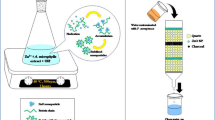
Abstract
In this study, nanoparticles (Ag, Cu and Ag–Cu) were synthesized from Blighia sapida through an ecofriendly biological approach. The study characterized and comparatively investigated the antibacterial potential of the nanoparticles (NPs) on pharmaceutical wastewater-isolated bacteria. The toxicological implications due to wastewater treatment with the NPs were also investigated on liver and kidney function indices of Wistar rats. The synthesized NPs were revealed to be un-agglomerated spheres (Transmission Electron Microscopy), with hydrodynamic diameter between 70 and 90 nm and an overall surface zeta potential of – 28 to – 31 mV (Dynamic Light Scattering). Judging by the zone of inhibitions, minimum inhibitory concentrations and the minimum bactericidal concentrations values obtained in this study, the NPs could be said to have potentiated significant antibacterial effects with the most profound effect observed with the Ag–Cu NPs relative to Ag- and Cu NPs at 50 µg/ml. For the sub-acute toxicity evaluation, the NPs at 200 µg/kg showed signals of progressive onset of toxicity, marked by reduction in alkaline phosphatase, aspartate transaminase and gamma glutamyl transaminase activities in the liver and kidney of the NPs-treated groups (p > 0.05) compared to the control group. This result suggested that the synthesized NPs may not be safe for direct application at 200 µg/kg, and it is therefore recommended that safety precautions be adhered in the use of silver and copper nanoparticles.
Graphical abstract






Similar content being viewed by others
Availability of data and materials
Research data are available at the repository of the department of medical biochemistry and pharmacology, Faculty of Science, Kwara State University, Nigeria. Data are also available on request.
References
Esakkimuthu T, Sivakumar D, Akila S (2014) Application of nanoparticles in wastewater treatment. Pollut Res 33(03):567–571
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N et al (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 10(8):1566. https://doi.org/10.3390/nano1008566
Zakar MZ, Zakar DR, Fischer F (2020) Climate change-induced water scarcity: a threat to human health. South Asian Stu 27(2).
Ma T, Siao S, Guangtao F, Jim WH, Yong N, Lihuan H et al (2020) Pollution exacerbates China’s water scarcity and its regional inequality. Nat Commun 11(1):650
Pang Z, Li Q, Jia Y, Yan W, Qi J, Guo Y, Hu F, Zhou D, Jiang X (2021) Controlling the pyridinium–zwitterionic ligand ratio on atomically precise gold nanoclusters allowing for eradicating Gram-positive drug-resistant bacteria and retaining biocompatibility. Chem Sci 12(44):14871–14882
Tortella GR, Rubilar O, Durán N, Diez MC, Martínez M, Parada J et al (2020) Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J Hazard Mater 390:121974
Obayiuwana A, Ogunjobi A, Yang M, Ibekwe M (2018) Characterization of bacterial communities and their antibiotic resistance profiles in wastewaters obtained from pharmaceutical facilities in Lagos and Ogun States, Nigeria. Int J Environ Res Public Health 15(7):1365
Al-Hakkani MF (2020) Biogenic copper nanoparticles and their applications: a review. SN Appl Sci 2(3):1–20
Nahar K, Aziz S, Bashar M, Haque M, Al-Reza SM (2020) Synthesis and characterization of silver nanoparticles from Cinnamomum tamala leaf extract and its antibacterial potential. Int J Nano Dimension 11(1):88–98
Lotfollahzadeh R, Yari M, Sedaghat S, Delbari AS (2021) Biosynthesis and characterization of silver nanoparticles for the removal of amoxicillin from aqueous solutions using Oenothera biennis water extract. J Nanostruct Chem 11:693–706
Ajani EO, Afolayan JS, Sabiu S (2021) Characterization of Blighia sapida synthesized-copper nanoparticles and its application in periodic pharmaceutical effluent treatment. J Environ Sci Health A 56(5):508–515
Moodley JS, Krishna SBN, Pillay K, Govender P (2018) Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv Nat Sci Nanosci Nanotechnol 9(1):015011
Zhang T, Guo J, Chen JF, Wang JM, Wan ZL, Yang XQ (2020) Heat stability and rheological properties of concentrated soy protein/egg white protein composite microparticle dispersions. Food Hydrocolloids 100:105449
Valodkar M, Bhadoria A, Pohnerkar J, Mohan M, Thakore S (2010) Morphology and antibacterial activity of carbohydrate-stabilized silver nanoparticles. Carbohyd Res 345(12):1767–1773
Saleh TA, Gupta VK (2012) Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep Purif Technol 89:245–251
Jemal K, Sandeep BV, Pola S (2017) Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J Nanomater 3:1–11
Tomaszewska E, Soliwoda K, Kadziola K, Tkacz-Szczesna B, Celichowski G, Cichomski M et al (2013) Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater. Article ID 313081, 10 pages.
Garcha S, Verma N, Brar SK (2016) Isolation, characterization, and identification of microorganisms from unorganized dairy sector wastewater and sludge samples and evaluation of their biodegradability. Water Resources Industry 16:19–28
Abiola C, Oyetayo VO (2016) Isolation and biochemical characterization of microorganisms associated with the fermentation of Kersting’s Groundnut (Macrotyloma geocarpum). Res J Microbiol 11(2–3):47–55
Amadi EC, Eze EA, Chigor VN (2020) Evaluation of parasites/biological indices as veritable indicators of faecal enterococcus contamination of surface waters: case study of Adada River, Nigeria. Asian J Biol Sci 13:53–61
Tallur PN, Sajjan DB, Mulla SI, Talwar MP, Pragasam A, Nayak VM et al (2016) Characterization of antibiotic resistant and enzyme producing bacterial strains isolated from the Arabian Sea. Biotech 6(1):28.
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharmaceutical Anal 6(2):71–79
Organization for Economic corporation and Development (OECD). Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 2008, 13 pages. ISBN: 9789264070684.
Adeyemi OT, Osilesi O, Adebawo OO, Onajobi FD, Oyedemi SO, Afolayan AJ (2015) Alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) Activities in selected tissues of rats fed on processed atlantic horse mackerel (Trachurus trachurus). Adv Biosci Biotechnol 6(03):139
National Research Council (2011) Guide for the Care and Use of Laboratory Animals, Eight. The National Academy Press, Washington, DC. ISBN 978-0-309-15400-0
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31(1):87–96
Gornall AC, Bardawill CJ, David MM (1949) Colorimetric method for total protein determination. J Biol Chemi 177(2):751–766
Malloy HT, Evelyn KA (1937) The determination of bilirubin with the photoelectric colorimeter. J Biol Chem 119(2):481–490
Wright PJ, Leathwood PD, Plummer DT (1972) Enzymes in rat urine: alkaline phosphatase. Enzymologia 42(4):317
International Federation of Chemistry and Laboratory Medicine (IFCC) (1980) Measurement of lactate dehydrogenase in serum. J Clin Chem Clin Biochem 18: 521.
Szasz G (1969) A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin Chem 15(2):124–136
Francis PS, Lewis SW, Lim KF (2002) Analytical methodology for the determination of urea: current practice and future trends. TrAC Trends Anal Chem 21(5):389–400
Masson P, Ohlsson P, Björkhem I (1981) Combined enzymic-Jaffé method for determination of creatinine in serum. Clin Chem 27(1):18–21
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Jyoti K, Singh A (2016) Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J Genetic Eng Biotechnol 14(2):311–317
Suresh AK, Doktycz MJ, Wang W, Moon JW, Gu B, Meyer HM III, Pelletier DA (2011) Monodispersed biocompatible silver sulfide nanoparticles: facile extracellular biosynthesis using the γ-proteobacterium, Shewanella oneidensis. Acta Biomaterialia 7(12):4253–4258
Li H, Zhang L (2017) Photocatalytic performance of different exposed crystal facets of BiOCl. Eds: Angelo Albini, Chuncheng Chen and Michela Sturini. Curr Opin Green Sustain Chem 6:48–56. ISSN 2452–2236.
Levak M, Burić P, Dutour SM., Domazet JD, Mikac N, Bačic N et al (2017) Effect of protein corona on silver nanoparticle stabilization and ion release kinetics in artificial seawater. Environ Sci Technol 51(3):1259–1266.
Mehta BK, Chhajlani M, Shrivastava BD (2017) Green synthesis of silver nanoparticles and their characterization by XRD. J Phys Conf Ser 836(1):012050.
Abdullahi AA, Ighalo J, Ajala O, Ayika SO (2020) Physicochemical analysis and heavy metals remediation of pharmaceutical industry effluent using bentonite clay modified by H2SO4 and HCl. J Turkish Chem Soc Sect A Chem 7(3):727–744
Saratale RG, Gandhi SS, Purankar MV, Kurade MB, Govindwar SP, Oh SE et al (2013) Decolorization and detoxification of sulfonated azo dye CI Remazol Red and textile effluent by isolated Lysinibacillus sp. RGS J Biosci Bioeng 115(6):658–667
Reda RM, Selim KM, El-Sayed HM, El-Hady MA (2018) In vitro selection and identification of potential probiotics isolated from the gastrointestinal tract of Nile tilapia. Oreochromis niloticus. Prob Antimicrobial Proteins 10(4):692–703
Eid H, Hanafy AS (2019) Antibacterial resistance of Aeromonas species isolated from fish and water of Manzala Lake. Suez Canal Veterinary Med J SCVMJ 24(2):217–230
Gomes IB, Madureira D, Simões LC, Simões M (2019) The effects of pharmaceutical and personal care products on the behavior of Burkholderia cepacia isolated from drinking water. Int Biodeterior Biodegradation 141:87–93
El-Bendary MA, Abdelraof M, Moharam ME, Elmahdy EM, Allam MA (2021) Potential of silver nanoparticles synthesized using low active mosquitocidal Lysinibacillus sphaericus as novel antimicrobial agents. Prep Biochem Biotechnol, 1–10. https://doi.org/10.1080/10826068.2021.1875236. Epub ahead of print. PMID: 33529084.
Ahsan N, Shimizu M (2021) Lysinibacillus species: their potential as effective bioremediation, biostimulant, and biocontrol agents. Rev Agric Sci 9:103–116
Mohammadi S, Jazani NH, Kouhkan M, Babaganjeh LA (2018) Antibacterial effects of microbial synthesized silver-copper nanoalloys on Escherichia coli, Burkholderia cepacia, Listeria monocytogenes and Brucella abortus. Iran J Microbiol 10(3):171
Peralta DP, Chang AY, Ariza-Hutchinson, Ho CA (2018) Burkholderia multivorans: a rare yet emerging cause of bacterial meningitis. IDCases 11:61–63.
Deng Y, Wu Y, Jiang L, Tan A, Zhang R, Luo L (2016) Multi-drug resistance mediated by class 1 integrons in Aeromonas isolated from farmed freshwater animals. Front Microbiol 7:935. https://doi.org/10.3389/fmicb2016.00935
Zdanowicz M, Mudryk ZJ, Perliński P (2020) Abundance and antibiotic resistance of Aeromonas isolated from the water of three carp ponds. Vet Res Commun 44:9–18. https://doi.org/10.1007/s11259-020-09768-x
Piotrowska M, Przygodzińska D, Matyjewicz K, Popowska M (2017) Occurrence and variety of beta-lactamase genes among Aeromonas spp. isolated from urban wastewater treatment plant. Front Microbiol 8:63. https://doi.org/10.3389/fmicb.2017.0086
Bankier C, Matharu RK, Cheong YK, Ren GG, Cloutman-Green E, Ciric L (2019) Synergistic antibacterial effects of metallic nanoparticle combinations. Sci Rep 9(1):1–8
Perdikaki A, Galeou A, Pilatos G, Karatasios I, Kanellopoulos NK, Prombona A, Karanikolos GN (2016) Ag and Cu monometallic and Ag/Cu bimetallic nanoparticle–graphene composites with enhanced antibacterial performance. ACS Appl Mater Interfaces 8(41):27498–27510
Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7:1831
Rai M, Kon K, Ingle A, Duran N, Galdiero S, Galdiero M (2014) Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol 98:1951–1961. https://doi.org/10.1007/s00253-013-5473-x
Fan X, Yahia LH, Sacher E (2021) Antimicrobial properties of the Ag, Cu nanoparticle system. Biology 10(2):137
Mudryk Z, Perliński P, Gackowska J (2015) Antibiotic resistance of Aeromonas spp. isolated from seawater and sand of marine recreation beach in the southern Baltic Sea. Baltic Coastal Zone 19:67–80
Stefańska I, Kwiecień E, Jóźwiak-Piasecka K, Garbowska M, Binek M, Rzewuska M (2021) Antimicrobial susceptibility of lactic acid bacteria strains of potential use as feed additives—the basic safety and usefulness criterion. Front Vet Sci 8:687071. Published 2021 Jul 1. https://doi.org/10.3389/fvets.2021.687071
Loo YY, Rukayadi Y, Nor-Khaizura MAR, Kuan CH, Chieng BW, Nishibuchi M, Radu S (2018) In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front Microbiol 9:1555
Mirjalili BBF, Khabnadideh S, Gholami A, Zamani L, Nezamalhosseini SM, Kalantari N (2020) Cu (OAc) 2 as a green promoter for one-pot synthesis of 2-amino-4, 6-diarylpyridine-3-carbonitrile as antibacterial agents. Bull Chem Soc Ethiop 34(1):149–156
Abootalebi SN, Mousavi SM, Hashemi SA, Shorafa E, Omidifar N, Gholami A (2021) Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against Acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on the human cell lines. J Nanomater. Articles ID 6676555, 12 pages.
Bhattacharya D, Nakum J (2022) Various green nanomaterials used for wastewater and soil treatment: amini-review. Front Environ Sci, p.420.
Raja MM, Raja A, Imran MM, Santha AM, Devasena K (2011) Enzymes application in diagnostic prospects. Biotechnology 10(1):51–59
Dong V, Nanchal R, Karvellas CJ (2020) Pathophysiology of acute liver failure. Nutr Clin Pract 35(1):24–29
Parhamifar L, Andersen H, Moghimi SM (2013) Lactate dehydrogenase assay for assessment of polycation cytotoxicity. In Nanotechnology for nucleic acid delivery 2013 (pp. 13–22). Humana Press, Totowa.
Kumar P, Nagarajan A, Uchil PD (2018) Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harbor Protocols 2018(6):pdb-rot095497.
Tang H, Xu M, Luo J, Zhao L, Ye G, Shi F, Lv C, Chen H, Wang Y, Li Y (2019) Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ Sci Eur 31(1):30
Djabir YY, Meylin ID, Tayeb R (2019) The protective effect of Paliasa (Kleinhovia hospita L.) leaf extract against elevated total bilirubin serum induced by toxic dose of antituberculosis in rats. Nusantara Med Sci J 4(2):43–47.
Hadrup N, Lam HR (2014) Oral toxicity of silver ions, silver nanoparticles and colloidal silver–a review. Regul Toxicol Pharmacol 68(1):1–7
Pohanka M (2019) Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl Lek Listy 120(06):397–409
Jeyasree J, Bupesh G, Vasanth S, Beulah JP, Pandian K, Anand AV, Vijayakumar TS, Narayanan L (2020) In-vivo Toxicological (Acute) characterization of bio-synthesized silver nanoparticles in Labeo rohita. Nano Biomed Eng 12(2):115–123
Cui Y, Liu H, Zhou M, Duan Y, Li N, Gong X, Hu R, Hong M, Hong F (2011) Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res Part A 96(1):221–229
Li M, Lin D, Zhu L (2013) Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ Pollut 173C:97–102
Acknowledgements
The authors acknowledge support on non-financial logistics from the Department of Medical Biochemistry and Pharmacology, Faculty of Science, Kwara State University, Nigeria. The Research and Postgraduate Support of the Durban University of Technology is duly acknowledged for the assistance toward the analytical characterization of the NPs.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EA was involved in research conception and supervision, SS was involved in experimental design, ASJ was involved in research implementation, and FDR and AAM were involved in literature reviews.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interests.
Ethical statement
The protocols adopted in this study conform with the Guidelines for the Care and Use of Laboratory Animals of the Institute for Laboratory Animal Research of the National Research Council, USA. Research facilities were also assessed and approved for the experiment by the designates of the Faculty of Science, Kwara State University, in accordance with the global standards.
Consent to participate and for publication
Authors’ participation in this study was voluntary, the authors have shared passion for collaborative research, and the submission of this research work for publication in the journal of Nanotechnology of Environmental Engineering has been endorsed by all contributors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afolayan, J.S., Ajani, E., Saheed, S. et al. Green synthesis of copper and silver nanoparticles and their comparative toxicity and antibacterial evaluation in pharmaceutical wastewater treatment. Nanotechnol. Environ. Eng. 8, 333–346 (2023). https://doi.org/10.1007/s41204-022-00286-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-022-00286-6