentration until it reaches (13.91%) at the highest ULU concentration (500 µg/ml). Thanh et al. (2016) used the cytotoxic activity against human breast cancer (IC50 25.09 ± 1.36 µg/ml), hepatocellular carcinoma (IC50 29.67 ± 2.87 µg/ml), and cervical cancer (IC50 36.33 ± 3.84 µg/ml) to calculate ULU’s anticancer activity. Amin and Abdelreheem (2020) estimated the cytotoxicity of ULU and found that it has a substantial impact on numerous cancer cell types. The cytotoxicity of ULU against prostate cancer cell lines is also shown in Table 2, however it is less severe than against lung cancer cell lines. The lowest viability of PC-3 cells was 17.58% with an IC50 of 237.02 ± 6.84 µg/ml at the greatest dose of ULU (500 µg/ml). Numerous indications point to the anticancer effects of bioactive chemicals produced from algae through several mechanisms, including the prevention of cancer cell growth, invasion, and metastasis as well as the induction of apoptosis in cancer cells (Farooqi et al. 2012). Either an intrinsic (mediated by mitochondria) or an extrinsic (mediated by death receptors) strategy can cause apoptosis (Brenner and Mak 2009; Mellier et al. 2010). Each of these mechanisms involves signal transduction and ultimately leads to apoptosis (Park et al. 2012).
ULU Antiviral Activity
The VERO cell line (Table 3) and MTT test (Table 4) were used to assess ULU’s antiviral activity against HAV-10 and adenovirus. Table 3 shows the cytotoxic activity of ULU with Mammalian cells from African Green Monkey Kidney (Vero) cells under these experimental conditions, with a cell cytotoxic concentration (CC50) of 325.59 ± 9.47 µg/ml. Table 4 shows that ULU has antiviral activity against all tested viruses at the maximum non-cytotoxic concentration (MNCC) (100 µg/ml).
This cytotoxic activity was weak (+) against the HAV-10 virus (13.94 ± 1.62% inhibition) and did not affect the Adenovirus (0%) inhibition. ULU’s best antiviral activity was against (HAV-10) but all results didn’t exceed the reference drug (Amantadine). According to Koenighofer et al. (2014), marine sulfated polysaccharides exhibit promising inhibitory effects on a variety of viral strains. These compounds work to stop virus particle internalization by interfering with interactions between the virus and its host cell receptors. The antiviral properties of ULU may result from polysaccharide adsorption on viruses or receptors found on cell surfaces (Yuhao et al. 2018).
Antibacterial Activity
The antibacterial activity of ULU varied significantly according to the tested microbes. Generally, it was lower than the effect of the tetracycline of the tested microbes (Table 5; Fig. 3a, b). Ulvan extract showed antimicrobial activity using the disc diffusion method toward Pseudomonas aeruginosa ATCC9027, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC29212, Klebsiella pneumonia ATCC 13,883, Vibrio damsela, Pseudomonas fluorescent ATCC13525, and Streptococcus agalactiae ATCC13813. These results are in agreement with others obtained by Ibrahim et al. (2022), who demonstrated a moderate inhibition zone ranged from 11 to 15 mm against P. aeruginosa ATCC9027, P. fuorescens ATCC17386, K. pneumoniae ATCC13883 and B. subtilis ATCC 6633. In addition, similar results were obtained by El Halmouch et al. (2023), who recorded the positive antibacterial activity of the purified exopolysaccharides (EPS) of Bacillus paralicheniformis ND2, varied between 10 mm against Streptococcus agalactiae ATCC 13,813 to 22 mm against Staphylococcus aureus ATCC 25,923.Also, the antibacterial activity by EPS extracted from B. subtilis SH1 was observed by Hassan and Ibrahim (2017) against Aeromonas hydrophila, Pseudomonas aeruginosa and Streptococcus faecalis.
Extracts of U. lactuca were also reported to show antiprotozoal and antimycobacterial activity by Spavieri et al. (2010). In the same context, the antibacterial activity of ulvan derived from U. reticulate was studied by Tran et al. (2018), who showed strong antibacterial action, with an inhibition zone diameter of 18 mm for E. coli and 20 mm for Enterobacter cloacae. Whereas our results showed no activity appeared toward Staphylococcus aureus ATCC25923, Escherichia coli ATCC8739, and Vibrio fluvialis. These results are inconsistent with others reported by Ibrahim et al. (2022), who found that the values of inhibition zones were 11 and 18 mm against E. coli ATCC8739 and S. aureus ATCC25923, respectively. The highest inhibition zones were observed Pseudomonas aeruginosa ATCC9027, and Klebsiella pneumonia ATCC13883, they were 12.7 and 12.3 mm, respectively.
Antioxidant Activity of ULU
Table 6 showed that ULU is a poor antioxidant, this polysaccharide activated both DPPH and FRAP, and the activity (%) increased in a concentration-dependent manner, but its antioxidant efficacy does not exceed that of vitamin C (reference drug). According to Duh (1998), reducing properties are generally related to the presence of reductions that prevent peroxide formation by reacting with certain peroxide precursors. The results show that ULU’s low antioxidant activity is due to its low reducing power. Some seaweed components may act as reductants by giving electrons and rejoining with free radicals to transform them to more stable products, thus terminate the free-radical chain reaction (El-Sheekh et al. 2021). According to Li et al. (2018b), ulvan from the alga Ulva pertusa (Chlorophyta) has significant antioxidant and antihyperlipidemic activities; nevertheless, the responsible group of heteropolysaccharides in these activities is unidentified. Rahimi et al. (2016) evaluated antioxidant activity of ulvan via using DPPH scavenging activity assay which measure protection against oxidative damage by cell extracts indicated that Ulvan have the ability to scavenge DPPH radicals, and this agree with our results.
Conclusion
ULU has valued components that can decrease the development of cancer cells; however, its cytotoxicity against the prostate cancer cell line was lower than that against lung carcinoma cell line. ULU showed antiviral activity against HAV-10 but has no activation against the Adenovirus. The antibacterial activity of ULU against the tested pathogenic bacteria (Staphylococcus aureus ATCC25923 Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC29212, Pseudomonas aeruginosa ATCC9027, Klebsiella pneumonia ATCC13883, Pseudomonas fluorescent ATCC13525, and Streptococcus agaloctiae ATCC13813) has promising results that indicated a good antibacterial agent. ULU also showed weak antioxidant activity. Further study on ULU and other natural products may value and support its biological activity with no side effects on the human beings as a natural source exploited in the environmental and pharmaceutical fields.
Data Availability
All of the material is owned by the authors and no permissions are required.
References
Abu-Ahmed S, Deyab M, AL-Afefy A, El-Adl M (2021) Ulva species blooms and its biochemical composition in relation to aquatic Environmental Properties at North Damietta- Egypt. Scient J Damietta Fac Sci 11(1):47–55
Aguilar-Briseno A, Cruz-Suarez LE, Sassi JF, Ricque-Marie D, Zapata-Benavides P, Mendoza-Gamboa E, Rodriguez-Padilla C, Trejo-Avila LM (2015) Sulfated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar Dru 13:697–712
Al-Salahi R, Alswaidan I, Ghabbour HA, Ezzeldin E, Elaasser MM, Marzouk M (2015) Docking and antiherpetic activity of 2-Aminobenzo[de]-isoquinoline-1,3-diones. Molecules 20:5099–5111
Aleem AA (1978) Contribution to the study of the marine algae of the red sea. I- the algae in the neighborhood of al-Ghardaqa, Egypt (Cyanophyceae, Chlorophyceae and Phaeophyceae. Bull Fac Sci King Abdulaziz Univ Jeddah 2:73–88
Aleem AA (1993) “Marine algae in Alexandria, Egypt. Alexandria Privately Published 1–135
Alves A, Caridade SG, Mano JF, Sousa RA, Reis RL (2010) Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. " Carbohydr Res 345(15):2194–2200
Amin HH, Abdelreheem MAT (2020) Comparative effect of Ulvan and Biosynthesized Silver Nanoparticles on different cell lines cytotoxicity and gene expression. OnLine J Biol Sci 20(2):125–133
Andrade FT, Silva D, Enrich-Prast A, Yoneshigue-Valentin A, Yocie, Oliveira V (2020) Structural characterization of Ulvan Polysaccharide from Cultivated and Collected Ulva fasciata (Chlorophyta). Advances in Bioscience and Biotechnology. 11:206–216. https://doi.org/10.4236/abb.2020.115016
Banerjee SK, Maulik SK (2002) Effect of garlic on cardiovascular disorders. Nutr J 19:1–4
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. Curr Opin Cell Biol 21:871–877
Canabady-Rochelle LL, Harscoat-Schiavo C, Kessler V, Aymes A, Fournier F, Girardet JM (2015) Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem 183: 129–135
Chen L, Huang G (2018) The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol 115:77–82
Colthup NB (1950) Spectra-structure correlations in the infra-red region. J Opti Soci Amer 40:397–400
Costa C, Alves A, Pinto PR, Sousa RA, Borges da Silva EA, Reis RL, Rodrigues AE (2012) Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carb Polym 88:537–546
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) “Colorimetric method for determination of sugars and related substances " Analytical Chemistry 28(3):350–356
Duh PD (1998) Antioxidant activity of Budrock (Arctium lappa L.): its scavenging effect on free radical and active oxygen. The J Amer Oil Chem Soci 75:455–461
El Halmouch Y, Ibrahim H, Dofdaa N, Mabrouk M, El-Metwally M, Nehira T, Ferji K, Ishihara Y, Matsuo K, Ibrahim M (2023) Complementary spectroscopy studies and potential activities of levan-type fructan produced by Bacillus paralicheniformis ND2. Carbohydr Polym 311:120743. https://doi.org/10.1016/j.carbpol.2023.120743
El-Kassas HY, Attia AA (2014) Bactericidal application and cytotoxic activity of biosynthesized silver nanoparticles with an extract of the red seaweed pterocladiella capillacea on the HepG2 cell line. As Pac J Can Prev 15(3):1299–1306
El-Sheekh MM, Hassan L, Morsi HH (2021) Growth enhancement of Spirulina platensis through optimization of media and nitrogen sources. Egypt J Bot 61(1):61–69
Farooqi A, Butt G, Razzaq Z (2012) Algae extracts, and methyl jasmonate anti-cancer activities in prostate cancer: choreographers of ‘the dance macabre’. Can Cell Inter. https://doi.org/10.1186/1475-2867-12-50
Filisetti-Cozzi TM, Carpita, NC (1991) Measurement of uronic acids without interference from neutral sugars. Anal biochem 197(1): 157–162
Geetha BR, Tuvikene R (2021) Potential antiviral Properties of industrially important Marine Algal Polysaccharides and their significance in fighting a future viral pandemic. Vir 13(9):1817–1838
Guiry GM (2020) In: Guiry MD, Guiry GM (eds) AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. https://www.algaebase.org
Hans N, Malik A, Naik S (2021) Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Biores Technol Rep 13:100623
Hassan SWM, Ibrahim HAH (2017) Production, characterization and valuable applications of exopolysaccharides from marine Bacillus subtilis SH1. Pol J Microbiol 66(4):449–461
Hernández-Garibay E, Zertuche-González JA, Pacheco-Ruíz I (2011) Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J Appl Phycol 23:537–542
Hu JM, Hsiung GD (1989) Evaluation of new antiviral agents I: in vitro prospective. Antivir Res 11:217–232
Hussein M, Hamouda R, El-Naggar N, Karim-Eldeen M (2015) Characterization, antioxidant potentiality and biological activities of the polysaccharide ulvan extracted from the marine macroalga ulva sp. J Agricul Chem Biotech 6(9):373–392
Ibrahim MIA, Amer MS, Ibrahim HAH, Zaghloul EH (2022) Considerable production of Ulvan from Ulva lactuca with special emphasis on its Antimicrobial and Anti-fouling Properties. Appl Biochem Biotechnol 194:3097–3118
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Dr 9:196–223
Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10:813–829
Koenighofer M, Lion T, Bodenteich A, Prieschl-Grassauer E, Grassauer A, Unger H, Mueller CA, Fazekas T (2014) Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Resp Med 9:57–68
Lahaye M, Jegou D (1993) Chemical and physical-chemical characteristics of dietary fibres from Ulva lactuca (L.) Thuret and Enteromorpha compressa (L.) Grev. J Appl Phycol 5(2):195–200
Lee S (2017) Chap. 4–Strategic Design of Delivery Systems for Nutraceuticals. In Nanotechnology Applications in Food. Oprea AE, Grumezescu AM Eds. Acad Pr Camb MA USA 65–86
Li W, Jiang N, Li B, Wan M, Chang X, Liu H, Zhang L, Yin S, Qi H, Liu S (2018a) Antioxidant activity of purified ulvan in hyperlipidemic mice. Int J Biol Macromol 113:971–975
Li W, Wang K, Jiang N, Liu X, Wan M, Chang X, Liu D, Qi H, Liu S (2018b) Antioxidant and antihyperlipidemic activities of purified polysaccharides from Ulva pertusa. J App Phycol 30. https://doi.org/10.1007/s10811-018-1475-5
Lipkin Y, Silva PC (2002) Marine algae and seagrasses of the Dahlak Archipelago, southern Red Sea. Nova Hedwigia 75(1/2): 1–90
Liu Z, Gao T, Yang Y, Meng F, Zhan F, Jiang Q, Sun X (2019) Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules 24:4286–4299
Lopes N, Ray S, Espada S, Bomfim W, Ray B, Galhardi L, Linhares R, Nozawa C (2017) Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int J Biol Macromol 102. https://doi.org/10.1016/j.ijbiomac.2017.04.043
Lopez V, Jager AK, Akerreta S, Cavero RY, Calvo MI (2011) Pharmacological properties of Anagallis arvensis L. (“scarlet pimpernel”) and Anagallis foemina Mill. (“blue pimpernel”) traditionally used as wound healing remedies in Navarra (Spain). J Ethnopharmacol 134:1014–1017
Lowry OM, Rosebrough NJ, Farr LA, Randall RJ (1951) Protein measurements with folin phenol reagent. J Biol Chem 193:265–275
Madany MA (2019) Fabrication of nanofibrous hybrid based on ulvanextracted from Ulva species (green algae) and its possible biotechnological applications. Ph D Thesis Alex Univ Fac Sci Eg
Mao W, Zang X, Li Y, Zhang H (2006) Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J Appl Phycol 18:9–14
Mellier G, Huang S, Shenoy K, Pervaiz S (2010) TRAILing death in cancer. Mol Asp Med 31:93–112
Mofeed J, Deyab M, Sabry A, Ward F. (2021) In Vitro Anticancer Activity of Five Marine Seaweeds Extract From Egypt Against Human Breast and Colon Cancer Cell Lines. 15 pp Res Squ. https://doi.org/10.21203/rs.3.rs-462221/v1
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immun Meth 65:55–63
Muthukumar J, Chidambaram R, Sukumaran S (2020) Sulfated polysaccharides and its commercial applications in food industries - a review. J Food Sci Technol 58(7):2453–2466
Ngo DH, Kim SK (2013) Sulfated polysaccharides as bioactive agents from marine algae. Inter J Biol Macromol 62:70–75
Olasehinde TA, Mabinya LV, Olaniran AO, Okoh AI (2019) Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Inter J Food Prop 22:100–110
Park AH, Sugiyama M, Harashima S, Kim YH (2012) Creation of an ethanol-tolerant yeast strain by genome reconstruction based on chromosome splitting technology. J Microbiol Biotechnol 22(2):184–189
Peasura N, Laohakunjit N, Kerdchoechuen O, Wanlapa S (2015) Characteristics and antioxidant of Ulva intestinalis sulfated polysaccharides extracted with different solvents. Inter J Biol Macromol 81:912–919
Pinto RM, Diez JM, Bosch A (1994) Use of the colonic carcinoma cell line CaCo-2 for in vivo amplification and detection of enteric viruses. J Med Virol 44:310–315
Rahimi F, Tabarsa M, Rezaei M (2016) Ulvan from green algae Ulva intestinalis: optimization of ultrasound-assisted extraction and antioxidant activity. J Appl Phycol 28:2979–2990. https://doi.org/10.1007/s10811-016-0824-5
Randazzo JW, Piqueras J, Diaz R, Aznar R, Sanchez G (2017) Improving efficiency of viability-qPCR for selective detection of infectious HAV in food and water samples. J Appl Microbiol 124:958–964
Reis RP, Carvalho JAA, Facchinei AP, Calheiros ACS, Castelar B (2018) Direct Effects of Ulvan and a Flour produced from the Green Alga Ulva fasciata Delile on the Fungus Stemphylium solani Weber. Algal Res 30:23–27
Rico JM, Fernández C (1996) Seasonal nitrogen metabolism in an intertidal population of Gelidium latifolium (Gelidiaceae, Rhodophyta). Europ J Phycol 31:149–155
Robic A, Bertrand D, Sassi JF, Lerat Y, Lahaye M (2009) Determination of the chemical composition of ulvan, a cell wall polysaccharide from Ulva spp (Ulvales, Chlorophyta) by FT-IR and chemometrics. J Appl Phycol 21:451–456
Saleh AM, Zaghloul EH, Ibrahim MIA (2020) Characterization of exopolysaccharide produced from marine-derived aspergillus terreus SEI with prominent biological activities. Egypt J Aquat Res 46:363–369
Salih AEM, Thissera B, Yaseen M, Hassane ASI, El-Seedi HR, Sayed AM, Rateb ME (2021) Marine Sulfated Polysaccharides as Promising Antiviral Agents: a comprehensive report and modeling study focusing on SARS CoV-2. Mar Drugs 19:406–437
Spavieri J, Kaiser M, Casey R, Hingley-Wilson S, Lalvani A, Blunden G, Tasdemir D (2010) Antiprotozoal, antimycobacterial and cytotoxic potential of some british green algae. Phytother Res 24:1095–1098
Sutharsingh R, Kavimani S, Jayakar B, Uvarani M, Thangathirupathi A (2011) Quantitative phytochemical estimation and antioxidant studies on aerial parts of Naravelia zeylanica DC. Inter J Pharmaceut Res II (II): 52–56
Thanh TT, Quach TM, Nguyen TN, Vu Luong D, Bui ML, Tran TT (2016) Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Inter J Biol Macromol 93(Pt A):695–702
Tian H, Yin X, Zeng Q, Zhu L, Chen J (2015) Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Inter J Biol Macromol 79:577–582
Toskas G, Heinemann S, Heinemann C, Cherif C, Hund RD, Roussis V, Hanke T (2012) Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts. Carboh Polym 89(3):997–1002
Tran TTV, Truong HB, Tran NHV, Quach TMT, Nguyen TN, Bui ML, Yuguchi Y, Thanh TTT (2018) Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat Prod Res 32:2291–2296
Vijayan P, Raghu C, Ashok G, Dhanaraj SA, Suresh B (2004) Antiviral activity of medicinal plants of Nilgiris. Ind J Med Res 120:24–29
Yaich H, Ben Amira A, Abbes F, Bouaziz M, Besbes S, Richel A, Blecker C, Attia H, Garna H (2017) Effect of extraction procedures on structural, thermal and antioxidant Properties of Ulvan from Ulva lactuca Collected in Monastir Coast. Inter J Biol Macromol 105:1430–1439
Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free radical and active oxygen species. J Agricult Food Chem 42:629–632
Yuhao S, Xiaolin C, Lin S, Song L, Huahua Y, Xueqin W, Yukun Q, Pengcheng L (2018) Antiviral activity against Avian Leucosis Virus Subgroup J of degraded polysaccharides from Ulva pertusa. BioMed Res Inter Article ID 9415965. https://doi.org/10.1155/2018/9415965
Zhao C, Guopeng L, Wu D, Dan L, Lijun Y, Petra H, Jesus S, Wang W, Galberto D, Yoshinori M, Daglia D, Haroon K, Rosanna F, Shaoyun W, Jianbo X (2020) The algal polysaccharide ulvan suppresses growth of hepatoma cells. Food Front 1:83–101
Zugazagoitia J et al (2018) Incidence, predictors, and prognostic significance of thromboembolic disease in patients with advanced ALK-rearranged non-small cell lung cancer. Europ Resp J 51:1702431. https://doi.org/10.1183/13993003.02431-2017
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Sahar O. Maray Carried out most of the practical experiments, prepared figures and tables, and co-write the manuscript text. Mohamed S. Abdel-Kareem suggested the problem of the work, and wrote the main manuscript text. Mona E. Mabrouk and Yasser El-Halmouch carried out the microbiological tests and wrote the microbiological part. Mofida E.M. Makhlof shared the problem’s suggestion, shared in the practical experiments, preparation of figures and tables, and co-write the manuscript text. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing interests
I declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maray, S.O., Abdel-Kareem, M.S.M., Mabrouk, M.E.M. et al. In Vitro Assessment of Antiviral, Antimicrobial, Antioxidant and Anticancer Activities of Ulvan Extracted from the Green Seaweed Ulva lactuca. Thalassas 39, 779–790 (2023). https://doi.org/10.1007/s41208-023-00584-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-023-00584-z