Abstract
This paper examines how researchers in biotechnology reflect on the challenges of turning microbes into what they call “cell factories”. These researchers use the tools of genome editing to harness the biochemistry of single cell organisms, such as bacteria, yeasts and microalgae, and tweak the enzymatic reactions of their metabolism. One research priority is to engineer microbes able to feed on agricultural residues and assemble drop-in compounds to be used in a range of commercial products, from drugs and food additives, to cosmetics, detergents and fuels. To justify financial support for such research, arguments about the need to move away from petroleum as a source of energy and feedstock for chemical synthesis are put forward, underpinned by concerns for climate change, resource renewability and energy security. Drawing on interviews with scientists, we explore what it means for them to make “cell factories” and discuss how they problematise the logic of carbon substitution that orientates their work. Biotechnology is expected to support a shift from one source of carbon, past life gone through slow geological cycles, to a different source of carbon, renewable biomass metabolised by living microbes. As scientists face unhappy cells, recalcitrant plant fibres and unfair competition from fossil-based processes, the promise of carbon substitution tends to be most convincing in the confined space of the lab where faith in biotechnology goes hand in hand with a pragmatic commitment to sustainability. We speculate that the researchers might be failed by the system that biotechnology seeks to (partially) replace, the conditions of which are shaped not around the material constraints of making “cell factories”, but around fossilised life cracked in ever-greater quantities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Inside these microbes, we have thousands of chemical reactions and thousands of metabolites. So, the task here is to redirect the pathway of these reactions towards the synthesis of the product of interest”, is how a metabolic engineer explained the main objective of their work to us (Interview 13). Researchers in the field of metabolic engineering use gene edition tools to harness the metabolism of bacteria, yeasts or microalgae, tweaking their enzymatic reactions to create what they call ‘cell factories’. A living organism becomes conceptualized as a “cell factory” when its complex biochemical machinery is modified to efficiently assemble a molecule, and the cell remains productive in the harsh environment of a bioreactor. In the last 20 years, one research priority has been to engineer microorganisms to produce compounds of interest from a variety of biological substrates, including non-edible agricultural residues (e.g., wheat straw, corn stover, sugarcane bagasse), at industrial scale. The compounds (obtained from non-biological sources) are already used in commercial products, ranging from food additives and cosmetics, to detergents, solvents and fuels. What scientists and engineers are after is a different way of producing the molecules. To justify financial support for such research, arguments are put forward about the potential of plant fibres and microbial fermentation to displace petroleum as liquid fuel and feedstock for chemical synthesis. These arguments have been underpinned by a mix of concerns for climate change, resource renewability, economic competitiveness and energy security (Liu et al., 2021; Peralta-Yahya et al., 2012; Stephanopoulos, 2007).
In this paper, we examine how researchers in the fields of metabolic engineering and biochemical engineering reflect on the challenges of turning microbes like yeasts into ‘cell factories’ in the hope of attenuating the dependence on fossil oil, both as a highly energetic fuel and for the myriad molecules produced from it. This highlights a particular facet of what the special issue editors have termed the “politics of decarbonisation”: we call it the logic of substitution. A common feature of supposedly climate-friendly markets and policies, beyond industrial biotechnology, we define the logic of substitution as the pursuit of technologies that would help to reduce CO2 emissions and that are presented as fitting within current habits, products and industries, which might thereby become further entrenched. Decarbonising the economy is expected to be only minimally disruptive, even though the extent to which things can effectively remain unchanged is unlikely. Therefore, we do not use the term substitution to describe how an actual transformation is occurring, the way it would be understood in transition studies (e.g., Geels & Schot, 2007). Instead, the logic of substitution refers to an orientation, which combines with the long history of human interventions in microbial life to frame the work of scientists and engineers seeking to transform microbes eating plant fibres into chemical producers en masse.
In industrial biotechnology, the logic of substitution is exemplified by the development of ‘drop-in’ biofuels and biomolecules, which are either identical to petroleum-based compounds or similar enough to be easily used as replacement. Polymers made of lactic acid derived from the fermentation of corn starch, cane sugar, or beet sugar by bacteria are a case in point. Although these bioplastics are chemically different than existing materials, they have been “promised to be able to replace oil-based products without changing the paradigm of disposable plastics and materials” (Béfort, 2021, p. 7). Mass production and consumption habits would continue as substitution is meant to happen at the level of the molecular fabric of the material and to operate, or compete, at the same industrial scale (see also Ulrich, 2023).Footnote 1 The aim is to shift from one source of carbon—past life gone through slow geological cycles—to a different source of carbon—renewable biomass metabolised by living microbes. This carbon substitution, we contend, hinges on the expectation, or perhaps the faith, that lab-based knowledge and technology can, and will, create biological manufacturing routes to make products that currently require petroleum and chemical synthesis.Footnote 2 Its justification as a response to the climate crisis is further built on the assumption that optimised microorganisms would be fed low value, abundantly available plant material (on the “vast supplies” rhetoric see MacKenzie, 2013).
Drawing on interviews with metabolic engineers and biochemical engineers, we unpack some of the challenges these researchers confront as they seek to realise the promised substitutions. Scholars in science and technology studies have shown that forward-looking statements, promises and expectations (and disappointments) are intrinsic to contemporary life sciences and their entrepreneurial spirit (e.g., Brown, 2003; Cooper, 2011; Marris & Calvert, 2020). A promissory politics has also been observed in response to climate change, as science, innovation and the market are called upon to mitigate the problem—from storing carbon in trees, soils and deep underground, to engineering the atmosphere (e.g., King et al., 2018; Stilgoe, 2016). Our paper contributes to these discussions by turning to researchers who use gene edition tools and chemical engineering processes to develop biotechnological products in an effort that claims to reduce greenhouse gas emissions by displacing fossil fuels and petrochemicals. Carbon substitution, we show, is a horizon that orientates the research, and it often remains just that as when a project fails the scientists pivot to the next one. Because they adhere to a logic that ought to sustain industrial-scale production, this scale makes it difficult for biotechnological advances to break through. We suggest, therefore, that the promise to re-carbonise the economy by harnessing the biological world might be reproducing its own limits.
The paper comprises five sections and a conclusion. First, we further conceptualise the logic of carbon substitution, before presenting how we conducted our research. Following this, we examine how the scientists we interviewed reflect on their work as they seek to modify the metabolisms of specific microorganisms to make them produce target chemicals, hopefully creating robust “cell factories”. The paper, then, turns to the costs of the envisioned sugar-powered economy, from unhappy cells and recalcitrant plant fibres, to unfair competition with petroleum-based production. The final section discusses what we identified among these researchers as a pragmatic commitment to the logic of carbon substitution and associated sustainability claims. The conclusion expands on this idea.
The bioeconomy as a living metabolism of carbon
In their “ontography” of carbon, Bensaude-Vincent and Loeve (2018, p. 14) explore the multiple ways in which carbon matters, from radiocarbon dating methods, to super-resistant nanomaterials, to carbon markets. Without denying the seriousness of global warming, the authors resist current tendencies to reduce carbon to a villain, even as they discuss the rise of petroleum, this incredibly powerful source of energy and versatile feedstock for chemical synthesis that has created a global dependence on hydrocarbons and flooded the world with plastics. One episode in the lives of carbon resonates particularly strongly with our study and the logic of carbon substitution: the promise of a bioeconomy in which microorganisms would ferment cellulosic fibres to replace petrochemistry (pp. 191–201). For the authors, “two metabolisms of carbon” (our translation, p. 191) are here pitted against each other, each with its own way of combining construction (anabolism) and degradation (catabolism). The first is today’s global economy fuelled by fossil oil: “an industrial metabolism plugged into the slow anabolic sedimentation processes of hydrocarbons and the fast catabolism of their frenetic consumption” (ibid.). The second is what happens in the biosphere: “a biological metabolism that joins the fast anabolism of organic carbon (nutrition, respiration, photosynthesis) and a slow catabolism inscribed in the longue durée of generations and evolution and, for a tiny fraction, in the sedimented time of soils” (p. 192). These two metabolisms depend on the same source of energy: “the carbonated chains of living organisms” (ibid.). Historically, chemistry has developed by replacing biological products with synthetic or chemically refined substitutes, thereby opening up novel uses (Bensaude-Vincent & Simons, 2008). The expectation now is that biology will provide new possibilities for substitution. The bioeconomy ought to “bypass the long-term sedimentation of fossil carbon and directly plug an industrial metabolism into the rapid cycles of the living metabolism” (Bensaude-Vincent & Loeve, 2018, p. 192). This living industrial metabolism would be made of crop residues, microbes, sugars, enzymes, metabolites, mixed in bioreactors and linked to new supply chains.
By taking carbon as their object of inquiry, Bensaude-Vincent and Loeve (2018) cast a gaze that cuts across biology, geology, technology and the economy. Theirs may be called a “chemical gaze”, to borrow a term coined by Landecker (2019). Materials and organisms are not seen in the way a naturalist would sort them out, into different species of plants, minerals, animals, fungi or bacteria. Under the “chemical gaze”, things are reduced to their chemical components and properties, like the carbon chains they consume, produce, store, and of which they are made. Landecker (2019) introduces the term to examine how, in the USA in the early 20th, agricultural residues (beet pulp and molasse) started being fed to farmed animals in the pursuit of “feed efficiency”. The byproducts came to be cheaper albeit impoverished sources of nutrients and energy; this, in turn, prompted the use of growth promoters, like arsenic and antibiotics, the long-term effects of which are now of great concern (Landecker, 2016, 2021). For Landecker (2019), the “chemical gaze” traced “a metabolic map of enzymatic and energetic conversions between different kinds of matter connecting one body to another across taxonomic boundaries”, and identifying substitutions along lines that were “simultaneously economic and scientific” (p. 531). The same reasoning attuned to chemical similarities and exchanges underpins the idea of a living metabolism of carbon displacing petrochemistry.
This bioeconomy is techno-optimistic, uncritically modelled on an ideal of industrial production supplied by cheap biological matter (for a critical analysis of this meaning of “bioeconomy” see Vivien et al., 2019; Levidow et al., 2012; Birch & Calvert, 2015). Beldo (2017) suggests that in industrial settings and narratives the vitality of living beings is often only acknowledged through “the disruptive capacity of excess life” (p. 109). Yet, excess life does something that scientific ingenuity and technology cannot replace: “The multiplication of porcine muscle cells, the photosynthetic growth of cornstalks, or the synergistic qualities of soil bacteria present themselves as both irreducibly generative and utterly indispensable to certain modes of capitalist production” (p. 115). The author points at the irreducibility of other-than-human beings and the contribution of their “metabolic labor” (pp. 118–19) to value creation in capitalist production systems. What we retain from the argument is that even when microbes are engineered to assemble molecules already obtained through petrochemistry, the making of “cell factories”—a machinic metaphor Beldo (2017) would move away from—depends on a metabolic vitality (or labor) that evades full control and understanding.
Despite the sense of novelty surrounding the bioeconomy, feeding microorganisms with agricultural residues or surpluses to make them industrially useful is not a new idea. Bud’s (1993) history of biotechnology details how throughout the twentieth century in countries like Germany, the UK, Japan, Denmark, Sweden, Hungary, France and the USA, fermentation specialists, biochemists, engineers and entrepreneurs, have tried to use microbes to manufacture fuels and chemicals.Footnote 3 These attempts gained popularity on occasion, especially when petroleum became scarce or expensive, in times of trade tensions, during the two world wars, and in response to the global oil shocks. The products, however, were seldom commercially viable in the longer term. Besides the food industry (bread, wine, beer, miso, cheese, kefir, soy sauce), only pharmaceutical companies seemed to have taken full advantage of the capacity of microorganisms to metabolise molecules of interest (e.g., antibiotics). There, biotechnology became entangled with proprietary genetic engineering techniques, as Hughes (2011) shows in the case of the biosynthesis of human insulin. When in the mid 1970s the recombinant DNA technology was invented, the press emphasised “the possibility of bacteria being transformed into ‘factories’ for the production of insulin and other drugs” (p. 21) While according to Hughes, the “‘microbe as factory’ idea” (ibid.) had purchase mainly on popular accounts, the metaphor of cells as factories is now common use among researchers, at least those we talked to, and in biotechnology publications. As a live metaphor (Keller, 2020), it exceeds reductive analogy (cells are just like factories), while at the same time its strong connection to the logic of substitution may foreclose research paths toward more radical possibilities.
The researchers we spoke with are contemporary practitioners of industrial biotechnology, bringing together knowledge and tools from biology, chemistry and engineering to develop industrial applications harnessing biological agents for the provision of goods and services. It is not easy to make recalcitrant plant cell walls fermentable and modify the intricate metabolism of yeasts so they can churn these sugars into compounds of interest, and ideally, commercial use. How effective are biomass-eating microbes at producing chemicals similar to those obtained from the cracking of fossil oil? Can the bioprocesses be scaled up? Is this cost-efficient? These are the questions raised by those we interviewed as we invited them to reflect on what it meant in practice to have their work orientated by the logic of carbon substitution.
Lab-based industrial biotechnology
The analysis presented here draws on in-depth conversations with academic scientists and engineers and published documentation (scientific articles, policy and technology roadmaps, press articles). Our interest in the science of industrial biotechnology developed as our attention was caught by recent and older headlines in the British press about the promises of environmental sustainability surrounding the engineering of microbes (e.g., Balch, 2015; Morosini, 2021; Redfern, 2013; Turns, 2020)—a popular genre also on biotech company websites (Karabin et al., 2021). We wanted to better understand how those doing the research underpinning biotechnological applications made sense of their work in relation to these expectations around sustainability. As we asked questions about the state of the field, its potentials, and the various projects of each scientist, we used the semi-directed interview method to tease out what the researchers saw as the major practical challenges of making microbes eat plant fibres to produce molecules of interest, in the lab and beyond. This article is not an evaluation of their work, nor an assessment of the technoeconomic viability of industrial biotechnology. Instead, we seek to pay attention to the ways in which the researchers problematised the logic of substitution.
Between May 2021 and February 2022, we carried out twenty interviews with scientists and engineers working in public universities and research institutions—except one, who was an independent consultant. The quotes that appear in the piece were said in response to open-ended questions during these interviews. Ten of our informants were employed in the United Kingdom (UK), the others were based across Europe, in Ireland, Sweden, Denmark, the Netherlands, Belgium, Germany, France, as well as in Australia and the United States (US).Footnote 4 Due to Covid-19 restrictions on travel and face-to-face meetings, the vast majority of the interviews were conducted online, but we were able to visit three university labs located in the UK in Autumn 2021. The people we talked to were affiliated to departments of chemical engineering, biochemical engineering, biological sciences, biotechnology, or biological engineering. All were senior tenured researchers and often principal investigator of their own team. Those trained as biologists—in microbiology, mycology, cell physiology, molecular biology or systems biology—tended to identify as metabolic engineers when asked to define their current research field, while some of them also considered that their research belonged to synthetic biology. Metabolic engineering is a field of research that stemmed from chemical engineering in the early 1990s. It aims to build on a biochemical understanding of cell metabolism to— improve the production of molecules by once-wild microbes (Raimbault, 2021). In contrast, synthetic biology is said to be modelled on electrical engineering and has been concerned with building standard parts and simplified liveable organisms (e.g., Calvert, 2010; Dan-Cohen, 2021; Roosth, 2017). STS scholars have discussed where to draw the contours of synthetic biology and the extent to which it generates new scientific knowledge about biological processes (Keller, 2009; O’Malley et al., 2008). None of our interlocutors seemed to think that their primary objective was to provide scientific explanations only for the sake of knowledge. If some marvelled at what remains unknown about the microbes they manipulate, their research questions and experiments appeared to be driven by what MacKenzie (2013) describes as an engineer’s ethos: solving problems by making things—“cell factories” and bioprocesses—that may somehow become useful and economical.Footnote 5
The researchers in this paper are mainly working with yeasts. Among the biologists by training, some had done research on pathogenic fungi and health-related applications before turning to the space of bioproducts. All our informants were at some point involved in projects expected to produce biofuels. Only for a few was this still a major topic of research. Most were trying to synthesise other carbon-containing compounds usually obtained from petroleum, and to a lesser extent plants or animals. The so-called target molecules were either bulk (commodity) chemicals to be mass produced, or speciality (fine) chemicals, either precursors for manufacturing other chemicals or directly blended into everyday products (food additives, cosmetics, detergents). All the scientists in our study were dependent for their research on public funding provided through their home institutions and project grants (e.g., from the Engineering and Physical Sciences Research Council and the Biotechnology and Biological Sciences Research Council in the UK; European Union research programmes; the US Department of Energy). Such government support is often conditional to demonstrating that research outcomes are applied and susceptible to be scaled up. All our interviewees, therefore, maintained frequent collaborations with industrial partners, ranging from large companies, such as yeast producers, chemical companies and oil majors, to smaller local firms specialised, for example, in cleaning or dairy products. Many had also been involved in start-ups, and all had their name on multiple patents in the entrepreneurial spirit of contemporary life sciences.
The nature of our study—focused on established scientists in the field, many of whom knew and had collaborated with or cited one another—means that we lack empirical granularity to compare the micro-dynamics of different institutional and national contexts, or to highlight the differences between those researchers who were first and foremost biologists and those who approached the topic as chemical engineers, and between those with successful business ventures and those happy to be primarily academics. While these differences likely exist, they are not the focus of this article. We are interested in what emerged from our conversations as a shared predicament for industrial biotechnology: that the promise of making microbes able to ferment crop residues might be most convincing in the confined space of the lab.
Making “cell factories”
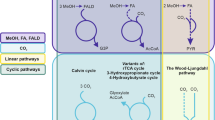
What does it mean to turn a cell into a “cell factory”? In metabolic and chemical engineering labs, the world of microbes is apprehended through a (bio)chemical gaze. Microorganisms are seen as living hosts for chemical reactions traceable as metabolic pathways, fluxes and networks. A pathway is a chain of steps whereby molecule A is transformed into molecule B and the flux is the passage of matter through these steps. The pathway called glycolysis, for example, is “the main highway of sugar metabolism, also in our own cells, in all living cells” (Interview 18). This is how cells use glucose for energy production and the biosynthesis of a molecule (pyruvate) that stores energy and is a precursor for other molecules (fatty acids, amino acids, ethanol). Inside living microbes, chemical reactions are fine-tuned, their rate, direction and speed highly regulated. Metabolic engineers develop experiments, measurement techniques, and computer models to decipher the movements of metabolites within and through cells (Raimbault, 2018; Stephanopoulos, 2002). It is by understanding and intervening into metabolic pathways that they seek to make microbes better at transforming what they eat—in the lab it tends to be monosaccharides like glucose—into something of interest.
The making of “cell factories” starts from the observation that microorganisms metabolise lots of molecules by and for themselves, but not sufficiently in quantity to mass produce them. Genome editing can be used to “streamline” their metabolism, by reducing the energy requirement of certain reactions, supressing the loss of some metabolites, or making it assimilate as much of the feedstock as possible (Interview 14). The cells are made to overproduce and reach high yield, measured as the quantity of target compounds generated per quantity of substrate used. They can be engineered to process unusual carbon sources like crop residues, or to assemble a compound they would not spontaneously synthesise. This is done by creating new pathways in their metabolism via genes introduced from other organisms—fungi, bacteria, plants or animals—crossing taxonomic boundaries (Landecker, 2019). Whether it is to make the organism more efficient or to make it do new things, scientists are interested in the genes coding for enzymes, the highly specialised proteins that activate and speed up chemical reactions inside cells (Kohler, 1973).
The researchers we talked to were specifically working with yeasts. When one says yeast, it usually means Saccharomyces cerevisiae, baker’s yeast, a key ingredient in long-existing food products and a model organism for the study of eukaryotes (on model organisms see Ankeny & Leonelli, 2020). Many firsts have been accomplished in baker’s yeast (Money, 2018). It was the first yeast genome to be sequenced in the 1990s, and today a synthetic version of that genome is being constructed by a consortium of synthetic biology labs (Calvert & Szymanski, 2020). S. cerevisiae is also widely used in industrial biotechnology, especially for biofuels. As one biotechnologist put it, “Saccharomyces cerevisiae, through its evolution, long before we got involved in it, is really specialized for producing ethanol” (Interview 11). Industry knows well how to harness the vitality of this microbe to produce ethanol fuels (Bud, 1993). Once-wild S. cerevisiae have adapted to industrial conditions over decades and ferment sugarcane and corn in Brazilian and American bioethanol plants (Gillon, 2010; Ulrich, 2023). Where genetic engineering is expected to make a difference is by making yeast strains able to use other feedstocks, such as crop residues (sugarcane bagasse or corn stover), or by redirecting S. cerevisiae’s relatively well-understood metabolism towards producing molecules other than ethanol. One achievement in metabolic engineering happened in the early 2000s at Berkeley, California, when baker’s yeast was engineered to manufacture artemisinin, an antimalarial drug precursor (Ro et al., 2006).
To create “cell factories”, scientists are facing a dilemma between starting from the well-known S. cerevisiae or selecting a different species with an innate metabolism better suited to the end product in sight:
“Some [organisms] will be now better positioned to produce something than others. If you choose only based on the one that is easier to manipulate, like, Saccharomyces cerevisiae or E. coli, maybe it's going to take a lot of effort to make that product. Lipid is a good example. [S.] cerevisiae is not very good at producing lipids. There has been a lot of research on lipid production in [S.] cerevisiae. All of the first omega three fatty acid production in microbes were in [S.] cerevisiae, because it was easier to characterise everything. But when it made it to the market it was Yarrowia [lipolytica].” (Interview 8)
This metabolic engineer referred to the commercialisation of a fish-free omega-3 oil that was sold as a nutritional supplement under the trademark “New Harvest” by the chemical company Dupont (Xue et al., 2013). The reason why the yeast Yarrowia lipolytica “made it to market” (at least briefly in the early 2010s as the product does not seem to be available anymore), is because its innate metabolism is extremely good at dealing with lipids. Y. lipolytica is not a newcomer to industrial biotechnology (Fickers et al., 2005). Dwelling in environments such as sewage, crude oil spills, cured meats and cheese, the yeast attracted attention in the late 1960s due to its ability to degrade oil, hence its name Y. lipolytica. Companies and research institutions used the yeast in so-called single-cell protein projects in Europe (British Petroleum was particularly active), Japan and China (Bud, 1993, pp. 133–9). It was hoped that microbes grown on petroleum-derived substrates could become a nutritional product for farmed animals (and even the poor). But as global fossil fuel prices rose and soy production took off in South America, these projects collapsed. Y. lipolytica’s popularity has recently rebounded as it appears to be a good host for microbial production of oleochemicals (e.g., omega 3) and biofuels (Blazeck et al., 2014).
A logic of substitution shapes the making of “cell factories” in that the choice of target molecules guides the engineering strategies, including the selection of which species to use. Like omega-3 fatty acids, often the biotechnological route is expected to provide a drop-in replacement. In the mid 2000s, the US Department of Energy published a report with a list of “building block chemicals” that could be obtained from biomass in the future (Werpy & Petersen, 2004). A handful of molecules in that list captured researchers’ attention, such as succinic acid, a precursor used in the synthesis of polyesters, acidic regulation in beverages, and pharmaceutical formulations (more later). The rational for targeting building blocks like succinic acid is that their versatility offers the most opportunities for substitution. Some of the researchers we interviewed cited the US Department of Energy’s report as an influential paper for the scientific community as it helped organise research around a few compounds. But they also gave us more ad hoc reasons for their choice of target molecule. In one case, a research contract with a cleaning products company focused the scientists’ work on surfactants and the engineering of a particular yeast, Starmerella bombicola, able to secrete promising compounds (Interview 10). In another, a team became specialised in the yeast Kluyveromyces marxianus through a collaboration with a nearby dairy company looking to get rid of its whey permeate waste. The microbe’s innate metabolism can handle lactose well and it spontaneously generates aromatic molecules, which became the target (Interview 11).
The examples of Y. lipolytica, S. bombicola and K. marxianus illustrate a broader trend in the field of metabolic engineering. A biotechnologist described it as “a widening of the repertoire of yeast strains that can be used” to make “cell factories” (Interview 11). For the scientist, the microbial repertoire is only going to expand, as “within reason, there’s almost no organism that if we put our mind to it, that we couldn’t bring the tools of synthetic biology to them” (ibid.). Reflecting the ideal of the factory applied to scientific research itself, researchers often used terms like “faster”, “easier”, “more precise”, “multiple combinations in one test tube”, “lots of experiments run in parallel”, to emphasize that lab work moves now at a different pace, and lower costs, thanks to new gene synthesis and editing techniques, robotisation and computation (and this is itself an industry). DNA sequences are more easily read, manufactured, introduced and deleted in living cells. Metabolic networks can be more quickly mapped out and modelled to provide insights into how genes and pathways may be turned on or off to redirect a microbe’s metabolism. Yet, as a metabolic engineer recalled, “it’s just tools, tools have become more convenient” (Interview 16). The scientists must still come up with effective strategies to improve the microorganisms, while many things remain unknown, even in the well-studied S. cerevisiae, a computational biologist insisted (Interview 15). Microbial life keeps exceeding human understanding, and to some extent, human researchers count on its irreducible vitality (cf. Beldo, 2017).
What the industry wants from “cell factories” are engineered microbes that act as self-replicating biocatalysts in a factory environment. Our interlocutors, therefore, all valued the “robustness” of the yeasts they work with (Interview 18). Microbes are expected to be “tough” (Interview 2), withstanding high temperatures as it is costly to cool down a bioreactor and enzymatic reactions are faster when it is hot. It is appreciated if the organisms tolerate acidic conditions and the presence of inhibitors, which often occur when the feedstock is crop residues (see next section). Being able to grow at high density is another plus. Wild strains may already display some of these qualities; if not, they can be forced to acquire them through directed laboratory evolution (whereby the organism is grown in a harsh environment and adapts to it) and genetic engineering using synthetic biology tools. In addition to pursuing specific applications, like producing a compound of interest, scientists also conduct “fundamental application-inspired research in the core machineries” of their yeasts, said one industrial microbiologist (Interview 18). For example, cells are made to survive the complete absence of oxygen, saving the cost of aerating the fermenter. Or cells are engineered to secrete a metabolite instead of keeping it inside their membrane which puts a limit on the quantity (Interview 10). Improved to live well in a bioreactor, a strain can be made into a so-called chassis (a machinic term which in common parlance refers to the base frame of a vehicle), or in other words a “pre-engineer cell” as a bioengineer described it: a platform organism “that is already optimized, not for a specific application, but for a broader application”, like producing a particular class of compounds (lipids, proteins, aromatic molecules) (Interview 9). Working with chassis is supposed to accelerate the development of industrially useful applications.
To summarise, making “cell factories” consists in engineering microbial metabolisms to have them move carbon to assemble specific molecules in great quantities. The substitution logic frames the whole endeavour. The microorganisms are expected to survive harsh conditions while churning out chemicals of commercial interest that may become substitutions at industrial scale. The metaphor of the “factory” shapes how research is organised by “charting [the scientists’] search for knowledge of a world not yet known” (Keller, 2020, 249).Footnote 6 It is, however, a live metaphor as the yeasts disrupt the ideals of industrial production it is hoped they will eventually embody, thereby challenging the ethos of efficiency, productivity and (partial) control that infuses this field of research.
The costs of a sugar-powered economy
Unhappy cells
In our conversations with metabolic and chemical engineers, promises of what Paxson and Helmreich (2014) call “microbial abundance” were articulated. Abundance would come from “cell factories” robust enough to be cultured en masse and ferment renewable biomass (see next). But as they create new yeast strains in an attempt to bring about a sugar-powered economy, the researchers work simultaneously with and against the microbes. One metabolic engineer presented the tension as follows:
“If you want to make a product, ideally you don't want the cell to grow. Because you have a carbon source, you want this carbon source to go for the production of the product, not for the growth of the cell. The cell wants to do exactly the opposite, wants to grow, does not want to produce, and you've got to find a way to optimally balance these two functions.” (Interview 13)
Another confirmed: “you need the cells to be alive and viable, but you don’t want to make too much of them” (Interview 18). Boosting production while ensuring growth and reproduction is a major challenge. These microbes are complex beings imperfectly understood. Engineering their metabolism is thwarted by unintended effects as it takes away energy, carbon and precursors for potentially vital biochemical reactions, making the organism less good at living. As a bioengineer summarised, “if you don’t have it to do the normal job, living, that will be dramatic!” (Interview 9). Yeasts that do not respond well to changes in their metabolism end up “not growing as happily as they used to,” another biological engineer told us (Interview 14). Unhappy cells become recalcitrant, no longer industrially useful, and they can even end up “burnt-out” (Interview 9).
A related concern voiced during the interviews is whether or not things can be scaled up in practice and the engineered organisms keep producing valuable molecules in large fermenters, which is necessary for real substitution. One chemical engineer provocatively asked:
“You know, it’s very nice to grow them in a lab under neat conditions. But what if you were going to throw them at 1000 litres or 200,000 litres? Will the organism behave the same under those conditions?” (Interview 2)
Moving from the millilitre-to-litre lab scale to the volumes needed to mass-produce molecules is not straightforward. In large fermenters, cells at risk of burnout struggle to survive and are outcompeted, one biotechnologist explained to us:
“Mutations arise all the time, every time a cell divides, most of them have no impact. If a mutation happens that causes a cell, let’s say, to produce less of your product or none of your product, that’s great for the cell, because now it doesn’t have that burden anymore and it can grow faster. That cell grows faster and it divides and it divides and it divides. Basically, what happens is that mutant becomes faster growing than your producing cell and outgrows it. And so if you’re in a large bioreactor where you're going to have many, many generations, essentially you’ll get the bioreactor full of non-producing cells.” (Interview 11)
Being outcompeted by mutants of their own kind is not the only threat to engineered yeasts placed in industrial conditions. Contamination by other microorganisms is also an issue, as is toxicity, which occurs when the compound a microbe is made to overproduce becomes lethal (Interviews 12; 14). For example, the fatty acids a yeast is engineered to synthetise might be of a different chain length and degree of saturation to the ones it metabolises for itself to build its cellular membrane. The unusual fatty acids are nevertheless incorporated into the membrane, changing its fluidity and threatening its integrity. The yeast is again less good at living, unable to fulfil its destiny as a “cell factory”.
In contrast to examples where the promise of microbes is in their unleashed liveliness,Footnote 7 the researchers in our study value microbial agency insofar as it can be improved for a specific application.Footnote 8 Too much agency and vitality (mutants) are no good. An engineered microorganism is “a tool to get a job done”, said a chemical engineer (Interview 2). This instrumental view resonates with the study by Granjou and Phillips (2019) of scientists specialised in soil metagenomics. The authors highlight a “hybridisation” in the discourse of these researchers who shared a “vision of soil and its microbiota as a lively, but controllable, resource-turned-technology in the service of humans,” soil as a “biological engine” (p. 406). Similarly here, “cell factories” are considered as hubs of enzymatic reactions occurring inside organisms that are kept alive in order to serve human (industrial) interests.
Recalcitrant plant fibres
“Cell factories” are envisioned as biocatalysts in a longer chain of chemical reactions, one component of a wider production line. The promised bioeconomy hinges on the assumption that tough optimised microbes convert abundantly available sugars into valuable compounds. Assuming biological abundance is deeply ingrained in the way the scientists we spoke with justified their work as contributing to an environmentally sustainable alternative to (petro)chemical synthesis. The following quote from a metabolic engineer is illustrative:
“What is the most abundant renewable feedstock? This is plant matter. If we look around us, that’s what’s made by photosynthesis, the trees and the carbohydrates and all of the green world around us. This is plant matter and plant matter in its great majority is basically sugars. […]. So, sugars are an abundant renewable feedstock and it should be at the core of any technology which aims at producing these molecules in a sustainable way. […] Microbes love sugars. They utilise sugars for their growth. And now here comes metabolic engineering. If we engineer the pathway of these microbes, then we can direct the conversion of these sugars into the products of interest to us.” (Interview 13)
Under the (bio)chemical gaze, the vegetal world becomes a source of biomass, matter, sugars. Though there might be plenty of sugars out there, these are not in a form readily processable by microorganisms. Fermentable sugars must, first, be released from the polymers called cellulose, hemicellulose and lignin that make plant fibres. These long and complex chains need to be broken down into smaller, simpler molecules. The process, a chemical engineer noted, already happens “in nature” but only very slowly because “if biomass was easy to break down, then our plants and trees would just melt in the field” (Interview 5).
Chemical engineers like to talk about the “recalcitrance” of the biomass to describe its resistance to degradation (Himmel et al., 2007). To release sugars from such recalcitrant matter, corn stover, wood residues, sugarcane bagasse, or wheat straw, are usually pre-treated with acids, heat, even sometimes microwaves, to disrupt their structure. Enzymes called cellulase obtained from rot fungi are then put to work, cleaving the long carbon chains and pulling away smaller sugars. These sugars can be assimilated by microbes, but still not as easily as glucose. Therefore, scientists seek to create strains able to stomach a wider range of sugar molecules as well as withstand the presence of inhibitors released from the lignin and survive the acidic conditions of the milieu. Research teams have engineered baker’s yeast, the spontaneous ethanol-producer, to use agricultural waste. In the US where large public investments have been made, cellulosic ethanol is gradually catching up (US DOE 2023). Additionally, research is carried out to develop more efficient enzymes, which are an expensive product, by genetically improving the fungus Trichoderma reesei that spontaneously produces cellulase and by looking at how “in the biosphere” microorganisms degrade biomass to recycle carbon and nutrients (Bischof et al., 2016; Bomble et al., 2017). This “living carbon metabolism” (cf. Bensaude-Vincent & Loeve, 2018) is an inspiration and resource for biotechnologists who might even try to engineer easier-to-ferment plant cells (see Levidow et al., 2012, p. 107–111).
The industrial focus means that new supply chains are needed to turn crop residues into feedstock for biotechnology. Massive quantities of material usually left in agricultural fields (or sometimes burned to generate electricity) must be moved to bioreactors. One scientist with ethanol-related patents licensed to industry pointed out that there are still “technological challenges more upstream, [such as] getting the material off the land without also bringing a lot of sand, stones into a very advanced equipment” (Interview 18). Another told us about a project focused on using municipal solid waste and where they found that too many non-productive microbes would “eat up” all the useful sugars before the waste-as-feedstock arrived to the fermenter (Interview 16). These examples resonate with the analysis made by Daniel (2022) of the difficulties in running water treatment plants to produce fertilisers and biogas due to the presence of toxic chemicals in household waste that constantly threatens the digestion work of bacteria. The logic of substitution, as another project of “waste metamorphosis” (p. 2) to generate value, can hold only if the supply of biomass is secured and the logistics sorted out to prevent uninvited living and non-living things from wreaking havoc the work of scientists and engineers. In other words, the assumed abundance of biomass is of little value on its own.
Unfair competition
Because the focus is on replacing existing products, substitution is about commensurability and competition. According to an industrial microbiologist who reflected on what happened with biofuels, the technical difficulties upstream “can be solved” (e.g., biomass pre-treatment, robust engineered yeasts, supply logistics), the problem is that “low oil prices […] have slowed that development” (Interview 18). The remark echoes MacKenzie’s (2013) argument: scientists and start-ups have framed their projects in a way that “directly connects microbial metabolism […] with the energy and material costs of running bioreactors, as well as with the changing costs and the energy density of other fuels such as natural gas or petroleum” (p. 82). This is the logic of carbon substitution. Microbes and biomass are supposed to compete with the “massive tonnage” of the global oil market and reckon with the “unpredictable fluctuations” of its prices (p. 84). One scientist who had worked on a project making jet fuel in partnership with an aircraft manufacturer, recalled how in the late 2000s-early 2010s, “there was a big interest in making biofuels” for planes and cars to replace kerosene, gasoline and diesel by using engineered bacteria, yeasts, or algae (Interview 8).Footnote 9 A few years later the interest had faded. The exploitation of still untouched fossilised life (e.g., tar sands), the roll out of new technologies (e.g., fracking), and the dynamic of supply and demand, brought down oil prices. In this context, research on microbial alternatives was “not viable anymore because now fossil fuels are very cheap again” (ibid.). The logic of substitution means that the economics of fermentation-based solutions is assessed against the market price of what they are supposed to displace.
To illustrate this uneven playing field, a metabolic engineer invited us to compare the global prices of crude oil and refined sugar—the best feedstock for fermentation so far:
“Currently, one ton of crude oil costs the same as one ton of sugar.Footnote 10 But if you look at the sugar and compare it to the oil, sugar has much more oxygen, while [in the oil], all the oxygen molecules are gone. Sugar has much less energy. Sugar is not particularly suitable for making fuel, or chemicals, such as polymers, fats particularly, because again it has this huge amount of oxygen and it’s quite energetically expensive to convert all of that into lipids. You can imagine that you have basically two products that cost the same, one is already more or less a finished product, you just need to crack it and distill it and you’re done, and the other one, you need to make a fermentation, theoretical yields will be maybe 60% or less, you then need to process all of that, the supply chain is not there...” (Interview 16)
Most researchers we spoke with were similarly quick to emphasize that cost is “a relation” (Interview 10): it depends as much on the chemical affordances of the raw material as it is shaped by “political decisions” (Interview 14). Many argued that the biotechnological way is not that costly but becomes so comparatively. There is no discussion that fossilised life is much more energetic than cane sugar or bagasse. For our interviewees, however, petroleum products would not be so cheap if it was not for the decades-long global support benefitting the fossil energy sectors, from technical optimisation and subsidies, to the absence of an effective carbon price (Interviews 9; 16). A similar argument was put forward about oleochemicals and monocrop agriculture. According to a biologist trying to engineer a yeast to obtain palm oil-like substitutes, if oil palms are “a fantastic biological way of generating a ton of oil”, the competitiveness of the sector is also indebted to “a political machinery behind that is always willing to make that cost also very low” (Interview 4). To be industrially viable, bioprocesses and “cell factories” are meant to compete, and substitute, on the basis of such costs.
How successful, then, has biotechnology been at displacing chemical synthesis? When we asked the question, the answers varied. For some researchers, “there are plenty of success stories”, such as 1,3 propanediol, amino acids and lactic acid, but “if you were to put them together and say what fraction of the chemical industry they represent, it is still a small fraction” (Interview 13). For others, that is precisely why these cannot be called successes. One example we heard about twice is succinic acid, one of the chemical building blocks identified by the US Department of Energy. In the mid 2010s, several companies managed to make cells ferment starch and molasse into this precursor otherwise made from fossil oil. Yet, as one biotechnologist put it, the succinic acid story ended as a “big disappointment” (Interview 11). It was not made commercially viable and companies shelved the innovation. Researchers in our study often had their own examples of patented processes that did not make it to market. One team had engineered yeast strains to metabolise octanoic acid usually obtained from palm and coconut oil. The scientists “hit the targets, but the price of octanoic acid continued to decrease as the project was done”, until the industrial partner (a chemical company) told them it was “no longer interested to produce it biotechnologically” (Interview 11).
We can see that tangible achievements in the lab, or large fermenters, are not token of commercial success. Even if biomass may in theory regenerate in shorter cycles, and robust yeast are engineered to assemble useful compounds, fossil resources continue to be abundant and cheap enough (the same applies to oil crops). Scientists’ desires and designs on the metabolisms of unhappy yeasts and their battles with recalcitrant plant fibres come too little if the economics are unfavourable.
Pragmatic commitments to substitution
The scientists we spoke with did not see this as reason to despair. New projects were always around the corner, building on unexpected findings from shelved technologies and new opportunities for collaboration with colleagues and industry. If they aimed to make things that work, our interlocutors were also keen to “learn along the way what [their] cells are doing”, as one system biologist phrased it (Interview 14); and though “people thought [metabolic engineering] would deliver earlier” on the promise of manufacturing chemicals biologically, it has certainly “delivered a lot of knowledge” (ibid.). In an ethnography of biochemical engineering research focused on sugarcane in Brazil, Ulrich (2023) mentions a scientist for whom biofuel is a good excuse to also develop broader applications, such as finding new types of sugar within the plant fibres. Similarly, the researchers we interviewed displayed what we problematise as a pragmatic commitment to carbon substitution in response to the economic, technological, and biological hurdles we have cited. Following Ulrich (2023), we do not mean that their attitude is cynical, nor purely strategic. Instead, their pragmatic disposition signals the limits of what can be achieved in research labs to address complex environmental problems (for a reflection of the nature of such problems see Barry, 2021). It also indicates that as applied as they wish or claim their work to be, our interlocutors remain scientists driven by a taste for possibilities.
An illustration of the researchers’ pragmatism is how easy it has been for some of them to change target molecules. After the decline of oil prices in the mid 2010s, different compounds and end uses have been pursued, as explained a metabolic engineer:
“All the funding agencies kind of switched from making lipids for fuels to making lipids for something else. And all the scientists are trying to reinvent what other lipids they can make that are valuable that are not fuels.” (Interview 8)
While not all the scientists we interviewed had turned away from optimising strains for advanced fuels, quite a few did switch to other lipids, and some further moved from bulk products to specialty chemicals:
“Industrial biotechnological processes do make sense […] for the production of chemicals that are either, let’s say, they’re very rare in nature, or they’re very difficult to obtain from nature, or if they are too expensive to chemically synthetise. So, where there’s basically no convenient and cheap option to get them another way than by biotechnology.” (Interview 16)
This metabolic engineer became invested in an application space where they foresaw a brighter industrial future: with so-called high value compounds, industrial biotechnology may succeed the way pharmaceutical biotechnology did.Footnote 11 Fragrances, food applications, antimicrobials and pheromones (used as pesticides) are examples we were given. For the scientists who did not stop working on bulk chemicals, the co-production of higher value molecules was seen as a way to cross-subsidise the products selling at lower prices. Hinting at a more radical shift, one biotechnologist told us how they even envisioned a possible “democratisation” of biotechnology, where it would be customised to the needs of local businesses looking to valorise a waste stream on a much smaller scale (Interview 11).
Although big breakthroughs that would have large environmental impacts, like the scale-up of microbial fuels and bulk biochemicals, are yet to happen, sustainability continues to be a promotional argument (Karabin et al., 2021). In public presentation of new business ventures, a blend of ecological qualities is often associated with biotechnology.Footnote 12 A couple of the researchers in our study have had their work showcased in the media as sustainability stories and most felt they had to engage in some kind of environmental branding. One computational biologist was being provocative when they told us that sustainability and innovation are claims one makes to “sell” a project to funding agencies (Interview 15). Another scientist explained that while they used to present their research on yeast as promising for health-related applications, “now we write into all our grants, […] we’re going to solve all the environmental problems, you do kind of maybe hype up your claims a bit” (Interview 11). The comments were not meant to dismiss the role that biotechnology could, or should, play to pave the way for a more sustainable future. Rather, they point to how changing political agendas reshuffle research priorities.
Climate change is one problem that carbon substitution via “cell factories” metabolising crop residues is expected to address. For example, the European Union Horizon 2014–2020 research funding call advocated for “harnessing the potential of biotechnology processes and bio-based products to reduce CO2 emissions, estimated to range from between 1 to 2.5 billion tonnes CO2 equivalent per year by 2030” (OJEU, 2013, p. 139). The call cited a 2009 report authored by the World Wildlife Fund in partnership with Novozymes, a major enzyme company (Buttazzoni, 2009). Drawing on published life cycle assessment analyses (e.g., Hermann et al., 2007), various biotechnological processes were compared with their petrochemical baselines and market uptake projections helped quantify the potential emissions reductions. What value to give to these numbers is unclear. The field of life cycle assessments, as Freidberg (2013) shows, is fraught with debates and biotechnology is not immune to controversy (on bioplastic see Ögmundarson et al., 2020). In the EU research funding call, the numbers are, nevertheless, taken at face value, providing a “rationale” for the “added value” of supporting biotechnology and fostering innovation through projects conducted in partnerships with industry (OJEU, 2013, p. 139). Whether chemical companies, which are expected to be interested research partners, are seriously committed to adopting biological feedstocks is another question (Geels, 2022).
The researchers we interviewed seldom cited precise numbers to justify the environmental benefits of industrial biotechnology. The possibility to use renewable plant fibres was seen as enough to establish that fermentation is better than existing (petro)chemical alternatives (on the idea that the biological is necessarily good, see Asdal et al., 2023). Bioprocesses do release greenhouse gases from the fermentation itself, the use of energy, and through other impacts. The assumption is that plant regrowth soaks up CO2 and offsets these emissions. Applied to first generation biofuels—biodiesel from oil palm, rapeseed and soy, and bioethanol from corn and sugarcane—this reasoning has been contested in European and US policy-making circles. Land use models indicate that growing fuel crops in one place could indirectly increase agricultural conversion and deforestation elsewhere, which in carbon terms outweighs the gains of not using fossil resources (Gillon, 2014; Levidow, 2013). Maybe optimised “cell factories” using agricultural residues perform better in the models, but if industrial biotechnology was to really scale up, its environmental impacts (on land cover, water use, and so on) would be hard to dismiss.
While we see that pragmatism prevails, in their labs where the focus on sustainability is here to stay, researchers continue to “dream” (Interview 8). One experienced scientist explained his new project to engineer Y. lipolytica “in a way that we eliminate the emission of any CO2 coming out of these organisms” (Interview 13). The research was supported by a programme of the US Department of Energy, the mandate of which is “to minimise the amount of CO2 emitted by a process” whatever the energy needed, because “the assumption is there’s going to be plenty of energy coming from photovoltaics from the sun essentially, then hydroelectric and nuclear” (ibid.). The requirement, the researcher noted, contrasts with the situation in the mid 1970s when they started their career as a chemical engineer and everybody was “worrying about the supply of energy” (ibid.). Also working with the oil-eating microbe, a younger metabolic engineer talked about creating “microbial consortia”, in which cyanobacteria would transform CO2 into biomass and oils that the yeasts would ferment to produce specific compounds (Interview 8). In the early 2010s, hopes had been high that cyanobacteria and microalgae could produce low carbon biofuels (MacKenzie, 2013). Although the outcomes proved disappointing due to scaling issues, biotechnology could not give up on microbes living on a CO2 diet.
Despite mixed experiences with industrial commercialisation, faith in biotechnology does not falter at the lab bench. The logic of substitution seems sufficient to provide an environmental justification that promises to replace fossil carbon with biological carbon, even as research shifts from biofuels, to commodity chemicals, to specialty compounds. Researchers adopt a pragmatic attitude towards the expectation that biotechnology would contribute to a low carbon society—if only it could scale up. But if it did, would consumers be ready to buy the products? As far as we can tell, the question did not bother our interviewees too much, perhaps because they were primarily academics. We did hear on several occasions that there is no GMO in the end products, which are molecules. The engineered organisms are just production tools safely (hopefully) kept in bioreactors, unlike genetically modified crops.Footnote 13 One metabolic engineer acknowledged that maybe companies are “a little bit afraid of GMOs because of customer perception” (Interviews 10). For example, Ecover ended up withdrawing its laundry detergents containing chemicals made from genetically modified microorganisms in response to an online campaign (Domen & Develter, 2014; Thomas, 2014). Another metabolic engineer told us that, in their start up, they never use the term GMO as it is “very unpopular in Europe” (Interview 16). Yet, overall, what “the public” made of real or imagined consumers is believed to think, and what regulators might do about it, did not appear to be a major concern for the scientists we spoke with. One may wonder the extent to which this relates to a lack of material—or biochemical—literacy on the part of mass consumption societies where raw materials and manufacturing processes are out of sight. With its desired effects of making changes as imperceptibly as possible, the logic of substitution will only reproduce this disconnect.
Conclusion
For metabolic and chemical engineers, even when yeasts are burnt out and biomass is recalcitrant, the logic of carbon substitution continues to be a potentiality lurking within microbes, plants, and the broader biological world. Scientists see single-cell organisms as enzymatic reactions to be optimised and crop residues as a source of carbon (and other chemical elements) to be metabolised into valuable compounds. Under the (bio)chemical gaze (Landecker, 2019), engineered yeasts eating fermentable sugars extracted from plant fibres are “cell factories” that could replace petroleum and its heavy chemistry in industrial-scale factories. Yet, researchers report various practical issues that prevent this from working at present.
Even when microorganisms are carefully selected and modified to be tough, getting them to produce the molecules of interest at scale is a challenge. Furthermore, as our interviewees repeatedly emphasised, and as shown in the historical literature (Bud, 1993), the commercial prospects of industrial biotechnology depend on the price of petroleum (and other feedstocks like palm oil). The logic of substitution, which we argue frames the researchers’ work, involves comparing the efficiency and economics of cracking fossilised life versus fermenting plant fibres, at the expense of the latter. The same logic, in turn, puts liquid fuels in competition with other sources of energy like electricity, and the development of electric batteries was mentioned to us as another factor justifying caution regarding the future of biotechnology for fuel applications.Footnote 14
In our study, the history of innovation through substitution in the chemical industry entwines with the even longer history of human interventions in microbial life, amidst ever-present fears of environmental breakdown and a research funding model that expects the potential for scale-up. It is hoped that a living carbon metabolism will plug into where the industrial fossil carbon metabolism is currently in place (Bensaude-Vincent & Loeve, 2018). Drop-ins must be cost-effective in comparison to what industry does, where decades of supportive policy, geopolitical arrangements and technical optimisation have ensured those products are mass-consumed today. Researchers are, to some extent, failed by the system their innovation would keep in place: while they adhere to a logic of substitution, the scale it imposes makes it difficult for biotechnological advances to break through. Therefore, as we argue, scientists manifest a pragmatic commitment to the question. In how they approach substitution, they are guided by their knowledge of particular yeasts, available technology, personal contacts within companies, and an engineering ethos (MacKenzie, 2013): they want to create something useful, hence the shift to specialty applications. Their work is further shaped by available funding streams and the interests of industry selecting for the target molecules. Without funding, labs cannot be stocked nor staffed, experiments do not happen, papers are not published, tenure is not passed. Today, promising to reduce CO2 emissions is a selling point and arguing that biological carbon substitutes for fossil carbon often seems enough to substantiate the claim.
Enormous breakthroughs have been achieved in the scientific understanding of the inner workings of microbes, the development of metabolic engineering tools, and technology to scale up production. The epistemic and technical limits that scientists face—between the recalcitrance of plant matter and the frustrations of domesticating cellular life—are generative as they lead to more research. Where researchers have little sway is when, by way of an industrial partner losing interest or a government changing its research policy, they find themselves indirectly confronted with the political economy, market strategies and infrastructures of the fossil fuel and petrochemical sectors (see Mitchell, 2009; on biofuels see Birch & Calvert, 2015). If the supply chains of the promised bioeconomy are unlikely to map onto the geography of crude oil and its byproducts, the logic of substitution forces the commensuration of the two by indexing the former on its ability to compete with the latter. Such is the “energopolitics” (Boyer, 2014) that dictates the conditions of possibility for the projects of scientists and their yeast-produced molecules, to succeed or fail in replacing today’s hydrocarbon uses. These conditions have been shaped not around the material constraints of making “cell factories”, but around fossilised life cracked in ever-greater quantities.
Notes
The logic of substitution, in which a new technology is conceived as a replacement and must compete with its predecessor, can be found in other domains. Brives and Pourraz (2020) provide a case in point as they unpack the regulatory and epistemological constraints placed upon phage therapy—the use of bacterial viruses to treat infections instead of antibiotics—to address microbial resistance. A main challenge for scientists working with phages is to meet evidentiary standards developed for industrial drugs, which are at odds with how these living things operate as cure.
We thank Jane Calvert for suggesting using the term “faith” in this context.
We identified two large research projects in chemical and metabolic engineering that we thought would give us an initial overview of the field. One was funded by a British research council, the second by the European Commission. We interviewed the researchers involved in those projects before expanding our sample using a snowball strategy.
On the blurred lines between science and engineering, understanding and making, and analysis and synthesis in synthetic biology, see Finlay (2013) and Schyfter and Calvert (2015). On problem solving and industry relevance in synthetic biology, see Balmer, Bulpin and Molyneux (2016) and McLeod, Nerlich and Mohr (2017).
“These microbes must be improved before their potential can be realised” (Stephanopoulos, 2002, p. 920).
Unlike advanced fuels which are (quasi-)identical to diesel and gasoline, ethanol is an imperfect substitute usually blended with petroleum-derived fuels.
This interview was conducted in the Autumn 2021 before the war in Ukraine.
Start-ups are also moving to speciality biochemicals, sometimes changing their name in the process to get rid of references to petroleum. For example, Biopetrolia, a spin off from a Swedish university, became Melt&Marble to reflect a new business angle focused on plant-based meat substitutes.
Melt&Marble’s website states that their proprietary biotechnology may help to prevent deforestation (caused by cattle ranching) and protect biodiversity (Melt & Marble, 2023). C16 Biosciences, a US-based start-up, argues that its yeast-produced palm oil-like lipids will “combat climate” and is “conflict-free” (C16 Biosciences, 2023).
In Europe, genetically modified crops have faced public backlash, leading anxious policy-makers to anticipate that ‘the public’ and its fears would be a major obstacle to synthetic biology and call on social scientists to advise how to deal with this issue (see Marris, 2015; Marris et al., 2015; Marris and Calvert, 2020).
The replacement of liquid fuel by electricity came up in several conversations (Interviews 9; 15; 6).
References
(OJEU) Official Journal of the European Union. (2013). Regulation (EU) No 1291/2013 of the European Parliament and of the Council of 11 December 2013 establishing Horizon 2020 - the Framework Programme for Research and Innovation (2014–2020) and repealing Decision No 1982/2006/EC (1), 104–173.
Ankeny, R. A., & Leonelli, S. (2020). Model organisms. Cambridge University Press.
Asdal, K., Cointe, B., Hobæk, B., Reinertsen, H., Huse, T., Morsman, S. R., & Måløy, T. (2023). ‘The good economy’: A conceptual and empirical move for investigating how economies and versions of the good are entangled. BioSocieties, 18(1), 1–24.
Balch, O. (2015). Scientists reveal revolutionary palm oil alternative: Yeast. The Guardian, 17 February 2015, available at: https://www.theguardian.com/sustainable-business/2015/feb/17/scientists-reveal-revolutionary-palm-oil-alternative-yeast. Accessed 24.08.2023.
Balmer, A., Bulpin, K., & Molyneux-Hodgson, S. (2016). Synthetic biology: A sociology of changing practices. Springer.
Barry, A. (2021). What is an environmental problem? Theory, Culture & Society, 38(2), 93–117.
Béfort, N. (2021). The promises of drop-in vs. functional innovations: The case of bioplastics. Ecological Economics, 181, 106886.
Beldo, L. (2017). Metabolic labor: Broiler chickens and the exploitation of vitality. Environmental Humanities, 9(1), 108–128.
Bensaude-Vincent, B. & Loeve, S. (2018). Carbone. Ses vies, ses oeuvres. Édition Le Seuil.
Bensaude-Vincent, B., & Simon, J. (2008). Chemistry: The impure science. Imperial College Press.
Birch, K., & Calvert, K. (2015). Rethinking ‘drop-in’biofuels: On the political materialities of bioenergy. Science and Technology Studies, 28(1), 52–72.
Bischof, R. H., Ramoni, J., & Seiboth, B. (2016). Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microbial Cell Factories, 15(1), 1–13.
Blazeck, J., Hill, A., Liu, L., Knight, R., Miller, J., Pan, A., ... & Alper, H. S. (2014). Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nature Communications, 5(1), 1–10.
Bomble, Y. J., Lin, C. Y., Amore, A., Wei, H., Holwerda, E. K., Ciesielski, P. N., ... & Himmel, M. E. (2017). Lignocellulose deconstruction in the biosphere. Current Opinion in Chemical Biology, 41, 61–70.
Boyer, D. (2014). Energopower: An introduction. Anthropological Quarterly, 87(2), 309–333.
Brives, C., & Pourraz, J. (2020). Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Nature Humanities and Social Sciences Communications, 6(1), 1–11.
Brives, C., Rest, M., & Sariola, S. (Eds.). (2021). With microbes. Mattering Press.
Brown, N. (2003). Hope against hype-accountability in biopasts, presents and futures. Science & Technology Studies, 16(2), 3–21.
Bud, R. (1993). The uses of life: A history of biotechnology. Cambridge University Press.
Buttazzoni, M. (2009). GHG Emission reductions with industrial biotechnology: Assessing the opportunities. WWF Denmark, available at: https://wwfeu.awsassets.panda.org/downloads/wwf_biotech_technical_report.pdf
C16 Biosciences. (2023). https://www.c16bio.com/. Accessed 24 Aug 2023
Calvert, J. (2010). Synthetic biology: Constructing nature? The Sociological Review, 58(1_suppl), 95–112.
Calvert, J., & Szymanski, E. (2020). A feeling for the (micro) organism? Yeastiness, organism agnosticism and whole genome synthesis. New Genetics and Society, 39(4), 385–403.
Cooper, M. E. (2011). Life as surplus: Biotechnology and capitalism in the neoliberal era. University of Washington Press.
Dan-Cohen, T. (2021). A simpler life. Cornell University Press.
Daniel, F. J. (2022). Industrializing bacterial work: Microbiopolitics, biogas alchemy, and the French waste management sector. Science, Technology, & Human Values, OnlineFirst, 1–22.
Domen, T. & Develter, D. (2014). Ecover is as green as ever! The Ecologist, 25 June 2014. Available at: https://theecologist.org/2014/jun/25/ecover-green-ever
Eaglin, J. (2022). Sweet fuel: A political and environmental history of Brazilian ethanol. Oxford University Press.
Fickers, P., Benetti, P. H., Waché, Y., Marty, A., Mauersberger, S., Smit, M. S., & Nicaud, J. M. (2005). Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Research, 5(6–7), 527–543.
Finlay, M. R. (2003). Old efforts at new uses: A brief history of chemurgy and the American search for biobased materials. Journal of Industrial Ecology, 7(3–4), 33–46.
Finlay, S. C. (2013). Engineering biology? Exploring rhetoric, practice, constraints and collaborations within a synthetic biology research centre. Engineering Studies, 5(1), 26–41.
Freidberg, S. (2013). Calculating sustainability in supply chain capitalism. Economy and Society, 42(4), 571–596.
Geels, F. W. (2022). Conflicts between economic and low-carbon reorientation processes: Insights from a contextual analysis of evolving company strategies in the United Kingdom petrochemical industry (1970–2021). Energy Research & Social Science, 91, 102729.
Geels, F. W., & Schot, J. (2007). Typology of sociotechnical transition pathways. Research Policy, 36(3), 399–417.
Gillon, S. (2010). Fields of dreams: Negotiating an ethanol agenda in the Midwest United States. The Journal of Peasant Studies, 37(4), 723–748.
Gillon, S. (2014). Science in carbon economies: Debating what counts in US biofuel governance. Environment and Planning A, 46(2), 318–336.
Granjou, C., & Phillips, C. (2019). Living and labouring soils: Metagenomic ecology and a new agricultural revolution? BioSocieties, 14(3), 393–415.
Helmreich, S. (2009). Alien ocean. University of California Press.
Helmreich, S. (2020). Not a metaphor: A comment on Evelyn Fox Keller’s ‘cognitive functions of metaphor in the natural sciences.’ Interdisciplinary Science Reviews, 45(3), 446–458.
Hermann, B. G., Blok, K., & Patel, M. K. (2007). Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environmental Science & Technology, 41(22), 7915–7921.
Himmel, M. E., Ding, S. Y., Johnson, D. K., Adney, W. S., Nimlos, M. R., Brady, J. W., & Foust, T. D. (2007). Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science, 315(5813), 804–807.
Hughes, S. S. (2011). Genentech: The beginnings of biotech. University of Chicago Press.
Karabin, J., Mansfield, I., & Frow, E. K. (2021). Exploring presentations of sustainability by US synthetic biology companies. PLoS ONE, 16(9), e0257327.
Keller, E. F. (1995). Refiguring life: Metaphors of twentieth-century biology. Columbia University Press.
Keller, E. F. (2009). What does synthetic biology have to do with biology? BioSocieties, 4(2–3), 291–302.
Keller, E. F. (2020). Cognitive functions of metaphor in the natural sciences. Interdisciplinary Science Reviews, 45(3), 249–267.
King, J. K. K., Granjou, C., Fournil, J., & Cecillon, L. (2018). Soil sciences and the French 4 per 1000 initiative – The promises of underground carbon. Energy Research & Social Science, 45, 144–152.
Kohler, R. E. (1973). The enzyme theory and the origin of biochemistry. Isis, 64(2), 181–196.
Landecker, H. (2016). Antibiotic resistance and the biology of history. Body & Society, 22(4), 19–52.
Landecker, H. (2019). A metabolic history of manufacturing waste: Food commodities and their outsides. Food, Culture & Society, 22(5), 530–547.
Landecker, H. (2021). Trace amounts at industrial scale: Arsenicals and medicated feed in the production of the ‘western diet.’ In A. N. H. Creager & J.-P. Gaudillière (Eds.), Risk on the table: Food production, health, and the environment (pp. 187–213). Berghahn.
Levidow, L. (2013). EU criteria for sustainable biofuels: Accounting for carbon, depoliticising plunder. Geoforum, 44, 211–223.
Levidow, L., Birch, K., & Papaioannou, T. (2012). Divergent paradigms of European agro-food innovation: The knowledge-based bio-economy (KBBE) as an R&D agenda. Science, Technology, & Human Values, 38(1), 94–125.
Liu, Y., Cruz-Morales, P., Zargar, A., Belcher, M. S., Pang, B., Englund, E., ... & Keasling, J. D. (2021). Biofuels for a sustainable future. Cell, 184(6), 1636–1647.
Mackenzie, A. (2013). The economic principles of industrial synthetic biology: Cosmogony, metabolism and commodities. Engineering Studies, 5(1), 74–89.
Marris, C. (2015). The construction of imaginaries of the public as a threat to synthetic biology. Science as Culture, 24(1), 83–98.
Marris, C., & Calvert, J. (2020). Science and technology studies in policy: The UK synthetic biology roadmap. Science, Technology, & Human Values, 45(1), 34–61.
Marris, C., Balmert, A., Calvert, J., Molyneux-Hodgson, S., Frow, E., Kearnes, M., ... & Martin, P. (2015). Taking roles in interdisciplinary collaborations: Reflections on working in post-ELSI spaces in the UK synthetic biology community. Science and Technology Studies, 28(3).
McLeod, C., Nerlich, B., & Mohr, A. (2017). Working with bacteria and putting bacteria to work: The biopolitics of synthetic biology for energy in the United Kingdom. Energy Research & Social Science, 30, 35–42.
Melt & Marble. (2023). https://www.meltandmarble.com/. Accessed 24 August 2023.
Mitchell, T. (2009). Carbon democracy. Economy and Society, 38(3), 399–432.
Money, N. P. (2018). The rise of yeast: How the sugar fungus shaped civilization. Oxford University Press.
Morosini, D. (2021). The super-natural world of bioengineered beauty. Financial Times, August 5, 2021, available at: https://www.ft.com/content/e0e1a9a7-f5a1-4688-93cf-efd1c6965941
O’Malley, M., Powell, A., Davies, J. F., & Calvert, J. (2008). Knowledge-making distinctions in synthetic biology. BioEssays, 30(1), 57–65.
Ögmundarson, Ó., Sukumara, S., Laurent, A., & Fantke, P. (2020). Environmental hotspots of lactic acid production systems. GCB Bioenergy, 12(1), 19–38.
Paxson, H. (2012). The life of cheese: crafting food and value in America. University of California Press.
Paxson, H., & Helmreich, S. (2014). The perils and promises of microbial abundance: Novel natures and model ecosystems, from artisanal cheese to alien seas. Social Studies of Science, 44(2), 165–193.
Peralta-Yahya, P. P., Zhang, F., Del Cardayre, S. B., & Keasling, J. D. (2012). Microbial engineering for the production of advanced biofuels. Nature, 488(7411), 320–328.
Raimbault, B. (2021). Dans l’ombre du génie génétique: Le génie métabolique. Natures Sciences Sociétés, 29(3), 262–273.
Raimbault, B. (2018). A l'ombre des biotechnologies: Reformuler la production de savoirs par la bio-ingénierie en France et aux Etats-Unis, Doctoral dissertation, Université Paris-Est.
Redfern, S. (2013). Genetically modified yeast turns crop wastes into liquid fuel. BBC News, 11 October 2013, available at: https://www.bbc.co.uk/news/science-environment-24489800
Ro, D. K., Paradise, E. M., Ouellet, M., Fisher, K. J., Newman, K. L., Ndungu, J. M., ... & Keasling, J. D. (2006). Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature, 440(7086), 940–943.
Roosth, S. (2017). Synthetic: How life got made. University of Chicago Press.
Schyfter, P., & Calvert, J. (2015). Intentions, expectations and institutions: Engineering the future of synthetic biology in the USA and the UK. Science as Culture, 24(4), 359–383.
Stephanopoulos, G. (2002). Metabolic engineering: Perspective of a chemical engineer. American Institute of Chemical Engineers. AIChE Journal, 48(5), 920.
Stephanopoulos, G. (2007). Challenges in engineering microbes for biofuels production. Science, 315(5813), 801–804.
Stilgoe, J. (2016). Geoengineering as collective experimentation. Science and Engineering Ethics, 22(3), 851–869.
Thomas, J. (2014). Ecover pioneers ‘synthetic biology’ in consumer products. The Ecologist, 16 June 2014, available at: https://theecologist.org/2014/jun/16/ecover-pioneers-synthetic-biology-consumer-products
Turns, A. (2020). How do you deal with 9m tonnes of suffocating seaweed? The Guardian, June 30 2020, available at: https://www.theguardian.com/environment/2020/jun/30/how-do-you-deal-with-9m-tonnes-of-suffocating-seaweed-aoe
Ulrich, K. (2023). The substitute and the excuse: Growing sustainability, growing sugarcane in São Paulo, Brazil. Cultural Anthropology, 38(4), 439–466.
(US DOE) United States Department of Energy (2023). Renewable fuel standard, alternative fuels data center. Available at: https://afdc.energy.gov/laws/RFS. Accessed 24 August 2023
Vivien, F. D., Nieddu, M., Befort, N., Debref, R., & Giampietro, M. (2019). The hijacking of the bioeconomy. Ecological Economics, 159, 189–197.
Werpy, T., & Petersen, G. (2004). Top value-added chemicals from biomass: Volume I -- Results of screening for potential candidates from sugars and synthesis gas. US Department of Energy, National Renewable Energy Lab., Golden, CO (US).
Xue, Z., Sharpe, P. L., Hong, S. P., Yadav, N. S., Xie, D., Short, D. R., ... & Zhu, Q. (2013). Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nature Biotechnology, 31(8), 734–740.
Acknowledgements
We would like to thank UCL School of Humanities and Social Sciences Dean’s Fund and UCL Anthropocene Initiative for awarding the funding for this research. UCL Institute of Advanced Studies are also thanked for hosting our talk on this topic, as are Lara Choksey, Jane Calvert and Andrew Barry for their thought-provoking responses at this event. AR also thanks the Leverhulme Trust for supporting her research. We both thank the two anonymous reviewers, the journal editors, the editors of the special issue, Céline Granjou, Vincent Banos, Arnaud Sergent and Sylvain le Berre, and the other contributors to this issue who attended the workshop organised in June 2022 for prompting us to think through the logic of substitution in terms of carbon and refine our argument. Finally, we warmly thank our interviewees for sharing their time and knowledge so generously. All the mistakes are ours.
Funding
This work was supported by University College London through the SHS Dean’s fund and the Anthropocene Initiative (small grant: “Anthropocene Substitution”). Rudge’s research was also supported by a Leverhulme Early Career Fellowship.
Author information
Authors and Affiliations
Contributions
VE and AR designed the study, conducted the interviews and performed data analysis together. VE wrote the first draft of the manuscript and AR edited and commented on it. VE and AR worked on the revisions together. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval, consent to participate, and consent for publication
The research was approved by UCL’s Research Ethics Committee and followed the Committee’s requirements regarding information, consent and anonymity.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ehrenstein, V., Rudge, A. The logic of carbon substitution: from fossilised life to “cell factories”. Rev Agric Food Environ Stud (2024). https://doi.org/10.1007/s41130-024-00206-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41130-024-00206-z