Abstract
The bamboo coral Isidella elongata is often associated with a diverse community, including commercial fish species, playing an important role in the deep-sea Mediterranean as a biodiversity hotspot. There has been a drastic decrease of the populations of this species since the twentieth century, mainly related to impacts of fishing, leading to its inclusion in the Barcelona Convention and the list of Mediterranean vulnerable marine ecosystems. However, the knowledge on its local scale distribution is still very limited. In this study, habitat suitability models were performed based on a dense population of I. elongata, located in the Mallorca Channel (western Mediterranean), to contribute to fill this knowledge gap. Generalized additive models, Maximum entropy models and Random Forest were combined into an ensemble model. Models showed that habitat is most suitable on smooth plains surrounding the seamounts of Ses Olives and Ausiàs March present in the study area. Furthermore, two models out of three showed a preference of the coral for flat areas. The predictions of the habitat suitability models presented in this study can be useful to design protection measures for this critically endangered species to contribute to the species’ and deep-sea fisheries management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isidella elongata is a cold-water coral (CWC) living on soft bottoms (Carpine and Grasshoff 1975). The species belongs to the family Keratoisididae, also known as “bamboo corals”. The family inherited its name due to its physical structure, with a candelabrum shape with ramifications consisting of brown organic nodes alternated by white carbonate internodes (Carpine and Grasshoff 1975). Isidella elongata is near-endemic to the Mediterranean Sea, but can also be found in the adjacent Atlantic waters of the Gulf of Cadiz and North Morocco (Bellan-Santini 1985; Bo et al. 2015b). Chimienti et al. (2019a) and González-Irusta et al. (2022) mapped the currently known distribution of I. elongata in the Mediterranean Sea, showing only a limited number of sites where living colonies of this species are still present. Healthy populations have been found mainly in areas where trawlers cannot operate due to (1) trawling limitations in depth (Fabri et al. 2014; GFCM 2005), (2) presence of steep slopes (Fabri et al. 2014), (3) rocky elevations (Bo et al. 2015a) or (4) protection due to the presence of submarine electricity cables nearby (Mastrototaro et al. 2017). However, a recent literature review that included unpublished sightings, suggested that the distribution of I. elongata in the eastern Mediterranean can be wider spread than previously thought (Gerovasileiou et al. 2019). Thus, recent research has contributed to enlarge our knowledge on the distribution of the species in the Mediterranean, as well as on the effects of bottom trawling for the species populations (Carbonara et al. 2022a).
Isidella elongata can be found on compact mud substrate (Pérès 1967) within a large bathymetric range between 170 and 1800 m (Bellan-Santini 1985; Fabri et al. 2014). The species is usually associated with flat areas (e.g., Bellan-Santini 1985; Pérès 1967), but it has also been found on sloping flanks up to 20° of submarine canyons in the Gulf of Lions (Fabri et al. 2014) and tributary canyons in the Catalan slope (González-Irusta et al. 2022). The species has, most probably, a low recovery capacity, as observed in other Keratoisididae species, which present slow growth rates (Andrews et al. 2009; Bo et al. 2017; Cartes et al. 2013; Maynou and Cartes 2011; Thresher et al. 2004). This, along with its unbending structure, makes the species very vulnerable to bottom trawlers (Cartes et al. 2013; Maynou and Cartes 2011; Carbonara et al. 2022).
Isidella elongata often presents a diverse associated fauna, such as anemones, shrimps, cephalopods and fish among others, generating environmental heterogeneity on the seafloor (Carbonara et al. 2020, 2022; Cartes et al. 2021, 2022; Mastrototaro et al. 2017; Pérès 1967). The threatened status of I. elongata as the only anthozoan in the Mediterranean Sea recognized as critically endangered species by the IUCN Red List (Bo et al. 2015b), contributed to its inclusion in the list of endangered or threatened species under the Specially Protected Areas and Biological Diversity (SPA/BD) Protocol to the Barcelona Convention (UNEP/MAP-SPA/RAC 2018). According to article 11 of the Protocol, protection measures are mandatory for Contracting Parties. This also led to recent resolutions that recognized I. elongata as a vulnerable marine ecosystem (VME) representative taxon by the International Council for Exploration of the Sea (ICES 2020) and recommended the establishment of a set of measures to protect VMEs formed by cnidarians by the General Fisheries Commission of the Mediterranean (GFCM 2019a). This resolution encourages contracting parties to a progressive implementation of measures to prevent significant adverse impacts of deep-sea fisheries activities.
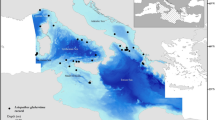
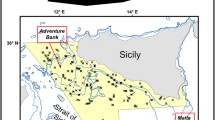
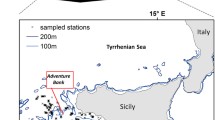
One of the densest (2300 – 2683 colonies ha−1), healthiest, and mature populations of I. elongata documented up to date, was found in the Mallorca Channel, in the Balearic Islands (Mastrototaro et al. 2017). A diverse associated community was observed, with up to 50 different identified taxa (Mastrototaro et al. 2016, 2017). The population was found between 38.59°–39.03°N and 1.75°–2.16°E (approx. 770 km2, Fig. 1), east of Ibiza, between two seamounts, Ses Olives and Ausiàs March, which form a positive relief aligned according to a NE-SW trend (Fig. 1B). Ses Olives seamount covers 59 km2 and has its summit at 250 m depth, flat on top, with flanks sloping up to 40° (Vázquez et al. 2015). Ausiàs March is larger (73 km2), with its summit at a shallower depth (86 m), moderate slopes on top (2–4°) and flanks sloping up to 32°. Both seamounts are characterized by slumps and slides that can cause debris flows and turbidity currents from the surrounding seafloor up to depths of 1000 m (see Acosta et al. 2003 for a complete description). The summit of the seamount Ausiàs March was protected from trawl fisheries under the Spanish fisheries legislation (Orden AAA/1504/2014). Furthermore, bottom trawling is forbidden near submarine electricity cables present in the area (Fig. 1; Acosta et al. 2004; Mastrototaro et al. 2017). These two measures are thought to have contributed to the preservation of this population (Acosta et al. 2004; Mastrototaro et al. 2017).
To date, the known spatial distribution of I. elongata is limited to single observations by trawl hauls, longline fishery, or remotely operated vehicle (ROV) transects (e.g., Carbonara et al. 2020, 2022; González-Irusta et al. 2022; Gerovasileiou et al. 2019; Ingrassia et al. 2019; Mastrototaro et al. 2017; Pierdomenico et al. 2018). Some studies have addressed distributional patterns on different Mediterranean regions (the Strait of Sicily, central Mediterranean Sea, Lauria et al. 2017; Southern Adriatic Sea, Carbonara et al. 2022), including large-scale patterns in the Balearic Sub Basin (González-Irusta et al. 2022). However, information on the distributional patterns at a local scale is lacking. Habitat suitability models (HSMs) have been widely used to map the spatial extent of organisms in the deep-sea, where data acquisition is costly (e.g., Burgos et al. 2020; Morato et al. 2020; Wang et al. 2022). The aim of our study is to develop HSMs and predictive maps for the occurrence of I. elongata in the Mallorca Channel, using the data available from the healthy and dense populations found in 2014 (Mastrototaro et al. 2017). The results will improve the current knowledge on the preferred habitat for this species and will contribute to the assessment and management of this critically endangered species.
Materials and Methods
Data Collection and Processing
Occurrences of I. elongata were recorded by means of non-invasive visual ROV surveys carried out by OCEANA on board the Ketch Catamaran Ranger in waters off the Balearic Archipelago (Fig. 1) from 2006 to 2014 (for technical details, see Mastrototaro et al. 2017). Mastrototaro et al. (2017) documented the occurrence of I. elongata colonies in five out of 58 ROV video transects with a total of 1043 presence records. In the study of Mastrototaro et al. (2017), presences and absences were recorded at a high spatial resolution, often less than 1 m in between colonies, covering a depth range between 480 and 615 m.
Bathymetric data were acquired using a EM12 Multibeam Echosounder (Kongsberg) on the R/V Hespérides in the framework of the "Exclusive Economic Zone (EEZ) Spanish Program" from 1995 to 1997. This program was led by Instituto Español de Oceanografía and Instituto Hidrográfico de la Marina (IEO-IHM). The echosounder was designed to operate in open waters, from 50 m to 11,000 m depth and uses a 13 kHz frequency with 81 beams. The beam width ranges from 1.8° to 3.5° with a swath width of up to 3.5 times the water depth. Backscatter data were not available due to acquisition configuration and therefore, backscatter facies could not be differentiated. The bathymetry was processed using CARIS SIPS and HIPS v10.4 and the gridded data (50 × 50 m) was projected to WGS 1984 UTM Zone 31N. Terrain variables were derived from bathymetric data. Slope, aspect and roughness were derived using the ‘raster’ package in R (Hijmans 2020). While slope defines the steepness of the seafloor, roughness describes the topographic rugosity in a given area (i.e., difference between a location and the eight corresponding surrounding cells), useful to identify areas with potential high diversity as presence of habitat-forming species (Lundblad et al. 2006). Aspect, which measures seafloor surface direction, was further processed into northness and eastness as measurements for the orientation of any particular location. The Bathymetric Position Index (BPI) classifies landscape structure (e.g., valleys, plains, hill tops) based on the change in slope position over two scales, therefore, describing the relative elevation of a seabed location compared to neighbouring areas (Lundblad et al. 2006, Walbridge et al. 2018). It was calculated as the difference between the cell value and average of the corresponding surrounding cells in a given radius (Lundblad et al. 2006) on a fine (at 15–45 cell radius) and a broad (at 300–600 cell radius) scale, using the Benthic Terrain Model tool in ArcGIS (Wright et al. 2005).
Oceanographic variables were also tested (supplement 1) as potential predictor variables, although they could not be used in the models due to their coarse spatial resolution. Vessel Monitoring System (VMS) data was analysed and processed into fishing days per year per grid cell (supplement 2). However, most of the I. elongata observations were located in areas with zero fishing days per year. Therefore, the fishing dataset was not informative for the modelling process, but it is further discussed.
A Priori Selection of Non-collinear Relevant Predictor Variables
Collinearity was evaluated between all terrain variables using Spearman's rank correlation coefficient and the Variation Inflation Factor (VIF, Zuur et al. 2009). A correlation coefficient ≤ 0.7 and VIF ≤ 3 (Zuur et al. 2009) was used to retain only uncorrelated predictor variables. Depth and slope were highly correlated with broad-scale BPI and roughness. Since preliminary models performed better using broad-scale BPI and roughness, depth and slope were excluded from the analysis. Finally, five terrain variables were selected, a priori, as predictor variables for I. elongata occurrence.
Habitat Suitability Modelling
Habitat suitability for the occurrence of I. elongata in the Mallorca Channel was assessed using three commonly used algorithms: (1) Generalized Additive Models (GAM, Hastie and Tibshirani 1986), (2) Maximum entropy modelling (Maxent, Phillips et al. 2006) and (3) Random Forest (Breiman 2001). The GAM was fitted using the ‘mgcv’ package in R (Wood 2011). A logit link function was used to fit the binomial error distribution of the response variable. Smooth terms were constrained to 4 knots to avoid overfitting (e.g., Morato et al. 2020; Rowden et al. 2017). Maxent was fitted using the ‘maxnet’ package in R, allowing all model features and including both presence and absence data (Phillips 2017). Random Forest was fitted using the ‘randomForest’ package in R with default settings (Breiman 2001; Liaw and Wiener 2002).
From the five a priori retained predictor variables, the most important ones were selected for each model. In GAM, forward and backward model selection were performed removing or adding one variable at a time, using the significant p-values (p < 0.05), the percentage of variance explained, and the Akaike Information Criterion (AIC) to compare the performance of the models. Maxent was trained with all variables and assigned a measure of importance to each one within the algorithm. For Random Forest, selection was done using the R package ‘VSURF’ (Genuer et al. 2019). This package uses a thresholding step, an interpretation step, and a prediction step to select variables (Genuer et al. 2019).
The importance of each predictor variable for each of the modelling approaches was assessed using a procedure adapted from Thuiller et al. (2009). Variable importance (unitless) was evaluated by calculating the correlation between the original prediction and a prediction based on a model whereby one variable is randomly permuted. This was done 50 times for each variable. In addition, this bootstrapping method for variable importance ensures that selected variables were significant and not selected not due to type I errors, emerged from spatial autocorrelation.
The relation between the predictor variables and the probability of I. elongata occurrence was assessed using response curves. For this purpose, the evaluation strip methodology of Elith et al. (2005) was followed, calculating the probability of presence across 500 values in the range of each variable. After models were fitted, a spatial prediction was made for habitat suitability in the study area. We restricted predictions to the depth range where observations were found.
To check the presence of spatial autocorrelation (SAC) in the residuals of the models, the Moran’s I index (I) was calculated (‘ape’ package in R, Paradis and Schliep 2018) as described in Dormann et al. (2007). Preliminary outcomes showed SAC in the residuals of all models except for Random Forest, which violate model assumptions preventing its correct application (Dormann et al. 2007). Therefore, an environmentally equidistant sub-sampling strategy adapted from de Oliveira et al. (2014) was used as an attempt to remove SAC in model residuals. The sub-sampling of the original dataset was done using the environmental Mahalanobis distance, calculated between all observations using the five terrain covariates. The two most distant observations were selected and added to a new dataset. Consecutively, the observation that is the most distant from the new dataset was added one by one. A balanced dataset of 150 records showed the best results to minimize SAC in the residuals.
Model performance was evaluated using k-fold cross-validation: all observations were randomly split into a training and a test dataset using 75% and 25% of the data, respectively. The model was fitted using the training set and its performance was tested based on its ability to predict the test dataset. This process was repeated 10 times (e.g., Rowden et al. 2017). For each training and test dataset, maximum true skill statistic (TSS; Allouche et al. 2006) and area under the curve (AUC; Swets 1988) were calculated. Model performances were considered good if TSS > 0.6 and AUC > 0.8 and poor when TSS < 0.2 and AUC < 0.7. Differences in evaluation metrics between models were tested using the Tukey’s Honest Significant Difference test (p < 0.05).
Model uncertainties are not homogeneously distributed in space. To highlight areas with higher uncertainty of prediction, a measure was calculated for grid cells in the study area, following the bootstrapping method used in Anderson et al. (2016). Presence-absence values were randomly redistributed among original observations with replacement, 100 times for each model. Predictions were used to calculate the percentage coefficient of variation (% CV, i.e., the ratio of the standard deviation and the mean) across every cell in the study area.
An ensemble model was used for the final habitat suitability projection (Rowden et al. 2017), combining the predictions of the GAM, Maxent and Random Forest algorithms, weighted by the model performance (AUC). Similarly, a weighted % CV was calculated as an uncertainty measure.
Results
The occurrences of I. elongata were present at a depth range of 463–612 m, on slopes of 0–10°. After equidistant sub-sampling in environmental space to remove SAC, the number of occurrences was reduced from 1043 presences and 1619 absences to 75 presences and 75 absences (Fig. 2).
All models showed good performance in predicting habitat suitability for I. elongata, scoring above 0.7 for both performance metrics used (Fig. 3). In general, Random Forest and the ensemble model performed better than the other models.
Model performance comparison using a True skill statistic (TSS) and b Area under the curve (AUC). Characters on top of the bars (a, b) denote the results of post-hoc Tukey’s Honest Significant Difference comparisons (p < 0.05). Models that do not include the same letter are significantly different for a given performance metric (e.g., Generalized Additive Models, GAM, and Random Forest do not include the same character on top of the TSS bar, so they are significantly different; while GAM and Maximum entropy models, Maxent are not significantly different for TSS)
From the five a priori selected predictors (Fig. 4), only roughness and broad-scale BPI were retained in GAM; while and roughness, fine-scale BPI and broad-scale BPI were selected in Random Forest (Table 1). The Maxent model retained all predictor variables, but northness, fine-scale BPI, and eastness had low importance. In all modelling algorithms, broad-scale BPI was the most important predictor, followed by roughness and fine-scale BPI.
Spatial autocorrelation was successfully removed from the residuals of Maxent (from I = 0.34 to I = 0.04) and the ensemble model (from I = 0.05 to I = -0.03), but it was still present in the residuals of GAM (I = 0.20).
Response curves from all models suggested that the probability of presence of I. elongata was high when broad-scale BPI values were low (Fig. 5A). More specifically, the optimal range of broad-scale BPI according to the three models was around zero or lower for GAM; from -60 to 130 for Maxent and lower than 120 for Random Forest, with a peak at broad-scale BPI values between 30 and 60 (Fig. 5A, B). The models GAM and Random Forest suggested higher probability of presence at low roughness values, from 3 to 20 (Fig. 5A, B). These results were confirmed by the ROV video footage, where I. elongata was observed in flat, muddy areas (Fig. 5C). In contrast, in the outcome of Maxent all roughness values were suitable except extreme values lower than 12 or higher than 65. Maxent and Random Forest included fine-scale BPI, which had a different effect in each model (Fig. 5A). Northness was included in Maxent and only the highest values were suboptimal for the presence of I. elongata (Fig. 5A).
Responses of Isidella elongata to the environmental variables. a Response curves, showing the relationship between probability of presence and the most important predictor variables for Generalized Additive Models, Maximum entropy models and Random Forest; b Schematic interpretation of the habitat preference; c Image of Isidella elongata on a muddy seabed near Ausiàs March (Source: OCEANA)
Spatial predictions of habitat suitability for I. elongata in the study area were mostly in agreement among the different models (Fig. 6). In general, Maxent showed clearer values for the spatial prediction, closer to either 0 or 1 (Fig. 6B). The spatial predictions of Maxent were different from those of GAM and Random Forest in an area located on the western to the northern side of Ausiàs March seamount (Fig. 6A-C). According to Maxent, this area was highly suitable, while the other models predicted lower suitability (< 0.5). In addition, in the area between the two seamounts, Random Forest predicted suitable habitat in smaller (0.05–2.5 km2) and more distinct patches than the other models (Fig. 6A-C). General spatial patterns of high suitability (> 0.9) in the individual models were similar and well captured in the ensemble model (Fig. 6D): patches of variable size (up to 10 km2) were predicted west of Ses Olives seamount, which corresponds to the corridor between the two seamounts where submarine cables are present (Fig. 1); a small patch (~4 km2) was predicted on the south eastern flank of Ses Olives seamount; and a large zone of about 24 km2 was predicted south of Ausiàs March, which is mostly flat or up to 5° of gently slopes.
Model uncertainty, given as coefficient of variation (% CV), was generally low for GAM, Maxent and the ensemble model, while uncertainty reached up to 75% CV in Random Forest (Fig. 6). Areas with higher uncertainty were present around Ses Olives seamount for all models (Fig. 6). This area corresponds to the steeper slopes of the seamount, with elevated roughness (Fig. 4A). The GAM predicted low uncertainty over the entire study area, while Maxent included four patches of higher uncertainty up to 60% CV. Random Forest had a higher and more homogenous uncertainty over the study area, with some areas with uncertainties up to 75% CV in small patches. This is likely due to the model extrapolating in areas where at least one environmental variable displays values above or below the sampled range. (Fig. 6C). Thus, the model predicts the same value as the last prediction made within the sampled range of this environmental variable.
Finally, the ensemble model showed highest uncertainty around Ses Olives and an area on top of Ausiàs March, similarly to the predictions of the three models. In general, areas with high uncertainty correspond to values of the predictor variables where no biological observations were available (Fig. 2 and 6).
Discussion and Conclusions
The HSMs constructed for I. elongata indicated that the preferred habitat for this species in the Mallorca Channel is characterized by areas not elevated compared with the wider surroundings, with a predicted preference for low bottom roughness as suggested by two out of three models (GAM and Random Forest). The area with most suitable habitat was located between Ses Olives and Ausiàs March seamounts, as well as on isolated patches surrounding them, but never on the summits. The unsuitability of elevated areas at Ausiàs March and Ses Olives seamounts for I. elongata agrees with existing sightings of this species on deep, flat and smooth mud bottoms across the Mediterranean (e.g., Bellan-Santini 1985; Gerovasileiou et al. 2019; Ingrassia et al. 2019). The rocky nature of Ses Olives and maërl beds on Ausiàs March (Vázquez et al. 2015) seems to be an unsuitable substrate for I. elongata to settle. However, most of the summit of Ses Olives is covered by a layer of soft sediment (OCEANA 2010), which could be suitable if this layer consisted of mud and was sufficiently thick for the CWC to grow on. Nonetheless, the summits of the two seamounts correspond to the upper limit of the depth range currently known for I. elongata in the Mediterranean Sea (Carbonara et al. 2020; Chimienti et al. 2019a).
The present spatial distribution and depth range of I. elongata is very likely to have been altered by historical fishing (Carbonara et al. 2022; Cartes et al. 2013; Fabri et al. 2014; González-Irusta et al. 2022; Pierdomenico et al. 2018). Although, the number of bottom trawlers around the Balearic Islands has been historically low compared to Iberian Peninsula (Quetglas et al. 2012), trawling activity below the insular continental shelf in the Mallorca Channel still targets important commercial species such as Norway lobster (Nephrops norvegicus), blue and red shrimp (e.g., Aristeus antennatus) and European hake (Merluccius merluccius, Quetglas et al. 2016). Hence, the complete potential range of distribution for this species could be masked by this anthropogenic effect as shown by González-Irusta et al. (2022). In their study, González-Irusta et al. (2022) found that deep and steep slope areas were the preferred habitat when modelling the distribution of I. elongata at regional scale for the Balearic Sub-Basin. However, the authors argue that this could be a consequence of the strong trawling impacts on the flat, soft bottom grounds (Cartes et al. 2013), which has restricted living populations to inaccessible areas for trawlers that in turn may not present the optimal habitat for the species. The differences in the spatial scales approached in González-Irusta et al. (2022) and the present study, as well as different trawling impacts on the species distribution (Bo et al. 2015a; Cartes et al. 2013; Fabri et al. 2014; Lauria et al. 2017; Pierdomenico et al. 2018) explain the contrasting results observed. Therefore, the characteristics (e.g. depth range, slope, BPI) of suitable habitat for I. elongata might be currently considered as a local adaptation of the species.
Trawl marks were observed in the study area during the ROV surveys (Mastrototaro et al. 2017) and the inspection of VMS (vessel monitoring system) data from bottom trawlers during the five years prior to sampling (2009–2014) confirmed that fishing was still occurring in the area (Supplement 2, Fig. 7). The overlap between fishing activity and the occurrence of I. elongata based on the ensemble model, is the highest at the south of Ausiàs March seamount (Fig. 7). A regular trawling haul with an average duration of 2 h (FAO 1996) using a common otter trawl of 3 m wide (Notti et al. 2013) and at a fishing speed of 2.5 knots (Hintzen et al. 2010), results in a swept area of 2.8 ha on average. Therefore, and considering the high densities of I. elongata colonies in the study area (2300 – 2683 colonies·ha−1, Mastrototaro et al. 2017), a single trawling haul could impact up to 6389–7453 colonies by damaging or removing them entirely (Cartes et al. 2013; Maynou and Cartes 2011). The impact of fishing activities on I. elongata forests (sensu Rossi et al. 2017) was already observed at 18 km west of the study area (Mastrototaro et al. 2017), with fishing rates as low as 0.5 fishing days·yr−1 according to VMS data (Supplement 2). The density of colonies found in this impacted area was 38 times lower (53–62 colonies·ha−1) than in the Mallorca Channel. Given the slow growth rate known for other bamboo coral species (~1.4 cm·yr−1 axial growth rate, Andrews et al. 2009), the average time needed for colonies of 20–30 cm high to recover from the direct impact of this single trawling haul would be between 15–21 years, without considering other disturbances.
Few studies have addressed HSM on I. elongata (e.g. Lauria et al. 2017; Carbonara et al. 2022; González-Irusta et al. 2022), and there is only one published for I. lofotensis (Burgos et al. 2020), a species from the same genus inhabiting the Nordic Seas. Isidella lofotensis is found in Norway and Greenland at depths between 200 and 700 m (Buhl-Mortensen et al. 2015) and, like I. elongata, inhabits soft sandy muds (Buhl-Mortensen and Buhl-Mortensen 2014) and its main habitat preferences are described by terrain variables (Burgos et al. 2020). However, Lauria et al. (2017), Burgos et al. (2020) and González-Irusta et al. 2022 found some oceanographic variables to be also relevant for predicting suitable habitat for Isidella species. For example, temperature and silicate concentration, which relate to the presence of different water masses and the type of sediment, seemed to be important factors for the distribution of I. lofotensis (Burgos et al. 2020). Furthermore, Lauria et al. (2017) found that sea bottom temperature, salinity and currents were important variables for the distribution of I. elongata in the Strait of Sicily. Indeed, this population seemed to develop better in areas that are exposed to strong currents (up to 0.15 m s−1) due to increased food availability and low sedimentation rates (Lauria et al. 2017).
In our study area, seawater temperature ranged from 13–14 °C and salinity was ~38.5 between 400 and 600 m, similar to the temperature and only marginally lower than the salinity range (38.7 – 38.9) found optimal for I. elongata by Lauria et al. (2017). The general circulation patterns around the Mallorca Channel (Barceló-Llull et al. 2019) and throughout the study area also could enhance the availability of food to the I. elongata population. In the Mallorca Channel, I. elongata at 463–612 m is mainly influenced by the Levantine Intermediate Water (LIW) (López-Jurado et al. 2008). The LIW passing through the Mallorca Channel is fed by dense waters originating in cascading events in the Gulf of Lions (Canals et al. 2006; López-Jurado et al. 2008; Puig et al. 2013). Here, during cold winter winds, water masses start to sink downslope, transporting turbid shelf water and re-suspended sediment (Puig et al. 2013). This dense water mixes partially with LIW and can reach as far as the Balearic Islands and the Mallorca channel (Puig et al. 2013). In addition, close to the study area, a south-eastward flow with a maximal velocity of 0.015 m s−1 is constantly present throughout the year, centred at 100 m, but reaching up to 600 m depth (Barceló-Llull et al. 2019). Although in our study water velocity is one order of magnitude lower than the observed velocity in the Strait of Sicily (Lauria et al. 2017), the constant slow flow could provide continuous food supply to the I. elongata population, as González-Irusta et al. (2022) also suggested. Indeed, prey capture rates of some CWCs are higher at low water velocities (Orejas et al. 2016; Purser et al. 2010). Including oceanographic variables would largely benefit HSM, obtaining more accurate and applicable results on the constraints of habitat preferences of I.elongata at all spatial scales, as they have been proved to exert large control in the distribution and other characteristics of cold-water corals (Puerta et al. 2020).
Protection measures for I. elongata are urgent for the whole Mediterranean Sea, as already claimed by different authors (Bo et al. 2015b; Carbonara et al. 2022; González-Irusta et al. 2022; Ingrassia et al. 2019; Mytilineou et al. 2014) and VME GFCM experts (FAO 2018; GFCM 2019b; 2022). Despite the urgency due to its current status (Bo et al. 2015b) and other threats derived from climate change and anthropogenic activities (Otero and Marin 2019), no management measures at regional or national level are currently in place in the study area. Considering the strong effects of fisheries and the overlap between fishing grounds and I. elongata distribution, closure areas could contribute to the conservation of the species and reduce significant adverse impacts to this VME taxa (Chimienti et al. 2019b; Wright et al. 2015; ICES 2020).
Data Availability
Biological data used for this study were collected, published and provided by the authors of Mastrototaro et al. 2017.
References
Acosta J, Canals M, López-Martı́nez J, Muñoz A, Herranz P, Urgeles R, Palomo C, Casamor JL (2003) The Balearic Promontory geomorphology (western Mediterranean): morphostructure and active processes. Geomorphology 49:177–204
Acosta J, Canals M, Carbó A, Muñoz A, Urgeles R, Muñoz-Martı́n A, Uchupi E (2004) Sea floor morphology and Plio-Quaternary sedimentary cover of the Mallorca Channel, Balearic Islands, western Mediterranean. Mar Geo 206:165–179
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Anderson OF, Guinotte JM, Rowden AA, Tracey DM, Mackay KA, Clark MR (2016) Habitat suitability models for predicting the occurrence of vulnerable marine ecosystems in the seas around New Zealand. Deep Sea Res Part I 115:265–292
Andrews A, Stone R, Lundstrom C, DeVogelaere A (2009) Growth rate and age determination of bamboo corals from the northeastern Pacific Ocean using refined 210Pb dating. Mar Ecol Prog Ser 397:173–185
Barceló-Llull B, Pascual A, Ruiz S, Escudier R, Torner M, Tintoré J (2019) Temporal and spatial hydrodynamic variability in the mallorca channel (Western Mediterranean Sea) from 8 years of underwater glider data. J Geophys Res Oceans 124:2769–2786
Bellan-Santini D (1985) The deep Mediterranean benthos: Reflections and problems raised by a classification of the benthic assemblages. In: Maraitou-Apostolopoulou M, Kiortsis V (eds) Mediterranean marine ecosystems, Springer Science & Business Media, pp 109–145
Bo M, Bavestrello G, Angiolillo M, Calcagnile L, Canese S, Cannas R, Cau A, D’Elia M, D’Oriano F, Follesa MC, Quarta G, Cau A (2015a) Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS ONE 10:e0119393
Bo M, Cerrano C, Antoniadou C, Garcia S, Orejas C (2015b) Isidella elongata. The IUCN red list of threatened species. eT50012256A50605973
Bo M, Otero MM, Numa C (2017) Overview of the conservation status of Mediterranean anthozoa. IUCN. https://portals.iucn.org/library/sites/library/files/documents/RL-2017-003.pdf
Breiman L (2001) Random forests. Mach Learn 45:5–32
Buhl-Mortensen L, Olafsdottir SH, Buhl-Mortensen P, Burgos JM, Ragnarsson SA (2015) Distribution of nine cold-water coral species (Scleractinia and Gorgonacea) in the cold temperate North Atlantic: effects of bathymetry and hydrography. Hydrobiologia 759:39–61
Buhl-Mortensen P, Buhl-Mortensen L (2014) Diverse and vulnerable deep-water biotopes in the Hardangerfjord. Mar Biol Res 10:253–267
Burgos JM, Buhl-Mortensen L, Buhl-Mortensen P, Ólafsdóttir SH, Steingrund P, Ragnarsson SÁ, Skagseth Ø (2020) Predicting the distribution of indicator taxa of vulnerable marine ecosystems in the arctic and sub-arctic waters of the Nordic Seas. Front Mar Sci 7:131
Canals M, Puig P, de Madron XD, Heussner S, Palanques A, Fabres J (2006) Flushing submarine canyons. Nature 444:354–357
Carbonara P, Zupa W, Follesa MC, Cau A, Capezzuto F, Chimieni G, D’Onghia G, Lembo G, Pesci P, Porcu C, Bitetto I, Spedicato MT, Maiorano P (2020) Exploring a deep-sea vulnerable marine ecosystem: Isidella elongata (Esper, 1788) species assemblages in the Western and Central Mediterranean. Deep Sea Res Part I Oceanogr Res Pap 166:103406
Carbonara P, Zupa W, Follesa MC, Cau A, Donnaloia M, Alfonso S, Casciaro L, Spedicato MT, Maiorano P (2022) Spatio-temporal distribution of Isidella elongata, a vulnerable marine ecosystem indicator species, in the southern Adriatic Sea. Hydrobiologia. https://doi.org/10.1007/s10750-022-05022-4
Carpine C, Grasshoff M (1975) Les Gorgonaires de la Méditerranée. Bull Inst Océanogr Monaco 71:1–140
Cartes JE, LoIacono C, Mamouridis V, López-Pérez C, Rodríguez P (2013) Geomorphological, trophic and human influences on the bamboo coral Isidella elongata assemblages in the deep Mediterranean: To what extent does Isidella form habitat for fish and invertebrates? Deep Sea Res Part I Oceanogr Res Pap 76:52–65
Cartes JE, Díaz-Viñolas D, Papiol V, Lombarte A, Serrano A, Carbonell A, Salas C, Gofas S, Parra S, Palomino D, Lloris D (2021) First faunistic results on Valencia (Cresques) Seamount, with some ecological considerations. Mar Biodivers Rec 14:17
Cartes JE, Díaz-Viñolas D, González-Irusta JM, Serrano A, Mohamed S, Lombarte A (2022) The macrofauna associated to the bamboo coral Isidella elongata: to what extent the impact on Isideidae affects diversification of deep-sea fauna. Coral Reefs 41:1273–1284
Chimienti G, Bo M, Taviani M, Mastrototaro F (2019a) Occurrence and biogeography of Mediterranean cold-water corals. In: Orejas C, Jiménez C (eds) Mediterranean cold-water corals: past, present and future. Springer Int Pub, pp 213–243
Chimienti G, Mastrototaro F, D’Onghia G (2019b) Mesophotic and deep-sea vulnerable coral habitats of the Mediterranean Sea: Overview and conservation perspectives. In: Soto L (ed) The Benthos Zone. IntechOpen, pp 1–20
de Oliveira G, Rangel TF, Lima-Ribeiro MS, Terribile LC, Diniz-Filho JAF (2014) Evaluating, partitioning, and mapping the spatial autocorrelation component in ecological niche modeling: a new approach based on environmentally equidistant records. Ecography 37:637–647
Dormann C, McPherson J, Araújo M, Bivand R, Bolliger J, Carl G, Davies R, Hirzel A, Jetz W, Daniel Kissling W, Kühn I, Ohlemüller R, Peres-Neto P, Reineking B, Schröder B, Schurr F, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628
Elith J, Ferrier S, Huettmann F, Leathwick J (2005) The evaluation strip: A new and robust method for plotting predicted responses from species distribution models. Eco Modell 186:280–289
Fabri M-C, Pedel L, Beuck L, Galgani F, Hebbeln D, Freiwald A (2014) Megafauna of vulnerable marine ecosystems in French Mediterranean submarine canyons: Spatial distribution and anthropogenic impacts. Deep Sea Res Part II Top Stud Oceanogr 104:184–207
FAO (1996) Selected papers presented at the third technical consultation on stock assessment in the Central Mediterranean, Tunis, Tunisia 8–12 november 1994. Food & Agriculture Org, Rome, Italy, pp 302
FAO (2018) Report of the second meeting of the Working Group on Vulnerable Marine Ecosystems (WGVME). Food & Agriculture Org, Rome, Italy, pp 57
Genuer R, Poggi J-M, Tuleau-Malot C (2019) VSURF: variable selection using random forests. R J 7:19–33
Gerovasileiou V, Smith CJ, Kiparissis S, Stamouli C, Dounas C, Mytilineou C (2019) Updating the distribution status of the critically endangered bamboo coral Isidella elongata (Esper, 1788) in the deep Eastern Mediterranean Sea. Reg Stud Mar Sci 28:100610
GFCM (2005) Management of certain fisheries exploiting demersal and deep water species and the establishment of a fisheries restricted area below 1000 m. REC.CM-GFCM/29/2005/1, General Fisheries Commission of the Mediterranea, pp 3
GFCM (2019a) Resolution GFCM/43/2019a/6 on the establishment of a set of measures to protect vulnerable marine ecosystems formed by cnidarian (coral) communities in the Mediterranean Sea
GFCM (2019b) Report of the third meeting of the Working Groups on Marine Protected Areas (WGMPA), FAO HQ, Italy, 18–21 Febrero 2019b, pp 53
GFCM (2022) Report of the Working Group on Vulnerable Marine Ecosystems and Essential Fish Habitats (WGVME-EFH). FAO HQ, Italy, 22–24 March 2022, pp 31
González-Irusta JM, Cartes JE, Punzón A, Díaz D, de Sola LG, Serrano A (2022) Mapping habitat loss in the deep-sea using current and past presences of Isidella elongata (Cnidaria: Alcyonacea). ICES J Mar Sci 79:1888–1901. https://doi.org/10.1093/icesjms/fsac123
Hastie T, Tibshirani R (1986) Generalized additive models. Statist Sci 1:297–310
Hijmans RJ (2020) raster: Geographic Data Analysis and Modeling. R package v3.0–12
Hintzen NT, Piet GJ, Brunel T (2010) Improved estimation of trawling tracks using cubic Hermite spline interpolation of position registration data. Fish Res 101:108–115
ICES (2020) ICES/NAFO Joint Working Group on Deep-water Ecology (WGDEC). ICES Sci Rep 2:188
Ingrassia M, Martorelli E, Bosman A, Chiocci FL (2019) Isidella elongata (Cnidaria: Alcyonacea): First report in the Ventotene Basin (Pontine Islands, western Mediterranean Sea). Reg Stud Mar Sci 25:100494
Lauria V, Garofalo G, Fiorentino F, Massi D, Milisenda G, Piraino S, Russo T, Gristina M (2017) Species distribution models of two critically endangered deep-sea octocorals reveal fishing impacts on vulnerable marine ecosystems in central Mediterranean Sea. Sci Rep 7:8049
Liaw A, Wiener M (2002) Classification and Regression by random forest. R News 2:18–22
López-Jurado JL, Marcos M, Monserrat S (2008) Hydrographic conditions affecting two fishing grounds of Mallorca island (Western Mediterranean): during the IDEA Project (2003–2004). J Mar Syst 71:303–315
Lundblad ER, Wright DJ, Miller J, Larkin EM, Rinehart R, Naar DF, Donahue BT, Anderson SM, Battista T (2006) A benthic terrain classification scheme for American Samoa. Mar Geod 29:89–111
Mastrototaro F, Aguilar R, Chimienti G, Gravili C, Boero F (2016) The rediscovery of Rosalinda incrustans (Cnidaria: Hydrozoa) in the Mediterranean Sea. Ital J Zool 83:244–247
Mastrototaro F, Chimienti G, Acosta J, Blanco J, Garcia S, Rivera J, Aguilar R (2017) Isidella elongata (Cnidaria: Alcyonacea) facies in the western Mediterranean Sea: visual surveys and descriptions of its ecological role. Eur Zool J 84:209–225
Maynou F, Cartes JE (2011) Effects of trawling on fish and invertebrates from deep-sea coral facies of Isidella elongata in the western Mediterranean. J Mar Biol Ass 92:1501–1507
Morato T, González-Irusta J, Dominguez-Carrió C, Wei C, Davies A, Sweetman AK, Taranto GH et al (2020) Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob Change Biol 26:2181–2202
Mytilineou C, Smith CJ, Anastasopoulou A, Papadopoulou KN, Christidis G, Bekas P, Kavadas S, Dokos J (2014) New cold-water coral occurrences in the Eastern Ionian Sea: Results from experimental long line fishing. Deep Sea Res Part II Top Stud Oceanogr 99:146–157
Notti E, Carlo FD, Brčić J, Sala A (2013) Technical specifications of Mediterranean trawl gears. Proceedings of the 11th International Workshop on methods for the development and evaluation of maritime technologies (DeMaT'13). Contrib Theory Fishing Gears Related Mar Syst 8:162–169
OCEANA (2010) Seamounts of the Balearic Islands. Proposal for a Marine Protected Area in the Mallorca Channel (Western Mediterranean), pp 64
Orejas C, Gori A, Rad-Menéndez C, Last KS, Davies AJ, Beveridge CM, Sadd D, Kiriakoulakis K, Witte U, Roberts JM (2016) The effect of flow speed and food size on the capture efficiency and feeding behaviour of the cold-water coral Lophelia pertusa. J Exp Mar Biol Ecol 481:34–40
Otero M, Marin P (2019) Conservation of Cold-Water Corals in the Mediterranean: Current Status and Future Prospects for Improvement. In: Orejas C, Jiménez C (eds) Mediterranean cold-water corals: past, present and future. Springer Int Pub, pp 535–545
Paradis E, Schliep K (2018) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Pérès JM (1967) The Mediterranean benthos. Oceanogr Mar Biol Annu Rev 5:449–533
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190:231–259
Phillips S (2017) maxnet: Fitting “Maxent” Species Distribution Models with “glmnet”. R package v0.1.2
Pierdomenico M, Russo T, Ambroso S, Gori A, Martorelli E, D’Andrea L, Gili JM, Chiocci FL (2018) Effects of trawling activity on the bamboo-coral Isidella elongata and the sea pen Funiculina quadrangularis along the Gioia Canyon (Western Mediterranean, southern Tyrrhenian Sea). Prog Oceanogr 169:214–226
Puerta P, Johnson C, Carreiro-silva M, Henry L, Kenchington E, Morato T, Kazanidis G, Rueda JL, Urra J, Ross S, Wei C, González-Irusta JM, Arnaud-Haond S, Orejas C (2020) Influence of Water Masses on the Biodiversity and Biogeography of Deep-Sea Benthic Ecosystems in the North Atlantic. Front Mar Sci 7:1–25
Puig P, Madron XD, Salat J, Schroeder K, Martín J, Karageorgis AP, Palanques A, Roullier F, López-Jurado JL, Emelianov M, Moutin T, Houpert L (2013) Thick bottom nepheloid layers in the western Mediterranean generated by deep dense shelf water cascading. Prog Oceanogr 111:1–23
Purser A, Larsson AI, Thomsen L, van Oevelen D (2010) The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J Exp Mar Biol Ecol 395:55–62
Quetglas A, Guijarro B, Ordines F, Massutí E (2012) Stock boundaries for fisheries assessment and management in the Mediterranean: the Balearic Islands as a case study. Sci Mar 76:17–28
Quetglas A, Merino G, González J, Ordines F, Garau A, Grau AM, Guijarro B, Oliver P, Massutí E (2016) Plan de implementación regional para pesquerías demersales de las Islas Baleares (Mediterráneo Occidental). http://www.repositorio.ieo.es/e-ieo/bitstream/handle/10508/11267/Myfish-RIP-WestMed-ESP.pdf?sequence=1&isAllowed=y
Rossi S, Bramanti L, Gori A, Orejas C (2017) Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots. Springer, Cham., p 1000
Rowden AA, Anderson OF, Georgian SE, Bowden DA, Clark MR, Pallentin A, Miller A (2017) High-resolution habitat suitability models for the conservation and management of vulnerable marine ecosystems on the Louisville Seamount Chain, South Pacific Ocean. Front Mar Sci 4:335
Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240:1285–1293
Thresher R, Rintoul SR, Koslow JA, Weidman C, Adkins J, Proctor C (2004) Oceanic evidence of climate change in southern Australia over the last three centuries. Geophys Res Lett 31:L07212
Thuiller W, Lafourcade B, Engler R, Araújo MB (2009) BIOMOD – a platform for ensemble forecasting of species distributions. Ecography 32:369–373
UNEP/MAP-SPA/RAC (2018) SAP/RAC : SPA-BD Protocol - Annex II: List of endangered or threatened species. https://www.rac-spa.org/sites/default/files/annex/annex_2_en_20182.pdf
Vázquez JT, Alonso B, Fernández-Puga MC, Gómez-Ballesteros M, Iglesias J, Palomino D, Roque C, Ercilla G, Díaz-del-Río V (2015) Seamounts along the Iberian continental margins. Bol Geol Min 126:483–514
Walbridge S, Slocum N, Pobuda M, Wright DJ (2018) Unified geomorphological analysis workflows with Benthic Terrain Modeler. Geosciences 8:94
Wang SQ, Murillo FJ, Kenchington E (2022) Climate-Change Refugia for the Bubblegum Coral Paragorgia arborea in the Northwest Atlantic. Frontiers in Marine Science 9
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc 73:3–36
Wright DJ, Lundblad ER, Larkin EM, Rinehart RW, Murphy J, Cary-Kothera L, Draganov K (2005) ArcGIS Benthic Terrain Modeler. https://resources.arcgis.com/en/communities/oceans/02pp00000007000000.htm
Wright G, Ardron J, Gjerde K, Currie D, Rochette J (2015) Advancing marine biodiversity protection through regional fisheries management: A review of bottom fisheries closures in areas beyond national jurisdiction. Mar Policy 61:134–148
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer-Verlag, New York, p 574
Acknowledgements
The authors wish to thank Dr. Giovanni Chimienti and OCEANA for the provision of the biological data, Secretaría General de Pesca, Ministerio de Agricultura Pesca y Alimentación (Spain) for the fishing data and Eric Goberville, Manuel Hidalgo, Safo Pïñeiro, Xisco Ordinas, Daniel Ottman, Olga Reñones for their valuable input and ideas. CO is deeply grateful to the Hanse- Wissenschafts Kolleg – Institute for Advanced Study (HWK) for a fellowship she obtained from October 2021 to July 2022. Many thanks to Ellen Kenchington and Jose Manuel González-Irusta for their suggestions to improve this manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by the European Union’s Horizon 2020 Research and Innovation Program, under the ATLAS project (Grant Agreement No. 678760) and iAtlantic project (Grant agreement No. 818123). This output reflects only the author’s view, and the European Union cannot be held responsible for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Contributions
P.P., C.O. and W.S. designed the concept of the study, W.S. and P.P. designed the structure, performed the analyses and primarily wrote the manuscript. B.R-S. contributed to the modelling selection and performance. R.A., P.M. and J.B. designed the field-work, and together with F.M., gathered and provided biological data and information. D.P., J-T.V. and O.SG. compiled and processed the bathymetric data. All authors were substantially involved in the literature search and writing of the manuscript, contributed to the final product in significant ways, and provided approval for publication.
Biological data used for this study were collected, published and provided by the authors of Mastrototaro et al. 2017.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Human and Animal Ethics
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Standaert, W., Puerta, P., Mastrototaro, F. et al. Habitat Suitability Models of a Critically Endangered Cold-water Coral, Isidella Elongata, in the Mallorca Channel. Thalassas 39, 587–600 (2023). https://doi.org/10.1007/s41208-023-00531-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-023-00531-y