Abstract
In this work, the role of gas kinetics in the growth of lobed graphene domains by atmospheric pressure chemical vapor deposition (AP-CVD) is elucidated by sandwiching Cu foil between Si/SiO2 wafers. Two different growths were carried out: (1) A Cu foil was placed at the center of a quartz tube in AP-CVD for graphene growth and (2) another Cu foil was sandwiched between Si/SiO2 wafers to alter the nucleation growth kinetics of graphene domains to mimic those in low-pressure chemical vapor deposition (LP-CVD). From the scanning electron microscopy (SEM) images, the graphene domains of the sandwiched Cu foil displayed mostly four-lobed, parallel-sided domains which are usually obtained under LP-CVD as compared to Cu foil without sandwiching which showed typical hexagonal graphene domains of AP-CVD. The Raman spectroscopy confirmed that the domains are single-layer graphene. An electron backscatter diffraction (EBSD) showed that the Cu foil is predominantly (001). The results of this study agree with the theoretical predictions of growth kinetics in graphene synthesis by CVD and showed that it is possible to obtain single-layer graphene domains which are usually obtained under LP-CVD by restricting the gas flux through the boundary layer.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Graphene, a monolayer of carbon atoms in a two-dimensional (2D) honeycomb lattice, has several unique properties that make it an ideal material for research as well as potential applications [1, 2]. Since the discovery of the first isolated graphene prepared by mechanical exfoliation of graphite [3], several chemical approaches for growing large-area graphene have been developed. Chemical vapor deposition (CVD) is an appealing approach to the production of graphene because of its capability for producing large-area, high-quality graphene samples. A spurt of interest in CVD growth of graphene was triggered by the seminal work published by Ruoff’s group using low-pressure chemical vapor deposition (LP-CVD) [4].
However, the CVD growth of graphene has revealed multiple challenges due to the need to explore enormous experimental parameter space due to the multiple components involved [5]. A vast majority of published results in this area have relied on the low-pressure CVD recipe for graphene growth. Indeed, low-pressure CVD approach has been shown to be scalable for large graphene film synthesis [6, 7]; however, it has challenges such as feeding the catalyst foil into a low-pressure reactor, severe evaporation of copper catalyst and requirement of a vacuum system compatible with flammable precursor gases [5]. The last two aspects do not constitute major problems for a research-oriented laboratory, but they do translate into substantial obstacles for industrial-scale graphene production and would contribute to increased production costs [5].
Although CVD graphene growth on Cu surface has a simplistic view, the growth mechanism or kinetics is complex and depends on growth background pressure. Bhaviripudi et al. [8] proposed a model for CVD graphene growth on low carbon solid solubility catalyst (Fig. 1) in which the carbon species first (1) diffuse through the boundary layer onto Cu surface (i.e., a low carbon solid solubility catalyst), (2) get adsorbed on the catalyst surface, and (3) decompose to form active carbon species which (4) diffuse on the surface of the catalyst to form nucleation sites and (5) attachment of active C species to nucleation sites to materialize graphene (C=C bonds formation), and (6) inactive species (such as hydrogen) get desorbed from the surface, form molecular hydrogen, and diffuse away from the surface through the boundary layer and are eventually swept away by the bulk gas flow. In this model, there are two fluxes of the active species that coexist: (1) flux of active species through the boundary layer and (2) the rate at which the active species are consumed at the surface of the catalyst to form the graphene lattice/domains (see Eqs. 1 and 2) below [8]:
where the \({J}_{\rm mt}\) is the flux of active species through the boundary layer (δ), \({J}_{\rm sr}\) is the flux of consumed active species at the surface, \({h}_{\rm g}\) is the mass transport coefficient, \({K}_{\rm s}\) is the surface reaction constant, \({C}_{\rm g}\) is the concentration of the gas in the bulk and \({C}_{\rm s}\) is the concentration of the active species at the catalyst surface. The slower of the two processes is the rate-limiting step during graphene growth. At steady state, three regimes result in which (1) \({h}_{\rm g}\gg {K}_{\rm s}\) (surface reaction-controlled regime), (2) \({h}_{\rm g}\sim {K}_{\rm s}\) (mixed regime) and \({h}_{\rm g}\ll {K}_{\rm s}\) (mass transport-limited regime) [8]. According to Ref.[8], under atmospheric pressure CVD (AP-CVD) at high growth temperatures (~ 1000 °C) the gas diffusion through the boundary layer is slower than the surface reactions (Ks > > hg) and this is the rate-limiting step. However, as the pressure is lowered, the boundary layer also increases, but the increase in gas diffusion through the layer is significantly higher compared to an increase in the thickness of the boundary layer [8]. Therefore, the overall effect is that the mass transport coefficient, \({h}_{\rm g}\), increases as a result of lowering the total pressure and the diffusion through the boundary layer is enhanced and no longer the rate-limiting step under normal growth conditions (\({h}_{\rm g}\gg {K}_{\rm s}\)). Under these conditions (i.e., LP-CVD), the surface reaction regime is rate limiting, and as long as the temperature is maintained uniform across the length of the wafer, the thickness of the graphene is uniform [8]. Therefore, the influence of the Cu surface and orientations are more noticeable in the early stage of graphene growth. Murdock et al. have demonstrated that under LP-CVD the growth of graphene domains is dictated by Cu crystallographic orientations [9].
a Processes involved during graphene synthesis using low carbon solid solubility catalysts (Cu) in a CVD process. b Mass transport and surface reaction fluxes under steady-state conditions. Reprinted with permission from Ref. [8]. Copyright (2010) American Chemical Society
In this study, we show that restricting the gas supply to the Cu foil by placing it between Si/SiO2 wafers under AP-CVD graphene growth alters the nucleation growth kinetics of graphene domains to mimic those in LP-CVD.
2 Experimental
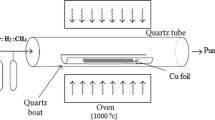
Cu foil samples (~ 40 × 40 mm2) were obtained from a high-purity (99.8%) 25-μm-thick annealed Cu foil for graphene growth from Alfa Aesar. Samples were annealed under Ar (300 sccm) and H2 (9 sccm) flow for 30 min at 1000 °C before the growth of graphene. Two different growths were carried out, as illustrated in Scheme 1. In the first growth process, Cu foil was placed at the center of a quartz tube in AP-CVD for graphene growth at 1000 °C from a mixture of gases, Ar (300 sccm): H2 (9 sccm): CH4 (10 sccm) for 10 s. Immediately after growth, the CH4 flow was stopped and samples were rapidly cooled down by pushing the quartz tube to the cooler region of the furnace and at less than 80 °C the samples were off-loaded from the tube. In the second growth process, a Cu foil was sandwiched between Si/SiO2 wafers and placed at the center of a quartz tube in AP-CVD for graphene growth at 1000 °C from a mixture of gases, Ar (300 sccm): H2 (9 sccm): CH4 (15 sccm) for 60 s. After growth, some of the samples were transferred from Cu foil to SiO2/Si substrates using bubbling method [10, 11] for further characterization. A WITec alpha 300RAS + confocal Raman system (WITec GmbH) was used for both imaging and spectroscopy with the laser power set below 2 mW to minimize heating effects. The excitation wavelength used was set to 532 nm (2.33 eV) through a numerical aperture of 0.9 and 100 × magnifications, which allows an image spatial resolution of about 360 nm. A high-resolution scanning electron microscope (SEM) Zeiss Ultra Plus 55 FE-SEM operated at 1.0 kV was used to investigate the surface morphology of the graphene samples. The topographical images were obtained using a Dimension Icon atomic force microscope (AFM) by Bruker AXS with NanoScope Analysis software in ScanAsyst mode. Electron backscatter diffraction (EBSD) analysis of a Cu foil was performed in a LEO 1525 field emission gun scanning electron microscope at an acceleration voltage of 25 kV using the Oxford INCA crystal software.
3 Results and discussion
In our experimental setup, the SiO2/Si slabs act as a barrier that eliminates the boundary layer and hence the rate-limiting reaction is the surface reaction (\({K}_{\rm s}\)) as in the LP-CVD. Therefore, the gas molecules diffuse through the limited space in the interface between the Si slabs and Cu surface with a low partial pressure compared to gas on the Cu foil without slabs, and thereafter, hydrocarbon decomposes on the Cu surface to supply carbon for graphene growth. As a result, the graphene domains on Cu foil between the slabs have a decreased growth rate and improved crystal quality. This hypothesis is further confirmed by the following experimental findings.
Figure 2 presents the SEM images of the graphene domains obtained from the two growth processes which display hexagonal graphene islands for the Cu foil without slabs (first growth) and lobed graphene domains (mostly four-lobed, parallel-sided domains) for sandwiched Cu foil (second growth) similar to those obtained under LP-CVD [9, 12, 13]. As discussed in Fig. 1, graphene growth mechanisms and results from LP-CVD and AP-CVD differ. Graphene growth on Cu in the LP-CVD method is a surface-limited diffusion process of carbon species [14]. Graphene domains, in this case, may show symmetric patterns as six-lobed snowflake-like structures [14,15,16] and four-lobed star-like structures [12, 14]. On the other hand, growth in the AP-CVD is not self-limiting and is likely to be a surface adsorption process in which extended growth time increases the number of graphene layers [8], and the graphene islands exhibit a hexagonal shape, suggesting a growth mechanism that is determined by the graphene structure rather than the underlying copper substrates [14, 17, 18].
Figure 3a shows an AFM image of one of the lobed graphene domains transferred onto SiO2/Si substrate. From AFM image, the height of the domain is ~ 1 nm which could be attributed to a single-layer graphene domain. The typical graphene wrinkles are also visible on the domain, and the white spots are residues from the transfer process. Moreover, Raman spectroscopy, a well-established tool to probe, among others, the number of graphene layers, and the interlayer interactions and stacking order in graphene samples, was used to characterize the graphene domains. Typically, the main Raman characteristic features of high-quality graphene are the G-band mode (~ 1590 cm−1) and the 2D-band mode (~ 2680 cm−1) which originate from the normal first-order Raman scattering process (the in-plane bond stretching displacements of sp2 carbon atoms, i.e., E2g symmetry) and the second-order double resonance Raman process that involves two in-plane transverse optical mode (iTO) phonons near the K-point, respectively [19]. However, in the defective graphene, these Raman features include the disorder-induced mode (D-band at ~ 1350 cm−1) which originates from a double resonance Raman process involving inter-valley scattering of iTO phonon and defect near the K-point [19]. Figure 3b displays the Raman G-peak intensity mapping of the lobed graphene domain, and the corresponding average spectra are shown in Fig. 3(c). It can be seen from the G-peak intensity mapping that the G peaks intensities scale range at the center of the domain is twice that of the overall domain which suggests that the domain is monolayer graphene with bilayer graphene starting to grow from the initial nucleation center. In graphene, the G-band intensity shows behavior that follows a linear trend as the number of layers increases from mono- to multilayer graphene [19]. Furthermore, the representative average Raman spectra of the domain (Fig. 3c) display a typical Raman spectrum of monolayer graphene and bilayer graphene (i.e., domain center). Figure 3d, e show Raman 2D peaks full width at half maximum (FWHM) mapping and the corresponding 2D-to-G peaks intensities ratio (I2D/IG) mapping of graphene domain, respectively. The FWHMs and I2D/IG ratios are around 30 cm−1 and ~ 3–4, and these indicate that the domain is monolayer graphene with a bilayer center (2D FWHM ≈ 50 cm−1 and I2D/IG ≈ 1). Fig. 3f displays the D-to-G peaks intensities ratio (ID/IG) mapping with an average value in the range of ~ 0.1‒0.2. This low ID/IG ratio indicates that the graphene domain is of high quality, as would be expected under LP-CVD conditions due to a high vacuum. Nevertheless, at higher growth temperatures (~ 1000 °C), AP-CVD grows high-quality graphene.
a An AFM image of graphene domain grown on Cu foil sandwiched between SiO2/Si slabs and transferred onto SiO2/Si substrate. b Raman G-peak intensity mapping of the graphene domain transferred onto SiO2/Si substrate. c The average Raman spectra of the G-peak intensity mapping in (a) (green (top) spectrum corresponds to monolayer graphene and red (bottom) spectrum to bilayer graphene). d Raman 2D-peak FWHM mapping. e I2D/IG mapping and f ID/IG mapping of the graphene domain in (b)
For a microstructural analysis of the annealed Cu foil, an SEM 10.0 kV primary electron beam was scanned across the foil surface to obtain its microstructural image, as shown in Fig. 4a, which displays the average grain size of ~ 30 μm. The surface normal projected inverse pole figure EBSD map of Alfa Aesar Cu foil (with color key inset) is presented in Fig. 4b. This figure reveals that the annealed Cu foil used in this study is predominantly (001). Previously published reports have shown that an annealed Alfa Aesar Cu foil for graphene growth is mainly (001) [9, 20]. Moreover, Murdock et al. demonstrated the influence of crystallographic orientations of polycrystalline Cu substrates on the formation of CVD graphene domains and identified four predominant cases under LP-CVD [9]:
-
(i)
Four-lobed, parallel-sided domains on Cu(101), orientated in one direction [− 101].
-
(ii)
Four-lobed, parallel-sided domains on Cu(001), orientated in two ~ 90° rotated directions with equal probability.
-
(iii)
Six-lobed, star-shaped domains on tilted and high-index Cu grains, e.g., Cu(103).
-
(iv)
Hexagonal-edged elongated domains on orientations close to Cu(111) and Cu(535), orientated in one direction.
Therefore, in our study, an observed lobed graphene domain on Alfa Aesar Cu(001) foil which is usually observed under LP-CVD confirms that the growth kinetics of graphene domains of a sandwiched Cu foil mimic those in LP-CVD.
4 Conclusion
In conclusion, we have demonstrated a simple reproducible method to alter the nucleation growth kinetics of graphene domains to mimic those in LP-CVD by restricting the gas supply to the Cu foil by placing it between Si/SiO2 wafers under AP-CVD graphene growth. Two different AP-CVD graphene growths were carried out on a Cu foil, i.e., on a Cu foil without sandwiching and on Cu foil sandwiched between Si/SiO2 wafers to alter the nucleation growth kinetics of graphene domains to mimic those in LP-CVD. The results of the graphene domains of the sandwiched Cu foil displayed lobed graphene domains which are usually obtained under LP-CVD as compared to Cu foil without sandwiching which showed typical hexagonal graphene domains of AP-CVD. Care was taken to have a Cu foil as flat as possible between the flat Si wafers, and the space between the Cu foil and the SiO2/Si slab was estimated to be approximately 50 µm. The size (~ 40 × 40 mm2) of the samples, i.e., Cu foil sandwiched between Si/SiO2 wafers, was limited by the inner diameter of the quartz tube; however, the sample size can be upscaled. Our findings are essential for the production of uniform high-quality, large-area graphene under AP-CVD conditions mimicking LP-CVD conditions.
References
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Xia F, Mueller T, Lin Y-M, Valdes-Garcia A, Avouris P (2009) Ultrafast graphene photodetector. Nat Nanotechnol 4:839–843. https://doi.org/10.1038/nnano.2009.292
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. https://doi.org/10.1126/science.1102896
Li X, Cai W, An J, Kim S, Nah J, Yang D, Piner R, Velamakanni A, Jung I, Tutuc E, Banerjee SK, Colombo L, Ruoff RS (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324:1312–1314. https://doi.org/10.1126/science.1171245
Vlassiouk I, Fulvio P, Meyer H, Lavrik N, Dai S, Datskos P, Smirnov S (2012) Large scale atmospheric pressure chemical vapor deposition of graphene. Carbon N Y 54:58–67. https://doi.org/10.1016/j.carbon.2012.11.003
Bae S, Kim H, Lee Y, Xu X, Park J-S, Zheng Y, Balakrishnan J, Lei T, Ri Kim H, Il Song Y, Kim Y-J, Kim KS, Özyilmaz B, Ahn J-H, Hong BH, Iijima S (2010) Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat Nanotechnol 5:574–578
Han GHEE (2011) Poly(ethylene co-vinyl acetate)-assisted one-step transfer of ultra-large graphene. NANO 6:59–65. https://doi.org/10.1142/S1793292011002342
Bhaviripudi S, Jia X, Dresselhaus MS, Kong J (2010) Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett 10:4128–4133
Murdock AT, Koos A, Ben Britton T, Houben L, Batten T, Zhang T, Wilkinson AJ, Dunin-Borkowski RE, Lekka CE, Grobert N (2013) Controlling the orientation, edge geometry, and thickness of chemical vapor deposition graphene. ACS Nano 7:1351–1359. https://doi.org/10.1021/nn3049297
Dodoo-arhin D, Fabiane M, Bello A, Manyala N (2013) Graphene: synthesis, transfer, and characterization for dye-sensitized solar cells applications. Ind Eng Chem Res 52:14160–14168. https://doi.org/10.1021/ie4017489
Fabiane M, Khamlich S, Bello A, Dangbegnon J, Momodu D, Charlie AT, Fabiane M, Khamlich S, Bello A (2013) Growth of graphene underlayers by chemical vapor deposition Growth of graphene underlayers by chemical vapor deposition. AIP Adv. https://doi.org/10.1063/1.4834975
Li X, Magnuson CW, Venugopal A, An J, Suk JW, Han B, Borysiak M, Cai W, Velamakanni A, Zhu Y, Fu L, Vogel EM, Voelkl E, Colombo L, Ruoff RS (2010) Graphene films with large domain size by a two-step chemical vapor deposition process. Nano Lett 10:4328–4334. https://doi.org/10.1021/nl101629g
Hwang C, Yoo K, Kim SJ, Seo EK, Yu H, Bir LP (2011) Initial stage of graphene growth on a Cu substrate. J Phys Chem C 115:22369–22374
Zhao P, Kumamoto A, Kim S, Chen X, Hou B, Chiashi S, Einarsson E, Ikuhara Y, Maruyama S (2013) Self-limiting chemical vapor deposition growth of monolayer graphene from ethanol. J Phys Chem C 117:10755–10763
Li X, Magnuson CW, Venugopal A, Tromp RM, Hannon JB, Vogel EM, Colombo L, Ruo RS (2011) Large-area graphene single crystals grown by low-pressure. J Am Chem Soc 133:2816–2819
Zhang Y, Zhang L, Kim P, Ge M, Li Z, Zhou C (2012) Vapor trapping growth of single-crystalline graphene flowers: synthesis, morphology, and electronic properties. Nano Lett 12:2810–2816
Graphene S, Foils C, Yan Z, Lin J, Peng Z, Sun Z, Zhu Y, Li L, Xiang C, Loı E, Kittrell C, Tour JM (2012) Toward the Synthesis of Wafer-Scale. ACS Nano 23:9110–9117
Robertson AW, Warner JH (2011) Hexagonal single crystal domains of few-layer graphene on copper foils. Nano Lett 11:1182–1189. https://doi.org/10.1021/nl104142k
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401
Madito MJ, Bello A, Dangbegnon JK, Oliphant CJ, Jordaan WA, Momodu DY, Masikhwa TM, Barzegar F, Fabiane M, Manyala N (2016) A dilute Cu ( Ni ) alloy for synthesis of large-area Bernal stacked bilayer graphene using atmospheric pressure chemical vapour deposition. J Appl Phys. https://doi.org/10.1063/1.4939648
Acknowledgements
This study is based on research supported by the South African Research Chairs Initiative of the Department of Science and Technology (SARChI-DST) and the National Research Foundation (NRF) (Grant No. 97994). M.F thanks the University of Pretoria and the NRF for financial support during his PhD studies. M.F acknowledges support from the LRSM, through the US National Science Foundation MRSEC, Grant No. DMR-1120901.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fabiane, M.S., Madito, M.J. & Manyala, N. Influence of growth kinetics on graphene domains shape under atmospheric pressure chemical vapor deposition. Graphene Technol 5, 75–81 (2020). https://doi.org/10.1007/s41127-020-00035-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41127-020-00035-z