Abstract
Patients with chronic pain develop peripheral neuropathy and experience sleep disturbance. Yokukansan is used to treat insomnia and control neuropathic pain. We studied if Yokukansan affects neuropathic pain and sleep disturbance using a rat model of chronic constriction injury (CCI). Male Wistar rats (4-week age) were divided into the following groups (n = 7, per group): CCI rats fed normal chow (CCI-0); CCI rats fed powdered chow mixed with 1% Yokukansan (CCI-1); CCI rats fed powdered chow mixed with 3% Yokukansan (CCI-3); and sham-operated control rats fed normal chow (SHAM). We examined sleep duration and quality using electroencephalograms and assessed pain using the von Frey and Hargreaves tests. Results were analyzed by one-way analysis of variance and Bonferroni post hoc tests. The CCI-0 group exhibited an increased wake period, decreased non-rapid eye movement (REM) sleep time, and no change in REM sleep time in comparison to the SHAM group. The CCI-1 group exhibited a decreased wake period, increased non-REM sleep time, and no change in REM sleep time compared to the CCI-0 group. The CCI-3 group exhibited increased non-REM sleep time but no changes in wake and REM sleep times compared to the CCI-1 group. The von Frey and Hargreaves test findings revealed an increase in the pain threshold in the CCI-1 group compared to the CCI-0 group. There was no difference in pain threshold between the CCI-1 and CCI-3 groups. In our rat model of CCI, sleep disturbance was reflected. Yokukansan inhibited CCI-induced sleep disturbance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of patients experience chronic pain due to peripheral neuropathy, and most of these patients report sleep disturbance, including an increase in sleep latency, higher level of wakefulness following sleep onset, decreased slow wave sleep, and changes in sleep stages [1,2,3,4]. Several recent studies have reported that sleep disturbance and chronic pain due to peripheral neuropathy have a bidirectional relationship [1, 3]. Chronic pain causes sleep disturbance and sleep disturbance worsens chronic pain. Concurrent sleep disturbance and chronic pain affect pain tolerance through their synergistic interactions [5]. Therefore, these symptoms should be immediately and concurrently treated. Agents that reduce chronic pain and improve sleep disturbance without an increased risk of adverse effects must be explored.
Yokukansan, which was first described in the Bao Ying Jin Jing Lu (written in 1550 BCE), is a Japanese traditional herbal medicine (Kampo; granules) that comprises seven herbs (Table 1).
Yokukansan showed consistency in yielding the concentration of active molecules in mice [6].
Yokukansan is widely used to treat insomnia, emotional irritability, and neurosis [7]. Several reports showed the effective use of Yokukansan against pain disorders, including headache and neuropathic pain [8, 9]. Yokukansan improves sleep disturbance in patients with behavioral and psychological symptoms caused by dementia [10]. Yokukansan also increased the total sleep time and tended to cause an increase in sleep efficiency and of stage 2 sleep, as well as a decrease of sleep latency and of stage3 + 4 sleep [11].
Yokukansan improves various clinical symptoms. A recent report indicated that Yokukansan effectively treated sleep disturbance in a rat model of cerebrovascular dementia [12]. Yokukansan also exhibited antiallodynic effects in a rodent chronic constriction injury (CCI) model [13]. However, there is little basic information regarding the effects of Yokukansan on pain-related sleep disturbance.
CCI, a technique described in an animal model by Bennet and Xie [14] for neuropathic pain, is commonly used in studies of neuropathy induced by long-term constriction of the sciatic nerve and is characterized by mechanical hyperalgesia and thermal allodynia [14]. In recent research, rats with CCI had qualitative and quantitative sleep disturbances [15]. In the present study, we examined if Yokukansan has any beneficial effects on sleep disturbance and neuropathic pain in a rat model of CCI.
Materials and methods
Animals
Appropriate approvals for using animals for the described experiments have been obtained from the Animal Care Committee of Teikyo University School of Medicine (Tokyo, Japan; Approval No. 17–019). Male, 4-week-old Wistar rats weighing 130–180 g were obtained from the Hino Breeding Center (Tokyo, Japan) and were maintained under conditions of controlled relative humidity (60% ± 2%), constant temperature of 24 °C ± 0.5 °C with controlled light/dark-schedule (light on from 6:00 a.m. to 6:00 p.m.). They had free access to food and water. The measurements were done on designated days. Rats were sacrificed after experiment by carbon dioxide (CO2) euthanasia.
The CCI model
Two weeks before the experiment, the rats were subjected to the CCI technique, which was developed by Bennett and Xie [14]. The CCI rat model is often employed for studying neuropathic pain and its treatment. After anesthesia using sevoflurane and sodium pentobarbital, the sciatic nerve on the right side was exposed in the middle part of the thigh by a direct dissection through the biceps femoris muscle. Four silk thread ligatures (4-0) were tied loosely around the exposed sciatic nerve at an interval of approximately 1-mm. By closing the muscles and skin, surgery was completed. In the control rats that have gone through sham operation, there was no ligation of the exposed right sciatic nerve. Autotomy was absent in all subjects.
Experimental groups
Rats were divided into the following four groups (n = 7 in each group): (1) CCI rats fed normal powdered chow (CCI-0); (2) CCI rats fed powdered chow mixed with 1% Yokukansan (CCI-1); (3) CCI rats fed powdered chow mixed with 3% Yokukansan (CCI-3); and (4) sham-operated control rats (SHAM) fed normal powdered chow. We examined the time and quality of sleep in these rats using electroencephalogram analysis and assessed pain using the von Frey and Hargreaves tests 2 weeks after inducing CCI or administering the sham surgery.
Feeding of Yokukansan
Yokukansan (Lot No. 2170054010; Tsumura and Co., Tokyo, Japan) at 1% or 3% concentration was mixed with powdered chow diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan; Table 1). The rats were fed the powdered chow 2 weeks after inducing CCI or administering the sham surgery. The SHAM group and CCI-0 group rats were given chow diet without Yokukansan. The concentrations were selected by referring to previous reports describing the doses of Yokukansan or other Kampo medicines that were effective [16, 17].
Electroencephalogram analysis
Five days before the experiment, sevoflurane and sodium pentobarbital anesthesia was administered to the rats and electroencephalogram (EEG) electrodes were implanted on the skulls of the rats with dental cement, and an electromyogram (EMG) electrode was placed in the muscle on the back of the head. For epidural EEG recordings, all rats were implanted with five stainless steel screws, by placing them over the frontal and occipital cortex, bilaterally. The placement of reference electrode was in the frontal bone rostral to the frontal cortex. For the EMG recordings, the muscle of the back of the head was inserted with two steel lines, bilaterally. The leads were attached to the socket, which was fixed using dental cement to the skull, along with the electrodes. For performing the analysis the left fronto-occipital lead was employed. The recordings of EMG and EEG were done for 5 h during the inactive stage in normal rats for 2 weeks after inducing CCI. The durations (in seconds) of the wake period, rapid eye movement (REM) sleep, and non-REM (NREM) sleep were compared between the groups.
Sleep stages for wake, NREM sleep and REM sleep, were visually scored in 4-s epochs using the Sleep Sign for Animals, version 3.2.0 (Kissei Comtec, Nagano, Japan), which is based on fast Fourier transform analyses of EEG signals divided in the δ (0.75–4 Hz), θ (6–10 Hz), and α (8–13 Hz) frequency bands and on other parameters such as locomotion and the EMG integral. Stages of wake and sleep were characterized as follows: wake was recognized by high EMG activity (EMG integral > 120 mV/s or locomotion > 0) and desynchronized low-amplitude, mixed frequency EEG activity (> 4 Hz); NREM was characterized by an EEG δ power higher than the threshold with decreased EMG activity (EMG integral < 0.7–120 mV/s; EEG δ > 173–2133 mV2; locomotion = 0); and REM was characterized by elevated EEG θ/(δ + θ) ratio (i.e., > 0.5–0.73) and locomotion = 0. Based on this scoring method, the alterations in REM sleep, wake, and NREM sleep time were calculated for 5 h.
Pain threshold
Pain was assessed before and 2 weeks after inducing CCI using the von Frey and Hargreaves tests. Mechanical hyperalgesia was evaluated by determining the threshold for the right hind paw withdrawal in response to a mechanical stimulus applied using von Frey filaments (0.008–300 g; DanMic Global, LLC, CA, USA) [18]. Rats were individually housed in a metal mesh cage that is raised 1 m above the ground. The animals were allowed to Acclimatization time for the rats was 30 min to the environment. The rigid tip of the von Frey filament, which was connected to the von Frey meter, was positioned on the right plantar surface from under the floor. Data from the von Frey test are shown as the percent control (% min).
Thermal allodynia was determined by examining the withdrawal latency of hind paw in against radiant heat response, employing a plantar test apparatus (SN# 42012-400; IITC Life Science Inc., Woodland Hills, CA, USA), according to the procedure of Hargreaves et al. [19]. Each rat was kept in an enclosed compartment on a glass surface, and through this glass plate, a beam of infrared radiation was directed upward. The beam heated the hind paw skin up to 37 °C. In the absence of a response to avoid damage to tissue, a 20 s cutoff time was imposed. Results from three separate trials with 5-min interval between them were used to determine the mean withdrawal latency for each measurement. Data from the Hargreaves test are expressed as the percentage maximum possible effect (%MPE). This value was determined using the formula given below:
Data analysis
All data were subjected to statistical analyses using the Excel Tokei 2012 program (Social Survey Research Information Co., Ltd., Tokyo, Japan). Results are shown as the mean ± standard deviation (SD). Data were compared using one-way analysis of variance, followed by Bonferroni tests. For all comparisons, differences were considered statistically significant at p < 0.05.
Results
Yokukansan had no effect on sleep disturbance, thermal allodynia and mechanical hyperalgesia in the sham-operated control rats in this study.
Effects of Yokukansan on sleep disturbance in the rat model of CCI
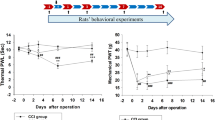
The CCI-0 group exhibited increased wake time and decreased NREM sleep time (wake time, 282.6 ± 15.5 min; NREM sleep time, 13.9 ± 11.3 min) compared with the SHAM group (wake time, 229.5 ± 25.9 min; NREM sleep time, 68.6 ± 25.1 min; p < 0.01). However, there was no difference in REM sleep time between the CCI-0 and SHAM groups (3.5 ± 6.3 versus 1.6 ± 2.2 min, respectively). These findings suggest that CCI rats may have sleep disturbances. The CCI-1 group exhibited decreased wake time and increased NREM sleep time (wake time, 249.3 ± 26.8 min; NREM sleep time, 47.5 ± 22.0 min) compared with the CCI-0 group (wake time, 282.6 ± 15.5 min; NREM sleep time, 13.9 ± 11.3 min; p < 0.01). However, there was no difference in REM sleep time between the CCI-1 and CCI-0 groups (3.2 ± 5.8 versus 3.5 ± 6.3 min, respectively). The CCI-3 group exhibited increased NREM sleep time (54.8 ± 29.8 min) compared with the CCI-1 group (47.5 ± 22.0 min). However, no differences existed between the CCI-1 and CCI-3 groups in REM sleep time (3.2 ± 5.8 versus 3.5 ± 4.2 min, respectively) or in wake time (249.3 ± 26.8 versus 241.8 ± 31.6 min, respectively; Fig. 1). All the results obtained in this study are shown in the supplementary information files.
Effects of Yokukansan on sleep disturbance. The durations of the wake, REM sleep, and non-REM sleep times were compared between the groups. The CCI-0 group exhibited increased wake time and decreased non-REM sleep time in comparison to the SHAM control group (p < 0.01). However, the REM sleep time did not significantly differ between the CCI-0 and SHAM groups. The CCI-1 group exhibited decreased wake time and increased non-REM sleep time compared with the CCI-0 group (p < 0.01). However, the REM sleep time did not significantly differ between the CCI-1 and CCI-0 groups. The CCI-3 group exhibited increased non-REM sleep time in comparison to the CCI-0 group (p < 0.05); however, the difference in wake or REM sleep time between the CCI-1 and CCI-3 groups was not significant
CCI-0, rats with CCI and fed normal chow; CCI-1, rats with CCI and fed powdered chow mixed with 1% Yokukansan; CCI-3, rats with CCI and fed powdered chow mixed with 3% Yokukansan; non-REM, non-rapid eye movement; REM, rapid eye movement; SHAM, control rats with sham surgery and fed normal chow + no Yokukansan.
Effects of Yokukansan on thermal allodynia and mechanical hyperalgesia
Thermal allodynia and mechanical hyperalgesia were examined by determining the withdrawal threshold 2 weeks after CCI. In the thermal allodynia test, which was assessed using the Hargreaves test, the pain threshold was lower in the CCI-0 group (− 49.22% ± 8.06%) than in the SHAM group (7.75% ± 21.91%). This finding suggested the presence of thermal allodynia (p < 0.01). The pain threshold was higher in the CCI-1 group (− 16.77% ± 18.32%) than in the CCI-0 group (− 49.22% ± 8.06%; p < 0.01) and was also higher in the CCI-3 group (− 10.21% ± 12.44%) than in the CCI-0 group (− 49.22% ± 8.06%; p < 0.01; Fig. 2).
Effects of Yokukansan on thermal allodynia. Data for the Hargreaves test are shown as the percentage maximum possible effect (%MPE). The pain threshold was less in the CCI-0 group compared to the SHAM group for allodynia (p < 0.01). The pain threshold was increased in the CCI-1 group in comparison to the CCI-0 group (p < 0.01) and in the CCI-3 group compared with CCI-0 group (p < 0.01). Results are shown as the mean ± SD
CCI-0, rats with CCI and fed normal chow; CCI-1, rats with CCI and powdered chow mixed with 1% Yokukansan; CCI-3, rats with CCI and powdered chow mixed with 3% Yokukansan; SHAM, control rats with sham surgery and fed normal chow + no Yokukansan.
In the mechanical hyperalgesia test, which was assessed using the von Frey test, the pain threshold was lower in the CCI-0 group (11.3% ± 7.7%) than in the SHAM group (58.3% ± 31%). This finding suggested the presence of mechanical hyperalgesia (p < 0.05). The pain threshold was higher in the CCI-1 group (29.7% ± 15.4%) and the CCI-3 group (29.3% ± 15.5%) than in the CCI-0 group (11.3% ± 7.7%; p < 0.05). However, the pain threshold did not differ between the CCI-3 (29.3% ± 15.5%) and CCI-1 groups (29.7% ± 15.4%; Fig. 3). All the obtained results in this study are presented in the supplementary information files.
Effects of Yokukansan on mechanical hyperalgesia. Data for the von Frey test are shown as percentage of control (% min). The pain threshold was increased in the CCI-1 and CCI-3 groups in comparison to the CCI-0 group (p < 0.05). However, there was no significant difference in the pain threshold between the CCI-3 and CCI-1 groups. Results are mean ± SD
CCI-0, rats with CCI and fed normal chow; CCI-1, rats with CCI and powdered chow mixed with 1% Yokukansan; CCI-3, rats with CCI and powdered chow mixed with 3% Yokukansan.
Data were compared using one-way analysis of variance, followed by Bonferroni tests. For all comparisons, differences were considered statistically significant at p < 0.05.
Discussion
In the present study, sleep disturbance was demonstrated by increased wake time and decreased non-REM sleep time in our rat model of CCI. We reported that feeding of Yokukansan improved sleep disturbance in the rat model of CCI. This improvement was reflected by decreased wake time and increased non-REM sleep time. The feeding of 3% Yokukansan improved sleep disturbance to a greater extent than that of 1% Yokukansan. Thus, Yokukansan may have a dose dependent sleep-inducing function in rats having neuropathic pain. Additionally, we demonstrated that treatment with 1% Yokukansan inhibited thermal allodynia and mechanical hyperalgesia in the rat model of CCI. However, the treatment effect did not differ between 1 and 3% Yokukansan, which implies that the effect of Yokukansan on thermal allodynia and mechanical hyperalgesia was not dose dependent. These results demonstrated that administering Yokukansan could be an effective way to treat sleep disturbance in patients suffering from neuropathic pain and also for treating neuropathic pain.
The CCI rats qualitatively and quantitatively exhibited poor sleep. These findings are in agreement with earlier reports demonstrating significantly increased wake periods and lowered non-REM sleep due to chronic neuropathic pain [15, 20].
In a previous animal research using the same CCI rat model as that used in our study, Yokukansan inhibited thermal allodynia and mechanical hyperalgesia [14]. Sleep disturbance in CCI rats is a result of abnormally hyperactive neural activity in the anterior cingulate region. The excess liberation of glutamate in the anterior cingulate region causes the activation of astrocytes in the anterior cingulate region and increases the uptake of gamma-aminobutyric acid (GABA) into cells. The reduction in the neurotransmission efficiency of the inhibitory system due to decreased GABA concentrations in the cell gap may cause sleep disturbances [15].
Elevated levels of glutamate in the extracellular space has been shown in the brains of thiamine-deficient rats. Yokukansan prevents the rise in the extracellular glutamate level [21].
The principal components of Yokukansan are Cnidii rhizome, Uncariae cum Uncis ramulus, Bupleuri radix, Poria, Atractylodis lanceae rhizoma, Glycyrrhizae radix, and Angelicae radix (Table 1). Of these seven components of Yokukansan, glycyrrhiza ameliorates the thiamine deficiency-induced reduction in the uptake of glutamate by astrocytes. Specifically, the decreased astrocyte glutamate uptake due to thiamine deficiency is ameliorated by glycyrrhizin and its metabolite 18 beta-glycyrrhetinic acid, which are among the eight components of glycyrrhiza. Uncariae cum Uncis ramulus has many pharmacological effects including stimulation of serotonin 1A receptor and blockade of serotonin 2A receptor. Furthermore, it antagonizes the N-methyl-D-aspartate (NMDA) receptor-mediated ion current [22].
Based on these findings, the effect of Yokukansan on sleep disturbance, thermal allodynia, and mechanical hyperalgesia in CCI rats may result from the inhibition of the excessive release of glutamic acid that occurs with the stimulation of chronic pain and from the possible involvement of NMDA receptor antagonism and serotonin 1 or serotonin 2. The components responsible for such effects of Yokukansan require further investigation.
Limitations
Sleep disturbance patterns of patients with neuropathic pain include an increased wake period and decreased non-REM sleep period [2]. This pattern is similar to the findings of the present study; therefore, we considered CCI rats to be an ideal animal model of neuropathic pain, for studying the above parameters. However, our findings do not imply that CCI rats are an appropriate animal model for studying neuropathic pain. A limitation of behavior tests is that inter-experimental variation and environmental conditioning may possibly affect the results, as shown in calibrated von Frey hairs [23].
Sleep disturbance confounds neuropathic pain treatment [24, 25]. Sleep disturbance and chronic pain in patients suffering from neuropathic pain should be treated concurrently. Antidepressants are the first-line of drugs for treating neuropathic pain. However, although antidepressants reduce pain, they do not improve sleep disturbance. In addition, paroxetine and duloxetine increase wake time and suppress sleep [26].
We did not measure the amount of food and water the rats intake accurately, so it cannot be denied that the different amount of food and water rats intake caused the lack of difference in treatment effect between the feeding of 1% Yokukansan and that of 3% Yokukansan.
Yokukansan has been clinically administered to patients for treating insomnia and neuropathic pain. Yokukansan is inexpensive and has few adverse effects. In the present study, Yokukansan improved sleep disturbance, thermal allodynia, and mechanical hyperalgesia in a rat model of CCI. These findings indicate that Yokukansan could clinically improve sleep disturbance and chronic pain in patients with neuropathic pain and does so without an increased risk of adverse effects. Therefore, Yokukansan could be successfully administered to patients with neuropathic pain. We also believe that by administering a dose of Yokukansan above the analgesic amount, sleep disturbance could be improved, and the quality of life of patients with neuropathic pain could be improved.
Thus, in our rat model of CCI, sleep disturbance was reflected by decreased sleep time and increased non-REM sleep time. Yokukansan inhibited CCI-induced sleep disturbance. It also inhibited thermal allodynia and mechanical hyperalgesia.
Data availability
The data used to support the findings of this study are included within the supplementary information files.
References
Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and managing sleep disturbance in patients with chronic pain. Anesthesiol Clin. 2016;34:379–93.
Ferini-Strambi L. Neuropathic pain and sleep: a review. Pain Ther. 2017;6:19–23.
Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311–4.
Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32.
Sivertsen B, Lallukka T, Petrie KJ, Steingrímsdóttir ÓA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156:1433–9.
Minoru T, Hiroshi T. Yokukansan, a traditional Japanese herbal medicine, alleviates the emotional abnormality induced by maladaptation to stress in mice. Phytomedicine. 2014;21(3):363–71.
De Caires S, Steenkanp S. Use of Yokukansan (TJ-54) in the treatment of neurological disorders: a review. Phytother Res. 2010;24:1265–70.
Nakamura Y, Tajima K, Kawagoe I, Kanai M, Mitsuhata H. Efficacy of traditional herbal medicine Yokukansan on patients with neuropathic pain. Masui. 2009;58:1248–55 ([In Japanese]).
Yamaguchi K. Traditional Japanese herbal medicines for treatment of odontopathy. Front Pharmacol. 2015;6:176.
Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, Tanji H, Fujiwara H, Seki T, Fujii M, Arai H, Sasaki H. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry. 2005;66:248–52.
Aizawa R, Kanbayashi T, Saito Y, Ogawa Y, Sugiyama T, Kitajima T, Kaneko Y, Abe M, Shimizu T. Yoku-kan-san-ka-chimpi-hange on the sleep of normal healthy adult subjects. Psychiatry Clin Neurosci. 2002;56(3):303–4.
Nagao M, Takasaki K, Nogami A, Hirai Y, Moriyama H, Uchida N, Kubota K, Katsurabayashi S, Mishima K, Nishimura R, Iwasaki K. Effect of Yokukansan on sleep disturbance in a rat model of cerebrovascular dementia. Tradit Kampo Med. 2014;1:19–26.
Suzuki Y, Mitsuhata H, Yuzurihara M, Kase Y. Antiallodynic effect of herbal medicine yokukansan on peripheral neuropathy in rats with chronic constriction injury. Evid Based Complement Alternat Med. 2012;2012:953459.
Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107.
Narita M, Niikura K, Nanjo-Niikura K, Narita M, Furuya M, Yamashita A, Saeki M, Matsushima Y, Imai S, Shimizu T, Asato M, Kuzumaki N, Okutsu D, Miyoshi K, Suzuki M, Tsukiyama Y, Konno M, Yomiya K, Matoba M, Suzuki T. Sleep disturbances in a neuropathic pain-like condition in the mouse are associated with altered GABAergic transmission in the cingulate cortex. Pain. 2011;152:1358–72.
Honda Y, Sunagawa M, Yoneyama S, Ikemoto H, Nakanishi T, Iwanami H, Suga H, Ishikawa S, Ishino S, Hisamitsu T. Analgesic and anti-stress effects of Yokukansan in rats with adjuvant arthritis. Kampo Med. 2013;64:78–85.
Goto M, Hayashi M, Todoroki T, Seyama Y, Yamashita S. Effects of traditional Chinese medicines (dai-saiko-to, sho-saiko-to and hachimi-ziogan) on spontaneously diabetic rat (WBN/Kob) with experimentally induced lipid and mineral disorders. Nihon Yakurigaku Zasshi Folia Pharmacologica Japonica. 1992;100:353–8 ([In Japanese]).
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88.
Takemura Y, Yamashita A, Horiuchi H, Furuya M, Yanase M, Niikura K, Imai S, Hatakeyama N, Kinoshita H, Tsukiyama Y, Senba E, Matoba M, Kuzumaki N, Yamazaki M, Suzuki T, Narita M. Effects of gabapentin on brain hyperactivity related to pain and sleep disturbance under a neuropathic pain-like state using fMRI and brain wave analysis. Synapse. 2011;65:668–76.
Ikarashi Y, Iizuka S, Imamura S, Yamaguchi T, Sekiguchi K, Kanno H, Kawakami Z, Yuzurihara M, Kase Y, Takeda S. Effects of yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine-deficient rats. Biol Pharm Bull. 2009;32:1701–9.
Kawakami Z, Ikarashi Y, Kase Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell Mol Neurobiol. 2011;31:1203–12.
Andrews K. The effect of changes in temperature and humidity on the accuracy of von Frey hairs. J Neurosci Methods. 1993;50:91–3.
Fishbain DA. Approaches to treatment decisions for psychiatric comorbidity in the management of the chronic pain patient. Med Clin North Am. 1999;83:737–60.
Onen SH, Alloui A, Gross A, Eschaller A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42.
Wichniak A, Wierzbicka A, Jernajczyk W. Sleep and antidepressant treatment. Curr Pharm Des. 2012;18:5802–17.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: RD, HS, and SS; Data curation: RD and MF; Formal analysis: RD and MF; Funding acquisition: RD, HS, and SS; Investigation and Methodology: RD; Project administration: RD, HS, and SS; Resources: RD, MF, and HS; Software: RD, MF, and HS; Supervision: RD, HS, and SS; Validation: RD and MF. Visualization: RD and MF; Roles/writing-original draft: RD and HS; Writing-review and editing: RD and HS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical Committee Permission
Appropriate approvals for using animals for the described experiments have been obtained from the Animal Care Committee of Teikyo University School of Medicine (Tokyo, Japan; Approval No. 17-019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deguchi, R., Fujimoto, M., Sekiyama, H. et al. Effect of Yokukansan on sleep disturbance and neuropathic pain in chronic constriction injury using a rat model. Sleep Biol. Rhythms 19, 277–283 (2021). https://doi.org/10.1007/s41105-021-00315-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-021-00315-y