Abstract
In this paper, the aging impact of desulphation (DeSO x ) procedures on lean NO x traps (LNT) was investigated. With accelerated aging procedures on an engine test bench and on a synthetic-gas test bench, LNTs were stressed with lean rich cycling under realistic desulphation conditions. Exhaust gas chassis dynamometer tests showed the impact on emissions of the lean rich treatment. High carbon monoxide (CO) slips were detected in NEDC tests during NO x regeneration (DeNO x ). With light-off tests, the pattern of damage was further investigated. A pronounced deactivation in CO-rich gas conversion was found to be the main reason for the carbon monoxide emissions in the chassis dynamometer tests. A correlation between DeSO x duration (cumulated duration of rich pulses) and the inhibited CO conversion was observed. Determinations of oxygen storage capacities of aged catalysts indicated that the lean–rich cycling mainly damaged the ceria oxide of the LNT. Variations of the rich gas components indicated that hydrogen in the feed gas as well as in situ generated hydrogen out of feed gas components (steam reforming, water gas shift) is accountable for the degradation in carbon monoxide conversion in rich purges. Investigations for lower desulphation temperatures showed that the effect is negligible for temperature <350 °C. Therefore, catalyst deactivation throughout NO x regeneration events with much lower temperatures than at DeSO x was not observed. As reference to other aging treatments used in the literature, samples aged hydrothermally at 750 °C, a phosphorus poisoned and hydrothermally aged LNT as well as a LNT sample from a vehicle endurance run were compared to the DeSO x aged catalysts. All LNTs had the same conventional LNT coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The share of diesel engines in new passenger cars for the western European market (EU15 + EFTA) was about 53.1% in 2014 corresponding to a report of the European Automobile Manufacturers Association (ACEA). Compared to 1990, where only 13.8% of new passenger cars had diesel engines, the increasing popularity of diesel-driven cars is obvious [1].
Beside the high demand of customers, the diesel engine is essential in the automobile manufacturer’s portfolio because of its advantages in thermodynamic efficiency and, therefore, lower CO2-emissions compared to conventional gasoline engines. Diesel engines are essential to lower the overall fleet CO2-emissions. A drawback of the diesel engines’ lean combustion process is, that conventional three way catalysts cannot be used to reduce engine out NO x [2,3,4]. To comply with the current European emissions legislation (EU6) and with further real driving emissions legislation (RDE), complex NO x exhaust aftertreatment systems are necessary. While the particulate matter problem was solved with the introduction of diesel particulate filters (DPF), current NO x limits are still a challenge. To achieve the NO x goals, BMW uses both Lean-NO x -Traps (LNT) and selective catalytic reduction systems (SCR-systems) [5,6,7].
The operating principle of Lean NO x Traps can be separated roughly into a cyclic storage phase of NO x under lean conditions, where NO x are adsorbed and bound in form of nitrates, and a reduction phase, where stored NO x are released and subsequently reduced to N2 [8, 9]. Depending on the washcoat formulation of the LNT, the maximum in NO x storage and as a consequence the interval between NO x -regeneration events varies strongly. When a defined NO x -storage is reached a regeneration event is necessary [8, 9]. The NO x regeneration—the so-called DeNO x event—takes place by a change in engine operating mode. A special combustion setting, the rich mode, leads to a rich exhaust gas with a lambda value <1 [4, 5].
LNTs typically contain NO x -storage components, like alkali or alkaline earth metals, and precious metals on aluminum oxide [8, 10]. Because of its chemical properties, NO x traps also operate as sulphur traps. The sulphur is bound as sulphates on the storage components similar to NO x . Sulphur can originate from fuel or lubricant oil [8, 9]. As both nitrate and sulphate formation takes place on the storage components, increasing sulphur poisoning decreases the maximum in NO x storage significantly as shown in [11,12,13]. The sulphating mechanism is reversible [14, 15]. To recover the NO x -trapping performance of LNTs after sulphur poisoning, another rich engine operation mode, similar to the NO x regeneration event, is necessary. As sulphates are much more stable than nitrates, higher regeneration temperatures are necessary compared to a DeNO x event. For efficient desulphation events (DeSO x ) different OEMs use periodically triggered particulate filter regeneration events, where LNT temperatures of about 580–630 °C are common. The DeSO x takes place during the DPF regeneration because no further catalyst heat up is necessary. Depending on the strategy of the particular OEM an substoichiometric exhaust gas (lambda <1) is set up for a defined cumulated time per DeSO x event with varying pulse duration and number of pulses to release the bound sulphur from the LNT [5, 16, 17].
Beside the so-called sulphur poisoning, the maximum catalyst temperature is an important criterion for Lean NO x Trap aging. Especially during DeSO x events it is important to not exceed maximum LNT temperatures [5, 16]. Thermal aging of LNTs primarily takes place at temperatures of 800 °C and above, like shown by Rohr et al. [18]. Despite keeping in the recommended DeSO x temperature range of LNT suppliers of approximately 700 °C, performance loss of LNTs can be observed over their lifetime. To grow the knowledge of aging effects and deactivation mechanisms during desulphation events, the impact of excessive DeSO x throughout cyclic lean–rich pulsing at relevant temperatures has been investigated on an engine test bench and on a synthetic gas test bench. To reveal the impact on the emission performance, chassis dynamometer tests were performed.
2 Experimental methods
2.1 Catalyst samples
Investigations were done on a conventional LNT technology. The LNT washcoat was built up with PGM components platin (Pt), palladium (Pd) and rhodium (Rh), NO x storage compounds barium oxide (BaO) and ceria oxide (CeO) and remainder aluminum oxide (Al2O3). The washcoat was supported on a cordierite monolith with a cell density of 400 cells per square inch.
2.2 Engine test bench aging and chassis dynamometer tests
To investigate the impact of excessive desulphation on a Lean NO x Trap in automotive usage, a conventional LNT–DPF combination system as used by BMW has been aged on a 3.0-l common-rail diesel engine. The engine was operated cyclically for 40 s in lean heating mode to set a temperature of approximately 620 °C following 14 s in rich mode. During rich mode, the LNT temperature increased because of exothermic reactions. The maximum LNT temperature did not exceed 710 °C to avoid a higher thermal degradation than during endurance runs (Fig. 4). This procedure was repeated for 1500 cycles to reach a cumulated rich time of 21,000 s. This amount is a bit higher than for typical endurance runs but was better suited to detect and understand the aging effects.
The synthetically DeSO x -aged LNT was afterwards installed in a test vehicle with a 3.0-l diesel engine. The LNT performance was determined on an exhaust chassis dynamometer in the New European Driving Cycle (NEDC).
2.3 Synthetic-gas test bench experiments
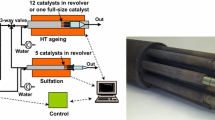
Furthermore, to determine the aging effects and mechanisms, fresh LNT cores with a diameter of 25.4 and 76.2 mm length from the same production process as the engine test bench DeSO x -aged full part sample were investigated on a synthetic-gas test bench (SG test bench). Figure 1 shows a schematic overview of the experimental setup on the synthetic gas test bench.
Mass flow controllers were used to mix the synthetic gases from gas cylinders. The carrier gas was nitrogen to adjust the defined volume flow. Water and decane were applied in fluid phase, vaporized by evaporator units and added to an additional nitrogen flow that was again added to the before mixed gaseous species. The mass flow controllers and evaporator bank were installed in a redundant circuit. Therefore, two different gas mixtures were available at the same time. Via a switching valve, and it was possible to switch very fast between the two gas mixes to simulate lean–rich cycling as it occurs, when a DeNO x or DeSO x event is triggered. The reactor consisted of a quartz-tube flow reactor in a furnace to get an isothermal temperature distribution across the entire LNT sample. To set up the gas temperature a N-type thermocouple was installed 10 mm upstream of the LNT inlet surface (green line in Fig. 1). LNT sample temperature was measured inside a channel with thermocouples placed 3 mm from the inlet as well as from the outlet surface. The thermocouple positions are shown in Fig. 2 in detail. Gas for analysis was taken downstream of the catalyst sample. Hydrocarbon species (C x H y ) and water content were analyzed with a FTIR spectrometer, total hydrocarbons with a flame ionization detector (FID), carbon monoxide (CO) and carbon dioxide (CO2) with a nondispersive infrared detector (NDIR), because of its better accuracy, and oxygen with a paramagnetic detector (PMA). Nitrogen oxides (NO x ) were analyzed with a chemiluminescence detector (CLD). For hydrogen determination through electron impact ionization a mass spectrometer was used.
Synthetic-gas test bench aging was analogous to the engine test bench aging. The availability of redundant MFC banks allowed a realistic reproduction of the lean–rich cycling. A gas temperature of 620 °C, for the lean gas as well as the rich gas mix, was set up to get a lean gas and LNT temperature comparable to the engine test bench aging. Lean and rich gas concentrations for the synthetic gas aging were similar to the engine and are listed in Table 1 below.
To determine the impact of desulphation DeSO x pulse length, the number of DeSO x pulses and cumulated DeSO x rich time have been varied. The DeSO x aged samples were compared to other aging procedures, to classify the aging effects resulting out of excessive desulphation. Two samples were hydrothermally aged for 20, respectively, 110 h at 750 °C on the synthetic gas bench. Another LNT was poisoned with phosphorus and further hydrothermally aged for 20 h at 750 °C, similar to the thermally aged sample. Phosphorus was deposited on the LNT via wet impregnation method with an aqueous solution of ammonium dihydrogen phosphate ((NH4)H2PO4), like that used in [19]. To get a reference to the performance after realistic in-car-usage, a LNT from a BMW endurance run with representative full useful life performance was uncanned and LNT samples were taken from the full monolith. This sample will be denoted with “ER”.
The test array with various DeSO x aging levels regarding number of pulses and pulse length as well as competitive other aging procedures is shown in Table 2 below. To show the influence of the DeSO x start temperature (the LNT temperature at the beginning of a rich purge), aging with 14 s pulse length was performed at two further temperatures. The possibility of a lower DeSO x temperature and the impact of DeNO x events were verified. Furthermore, rich gas species have been varied in three further agings with 14 s rich pulse length.
DeSO x aged samples were tested in a defined sequence. In a first step, the samples were aged to reach a defined cumulative rich time. Following this, the sample was tested in a lean gas light-off test, two water gas shift light-off tests with different CO concentrations, DeNO x efficiency tests (not discussed in this paper) and a determination of the oxygen storage capacity of the catalyst sample. All tests were conducted with a gas hourly space velocity (GHSV) of 40,000 h−1. Before each of the tests the catalyst sample was pretreated with seven lean–rich cycles (120/24 s) at a LNT temperature of 400 °C to get a defined slightly deactivated LNT state. When all tests for one aging iteration were completed, the sample was aged again to reach the next aging state. Figure 3 shows the test sequence. Test conditions for all tests are listed in Table 3.
The hydrothermally aged samples were tested in the same sequence, with hydrothermal treatment at 750 °C instead of DeSO x aging. The endurance run sample was tested without the aging step.
3 Results
The following section shows the results of engine test bench aging procedure, emission results of tests on the exhaust chassis dynamometer and the results of the synthetic-gas test bench experiments.
3.1 DeSO x aging procedure on engine test bench
Figure 4 illustrates the LNT in brick temperatures during catalyst aging on the engine test bench. The thermocouple positions are different to the SG test bench setup. The first thermocouple was applied after one-third of the total LNT length, and the second one after two-thirds of the brick length. Throughout aging, the LNT-temperature slowly increased to a maximum bed temperature of 715 °C. The reached maximum temperature is within the recommended operating temperature for this technology. In the literature significant deactivation effects by high-temperature operation were observed at treatments of 5 h at temperatures of 800 °C and above [18]. Thus, a LNT deactivation caused by high temperatures was avoided.
3.2 Emission results of exhaust chassis dynamometer tests
The aged catalyst performance was evaluated in a test vehicle in a NEDC. The test was repeated to avoid inaccuracies of the gas analytics. Figure 5 shows scaled cumulated emissions during a NEDC with the DeSO x aged LNT. As one can see, the weighted NO x results in the NEDC are uncritical but CO values are significantly higher than the set targets for long-term emission stability. A detailed consideration of cumulated CO trends in the NEDCs shows that two effects caused the carbon monoxide emissions. First, the CO light off is postponed, so CO is poorly oxidized in the beginning of the test. The second and much more relevant reason for the high CO values was found during the LNT regeneration phase.
Figure 6 shows the DeNO x event in detail. The upper part of the figure shows the lambda trends during the NO x regeneration event. The red signal is the lambda value upstream of the LNT, the blue one the downstream lambda signal. A complete NO x regeneration leads to a sharp decrease in downstream lambda as one can see in the third rich pulse in Fig. 6 (arrow 2). This phenomenon is used to detect a complete DeNO x event. The behavior of the downstream lambda signal is very untypical (arrow 1). After at least 10 s, a sharp decrease in downstream lambda caused by slipping rich gas, like CO and mainly hydrogen, would have been expected. As the downstream lambda signal strongly correlates with the hydrogen content in the exhaust gas during a DeNO x event, increasing hydrogen concentrations would have led to a sharp decrease in the lambda value of the oxygen sensor downstream of the catalyst. Obviously, the LNT was not able to form hydrogen out of the rich gas CO via water gas shift mechanism (explained in detail in Sect. 3.5), leading, as a result, to the high CO slip during the DeNO x event. CO started to slip very early and nearly reached the feedgas emissions at the end of the second DeNO x purge as marked with arrow 3.
Scaled CO concentrations of feedgas (green full line) and tailpipe (green dashed line) emissions during the NOx regeneration event of NEDC (Fig. 5) and LNT upstream (red) and downstream (blue) lambda of the DeSOx aged LNT after 1500 pulses of 14 s
3.3 Transfer of the aging procedure to the synthetic-gas test bench
To study the deactivation mechanisms the engine test bench procedure was transferred to the synthetic-gas test bench. Figure 7 shows gas and LNT temperatures during one of the DeSO x aging procedures on the gas test bench. The green line corresponds to the gas temperature upstream of the LNT, the red one to the LNT inlet and the blue one to the LNT outlet temperatures (see Fig. 2) during the aging iterations with a DeSO x pulse length of 7, 14 and 21 s. For the tests with 7 and 21 s pulses, the gas temperature was set to the same level as during the aging tests with 14 s pulse length, because the DeSO x start temperature does not depend on the pulse duration when a DeSO x event is triggered in the car. Exothermic reactions led to different LNT inlet and outlet temperatures at the end of the rich gas purges. Figure 7 shows a comparison of maximum temperature peaks depending on DeSO x pulse duration. A maximum LNT temperature of 675 °C was not exceeded. A decreasing pulse length led to a lower LNT inlet and outlet temperature. The peak temperature difference between 7 and 21 s DeSO x pulses was 23 °C.
3.4 Lean gas light off
In Sect. 3.2, a postponed lean gas light off and a strongly inhibited water gas shift reaction during the DeNO x event are shown for the engine test bench aged sample. To evaluate the influence of DeSO x pulses on the oxidation behavior, light-off experiments were conducted with a realistic lean gas composition—for details, see Table 3—at a gas hourly space velocity of 40,000 h−1. After lean/rich pretreatment, the LNT was cooled down to 120 °C under pure N2, then the lean gas was added and the LNT was heated up to 550 °C with 5 °C per minute. Figure 8 shows the light-off temperature for different stages of DeSO x aging compared to other aging treatments. The shown light-off temperatures are LNT inlet temperatures, where 50% of the dosed gas species (CO or C3H6) were oxidized.
Within the group of DeSO x aged samples, there was no significant difference in CO light-off performance noticeable. Neither the pulse number, nor the pulse length, nor the cumulated rich time had an effect on the light-off temperature. Even after 7000 s DeSO x , all samples showed a comparable performance. Only the sample with 3500 s rich time had a better CO oxidation performance, but worse than the 20-h hydrothermally treated LNTs. The lowest CO light-off temperature was observed with the 20-h hydrothermally aged sample. Although the duration of high-temperature exposure was comparable to the DeSO x aged samples with 21,000 s cumulated rich time, the 20-h at 750 °C HT aged LNT had a significant earlier light off. Extended hydrothermal aging with 110 h at 750 °C led to a slightly better CO oxidation than after more than 7000 s DeSO x -rich treatment. The effect of phosphorus poisoning and HT aging was similar to the only HT aged LNT. The small amount of 0.2 g/l phosphorus had only a minor impact on the light-off result. Compared to the realistic aged LNT sample after the endurance run, the DeSO x aged LNTs showed a similar to slightly better light-off performance. Despite a much lower DeSO x exposure of approximately 7500 s cumulated rich time, the endurance run sample showed a marginal higher CO light-off temperatures. This behavior can be explained by longer thermal treatment because of DPF soot burning operation, where the LNT reaches approx. 700 °C, and further residual sulfur and phosphorus poisoning, which had been identified by ICP analyses.
The results of propene light-off tests were similar to the CO oxidation results. There is no significant effect of DeSO x pulse duration, pulse number and cumulated rich time noticeable. The endurance run LNT had the highest light-off temperatures, whereas the 20-h hydrothermally aged sample had the best oxidation characteristics. Only the 110-h hydrothermally aged sample had a performance comparable to the ER sample.
3.5 Hydrogen formation
The main reason for the high carbon monoxide emissions in the exhaust chassis dynamometer tests in Sect. 3.2 was attributed to a poor rich gas conversion during the NO x regeneration event. As described below, an inhibition of the hydrogen (H2) formation mechanism was supposed, as no CO was converted and no decrease in downstream lambda was observed. On the one hand, H2 is a very effective reducing agent, especially at very low DeNO x temperatures [20,21,22,23]; on the other hand, it plays an important role in the detection of a complete NO x regeneration because of the hydrogen sensitivity of the oxygen [30] sensor downstream of the LNT. Catalytic hydrogen formation over a LNT with a comparable formulation was frequently mentioned in the literature [22, 24,25,26,27,28,29] and takes place according to the water gas shift (WGS) mechanism (1):
The WGS-mechanism is catalyzed by platin and ceria oxide. With palladium and rhodium as PGM components, good WGS activity was observed too [27]. As Pt is the main PGM component on the investigated LNT washcoat, primarily platin will influence the WGS activity and in the following equations, only Pt is applied. There are two theories discussed in literature how H2 formation takes place on a LNT. In [27,28,29] a two-stage redox mechanism is supposed. First, CO adsorbs on platin out of the gas phase (2) and is further oxidized to CO2 by oxygen from ceria oxide (CeO2) (3). Over reduced ceria (Ce+3) an O atom is separated from a water molecule and hydrogen gets formed (4).
A second approach originates from a dissociation of H2O to OH and H and subsequent formation of HCOOH surface formates with CO that was adsorbed on Pt. With H2O present in the gas phase, surface formates decompose and H2 and CO2 are formed [28, 29]. Jain et al. [29] found that H2 is formed via both described mechanisms. They came to the conclusion that smaller ceria particles led to lower activation energies regarding H2 formation. They also proposed that the Pt–CO interaction as well as imperfections in the ceria oxide crystallite (in this case O vacancies) improves water gas shift activity [29]. A determination whether path 1 or path 2 accounts for the investigated LNT formulation cannot be done. It is very likely that both the state of Pt and also the ceria crystallite are responsible for the WGS activity.
To evaluate the effects of DeSO x , hydrothermal aging and chemical poisoning, a WGS light-off test similar to the lean gas light off was carried out. The LNT was pretreated at 400 °C with seven lean–rich cycles, cooled down to 120 °C in nitrogen and then heated up to 500 °C at a rate of 5 °C/min. To investigate only the WGS activity solely CO and H2O in a balance of N2 were supplied at a GHSV of 40,000 h−1. The test was done with two different CO to H2O ratios of 0.51–5.6 and 2.15–11.8%, where the second one is more realistic to a DeNO x purge in a car.
As shown in Eq. (1) the stoichiometric coefficients of CO2 and H2 are the same. It can be assumed that the formation of one CO2 molecule coincides with one H2 molecule. Because of the lower drift of the NDIR compared to the HSense during long tests, the CO2 signals during the WGS light off were compared. From Eq. (1), hydrogen formation during the test is concluded. Figure 9 shows one of the WGS light-off tests in detail. The upper part of the figure shows the gas and LNT temperatures. The curves below show CO, CO2 and H2. It can be clearly seen that with increasing temperature, more CO was converted and higher CO2 concentrations were detected. The increase in CO2 comes with the same stoichiometric increase in hydrogen as proposed.
Figure 10 shows the CO2 formation over the LNT inlet temperature of all DeSO x aged samples. The samples are separated into groups of their cumulated DeSO x rich time. Red lines show the tests with 21,000 s, blue lines 10,500 s and green line 7000 s or less DeSO x rich time. Figure 10 contains the test results with the high CO to H2O ratio. Results of the second CO/H2O ratio are not shown but had similar results with lower light-off temperatures for all samples.
One can see that WGS activity strongly depends on the cumulated rich time of the DeSO x aging treatment. The effect of pulse length and total number of DeSO x rich pulses is negligible compared to the cumulated rich time as will be seen in detail later. To classify the impact of desulphation stress, the DeSO x aged LNTs were compared to hydrothermal treatment, a combination of phosphorus poisoning and hydrothermal treatment and the endurance run aged LNT in Fig. 11. An evaluation of the ECU data of the endurance run resulted in a cumulated DeSO x rich time of 7500 s. The result of the endurance run regarding hydrogen formation is in the range of the results of 7000 s DeSO x aging. The 20-h hydrothermally aged LNT showed a better performance than all other samples. After excessive hydrothermal aging of 110 h at 750 °C, the results were comparable to those after 7000 s DeSO x or the endurance run. 110 h at temperatures of 750 °C and above are not realistic for a LNT in real driving operation. Therefore, the effect of thermal aging is minor for the inhibited CO conversion like that shown in the DeNO x event in the NEDCs in Sect. 3.2. In addition, phosphorus poisoning with realistic phosphorus amounts and additional 20 h hydrothermal aging did not lead to same aging in WGS activity as after the 10,500 s and 21,000 s of DeSO x -rich treatment.
To show the aging effect in WGS activity during realistic DeNO x purges like in the New European Driving Cycle, the DeSO x aged samples with 7 s pulse length were compared to the 20-h@750 °C hydrothermally aged sample in the relevant temperature window (Fig. 12). At a catalyst temperature of 280 °C (vertical black line in Fig. 12) the WGS activity between the four aging iterations strongly differs. The hydrothermal sample converted 27% of the dosed CO to CO2, what means that 27% of the engine out carbon monoxide is converted to hydrogen with respect to Eq. (1). So, approximately 5800 ppm H2 was formed, when the feed gas contained 2.15% CO. After 21,000 s cumulated DeSO x rich time only 14% of the feed gas CO was converted, what led to 3000 ppm H2. The difference of 2800 ppm of hydrogen means that there is less of the most effective reductant available for NO x reduction. In addition, if a hydrogen sensitivity of 0.01 in lambda for 1500 ppm H2 is assumed, the deactivation in WGS activity causes a slower lambda decrease and further avoids the detection of a complete DeNO x event. Therefore, DeNO x event duration is longer than necessary and the engine out CO slips unconverted and leads to high CO tailpipe emissions.
A detailed comparison of the WGS light-off temperatures shows the addressed correlation between cumulated rich time and WGS activity. It can be clearly seen that increasing rich time causes a drastic shift of hydrogen formation towards higher catalyst temperatures. Compared to the synthetic aging treatments, none of those harmed the LNT in the scale of lean–rich aging at 620 °C. Only the LNT sample from the endurance run showed a comparable result. Different CO to H2O ratios led to different absolute results but the relative trend stayed unchanged (see Fig. 13).
3.6 Impact of DeSO x aging on the oxygen storage capacity
Ceria oxide and platinum play an important role for the water gas shift mechanism as mentioned before [27]. A determination of the oxygen storage capacity (OSC) of the LNTs was used as indication whether degradation in ceria oxides occurred. Oxygen storage capacities were determined in a test, where the LNT sample was kept under isothermal conditions at 300 °C. First, the sample was purged with 4% O2 in a balance of N2 for 120 s and after 60 s in pure N2 purged with 5000 ppm CO in N2 for 15 s. During the phase at which the LNT was purged with oxygen the ceria oxide could oxidize to CeO2 (Ce4+). The 15-s CO purge led to an oxidation of CO to CO2 by stored oxygen and a reduction of cerium to Ce2O3 (Ce3+). CO was used as reductant as the CO2 signal quality was better than the H2O signals from FTIR, when using H2 as reductant. The higher the OSC of the catalyst the more CO2 was formed. The forced oxidation and reduction of the cerium was examined in 16 cycles. For a better reproducibility, the last three cycles were evaluated. Figure 14 shows one full cycle of the OSC test procedure (left) and the CO purge in detail on the right side. The influence of DeSO x aging with 21 s rich purges in contrast to the 20-h hydrothermal treatment is apparent in this graph.
With increasing cumulated DeSO x rich time the formed CO2 decreased (Fig. 14 right), what indicated lower oxygen storage capacities. While the LNT aged for 20 h at 750 °C hydrothermally was able to oxidize nearly 50% of the CO, the DeSO x aged samples had much higher CO slips and, respectively, less CO2 was formed. The lower oxygen storage capacities with increasing DeSO x rich time correlates very well with the results from the WGS light-off tests. A detailed view on the maximum CO2 peaks in the OSC tests showed the same trend as for the WGS light-off charts. The LNT that was aged in the endurance run showed a similar result to that of the WGS light-off tests. Hydrothermally aged samples had much better oxygen storage capacities than DeSO x aged catalysts. The phosphorus poisoned sample did not cause a degradation of the OSC. A comparison of the CO2 peak concentration during the CO purge was chosen to be more expressive than the effective amount of CO2 in mol, as slightly different sample volumes and valve timings could have caused misinterpretation of the data (see Fig. 15).
3.7 Impact of rich gas and aging temperature on WGS activity
To reduce the shown aging effect, three “variables” in the DeSO x strategy could be adapted. One option is to reduce the cumulated rich time. This will be effective as the deactivation strongly correlates with the cumulated rich time as described in the sections before. Another one would be to lower the DeSO x start temperature or to change the rich combustion settings, if a specific gas species was reliable for the deactivation effect. Because of this, further tests with different gas mixes as well as lower DeSO x temperatures have been examined.
3.7.1 Rich gas variation
In a first step, hydrogen and propene were removed from the rich gas mix. Due to the concept of the synthetic-gas test bench, the gas was heated up to approx. 700 °C in the gas heater upstream of the reactor section (see Fig. 1). Even with no hydrogen in the feed, but CO, CO2 and H2O an equilibrium reaction in the gas phase led to hydrogen and carbon monoxide equilibrium in the gas feed. So in a first step a gas mix containing CO and lower amounts of hydrogen was used in the lean rich aging. After 830 cycles, the water steam was removed from the feed to prevent hydrogen formation in the gas heater. Therefore, CO was the only rich gas component in the remaining 670 rich cycles. As no water steam was available, hydrogen formation over the LNT was inhibited too for the remaining 670 rich purges. Another test was done with propene as only rich gas component in the rich gas mix. Because of the presence of water in the feed hydrogen was formed over the LNT over the aging procedure. Figure 16 shows the results of the rich gas variations.
The WGS light-off tests for the different aging procedures showed two effects. On the one hand, a different mixture of CO and H2 led to the same degradation as it was seen with the original rich gas (gray and orange bar after 10,500 s rich time). In the absence of hydrogen in the second aging iteration from 10,500 to 21,000 s DeSO x rich time, the shift of WGS light off was significantly lower compared to the standard rich gas (gray bar in Fig. 16 with 21,000 s rich time from cycle 830 to 1500). Tests with H2 as major rich gas component (about 2000 ppm CO in the rich gas) resulted in elevated WGS light-off temperatures compared to the original gas mix. The aging treatment with C3H6 as single rich gas component led to hydrogen formation over the LNT, without H2 in the feedgas. This aging treatment had the strongest impact on the WGS light-off temperature, respectively, to the rich gas conversion of the LNT. Summarized, hydrogen seems to have a strong impact on the LNT degradation. H2 in the feed as well as hydrogen formation over the LNT both harmed the WGS activity of the LNT, whereas catalytic H2 formation had the strongest effect.
Since in real exhaust gas CO and unburned hydrocarbons always come along with CO2 and H2O (the main products of the combustion of hydro carbons), hydrogen formation can not be prevented. A change in the rich combustion will not solve the problem of catalyst deactivation caused by the DeSO x . In addition, hydrogen cannot be removed from real exhaust gas during rich mode.
3.7.2 Reduction of DeSO x aging temperature
To further investigate whether DeNO x events also cause the same deactivation as shown with the DeSO x pulses or a reduction of the DeSO x temperature avoids the deactivation effect, two more DeSO x agings at lower catalyst temperatures have been carried out. One test was carried out with a LNT temperature of 350 °C and another one with 500 °C. 500 °C was chosen as very low DeSO x temperature without considering whether a DeSO x event will be efficient at these low temperatures. Figures 17 and 18 show detailed WGS light-off results with lowered LNT temperatures.
Figures 17 and 18 show that lean–rich cycling at typical DeNO x temperatures of 350 °C did not lead to a shift in WGS light off. Although hydrogen formation over the LNT took place at 350 °C during the lean–rich cycling, no degradation effect was noticeable. DeNO x operation does not harm the LNT. A reduction of the DeSO x temperature to 500 °C results in a better WGS activity compared to the LNT aged with lean–rich cycles at 620 °C. Compared to the other samples the degradation effect is still significant. 10,500 s cumulated rich time with a DeSO x start temperature of 500 °C led to the same WGS light off that was shown for the endurance run LNT. A reduction of the DeSO x temperature would not have the desired effect of maintaining a sufficient rich gas conversion.
4 Summary and discussion
Accelerated DeSO x treatment of a LNT on an engine test bench caused high CO emissions in a NEDC on a chassis dynamometer. The reasons were found in a late CO lean light off and mainly in a bad CO conversion during the NO x regeneration of the LNT. The slope in the lambda signal downstream of the LNT suggested very low hydrogen concentrations. As CO conversion in the rich purge and hydrogen formation occur concurrently relative to the water gas shift mechanism, a deactivation of this specific LNT function was assumed. The deactivation effect of lean–rich cycling at DeSO x conditions was investigated on a synthetic-gas-test-bench in lean gas light off, WGS light off and oxygen storage capacity tests. The aging procedure of the engine test bench was transferred to the synthetic-gas test bench. To get a reference to the synthetic DeSO x treatment, the results were compared to a LNT aged in an endurance run under real driving conditions and to other synthetically aged samples, as used in most of the literature studies. The sample aged under realistic conditions in a vehicle endurance run was exposed to approximately 7500 s of DeSO x rich time. The synthetic aged samples were treated hydrothermally for 20 and 110 h at 750 °C. Another sample was chemically poisoned with 0.2 g/l phosphorus, which represents the same quantity as found on the endurance run sample, and additionally hydrothermally aged for 20 h at 750 °C. Although temperatures in DeSO x aging procedures on the synthetic gas bench were lower than during the DeSO x on the engine test bench, a significant impact of the lean–rich treatment was found. While the impact on the propene light off was insignificant, a cumulated rich time of 7000 s led to a shift to higher CO light-off temperatures. Hydrothermal pretreatment with a comparable exposition duration at high temperatures could not reproduce the degradation effect as it was shown with lean–rich cycling. Only at very high expositions like 110 h at 750 °C the deactivation of CO oxidation in a lean gas was comparable. The endurance run sample had the worst CO conversion in lean gas, what was explained by the combination of poisoning, thermal degradation and DeSO x . While the impact on CO light off happened in the first part of the DeSO x aging, the lean–rich cycling at high temperatures led to a drastic deterioration of CO rich conversion to CO2 and H2. Similar to the lean gas light off, there was no clear correlation between pulse number and pulse length. Other than that, the cumulated DeSO x -rich time was a relevant factor regarding hydrogen formation. It is suggested that after more than 21,000 s of cumulated rich time a stronger deactivation would take place. Compared to the hydrothermally aged LNTs, the phosphorus and HT aged sample, none of these samples showed a similar behavior. The endurance run aged LNT fits very well regarding the DeSO x treatment that has occurred during the endurance run. To prove that lean–rich cycling harms ceria oxide, a test to determine the oxygen storage capacity was done. The results out of OSC tests led to the same trend as the WGS light-off tests. Increasing DeSO x rich time led to a lower OSC. Samples without lean–rich aging did not show a significant OSC degradation. To show the connection between the OSC of the catalyst and its WGS light-off temperature both values were correlated in Fig. 19.
The correlation between the oxygen storage capacity and the WGS light off can be clearly seen especially for the DeSO x aged samples. Based on these facts the assumption that the DeSO x treatment mainly deactivates the ability to convert CO under rich conditions—what is caused by the shift in WGS—was supported.
As DeSO x is unavoidable when using LNTs, an investigation of the effect of different rich gas mixes was done at 620 °C gas temperature. A test with CO and lower H2 concentrations led to a similar deactivation effect than with the initial rich gas. By switching off the water dosing unit, hydrogen could be removed from the feed gas. The aging with carbon monoxide as single rich gas component did not lead to a significant further degradation after 670 pulses with 14 s pulse length. As there was no water in the feed, no hydrogen could consequently be formed over the LNT too. A further DeSO x -aging with propene as only rich gas component and no hydrogen in the feed yielded a significant inhibition of the WGS mechanism. Propene, CO2 and H2O in the feed gas led to H2 formation over the LNT. The results from rich gas variations suggested that mainly hydrogen damages the ceria oxide of the lean NO x trap. Hydrogen formation over the LNT led to an increased performance loss compared to the aging with H2 in the gas feed. As H2, CO2 and H2O cannot be removed from the engine exhaust gas, the shown deactivation cannot be eliminated by a change in the rich combustion for DeSO x events.
To investigate the impact of the gas temperature on the LNT deactivation, i.e. whether a reduction in DeSO x temperature could reduce the aging effect, the lean–rich cycling with 14 s pulses was also done at 350 and 500 °C. While at 350 °C no degradation was observed, 500 °C led to slightly better results than after DeSO x aging at 620 °C. Although 500 °C is much too low for an efficient desulphation, the result showed that also a reduction of the DeSO x start temperature could not solve the problem as the catalyst deactivation was much more pronounced than after hydrothermal aging.
Based on these results the absolute necessity of a very efficient DeSO x strategy was shown. To lower the degradation effect of the LNT, the cumulated DeSO x -rich time per desulphation event was shortened. An effective strategy regarding pulse number and length is under investigation. Further, a Lean NO x Trap technology that is much more resistant to lean–rich cycling at DeSO x temperatures was developed by the coating supplier and is currently being tested in endurance runs.
Abbreviations
- Al2O3 :
-
Aluminum oxide
- BaO:
-
Barium oxide
- C3H6 :
-
Propene
- CxHy :
-
Hydrocarbon consisting of x carbon- and y hydrogen-atoms
- CeO:
-
Ceria oxide
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- DeNO x :
-
Regeneration mode to desorb and reduce stored nitrates from the LNT
- DeSO x :
-
Desulphation operation mode to remove sulphur from lean NO x traps
- ECU:
-
Engine control unit
- ER:
-
Endurance run
- EFTA:
-
European Free Trade Association
- EU6:
-
Stage 6 of the European exhaust emissions regulation
- EU15:
-
Member states of the European Union since the eastern enlargement 2004
- FTIR:
-
Fourier Transform Infrared spectrometer
- GHSV:
-
Gas hourly space velocity
- H2 :
-
Hydrogen
- H2O:
-
Water/water steam
- HC:
-
Hydrocarbons
- HCOOH:
-
Formic acid
- HT:
-
Hydrothermal
- LNT:
-
Lean NO x Trap
- MFC:
-
Mass flow controller
- N2 :
-
Molecular nitrogen
- NDIR:
-
Non dispersive infrared detector
- NEDC:
-
New European Driving Cycle
- (NH4)H2PO4 :
-
Ammonium dihydrogen phosphate
- NO:
-
Nitrogen monoxide
- NO2 :
-
Nitrogen dioxide
- NO x :
-
Collection of all nitrogen oxides (NO, NO2,…)
- PGM:
-
Platin-group-metal
- PMA:
-
Paramagnetic detector
- OEM:
-
Original equipment manufacturer
- O2 :
-
Oxygen
- OSC:
-
Oxygen storage capacity
- Pd:
-
Palladium
- Pt:
-
Platinum
- Redox:
-
Reduction oxidation reaction
- RDE:
-
Real driving emissions
- Rh:
-
Rhodium
- SCR:
-
Selective catalytic reduction
- THC:
-
Hydrocarbons
- T:
-
Temperature
- T50:
-
Temperature at 50% conversion in light-off tests
- WGS:
-
lean–rich-aging
- (g):
-
Gaseous species
- (s):
-
Surface species
References
European Automobile Manufactorers Association: Share of Diesel in New Passenger Cars, 2015. http://www.acea.be/statistics/article/Share-of-diesel-in-new-passenger-cars. abgerufen am: 13.01.2016
Mollenhauer, K. U., Tschöke, H.: (Hrsg.): Handbuch Dieselmotoren. VDI-Buch. Springer, Berlin, Heidelberg (2007)
Pischinger, S.: Vieweg Handbuch Kraftfahrzeugtechnik. ATZ/MTZ-Fachbuch. Springer Fachmedien Wiesbaden, Wiesbaden (2016)
Hausberger, S.: Skriptum Schadstoffbildung und Emissionsminimierung bei KFZ Teil II. Technische Universität Graz, Graz (2017)
Brüne, H.J., Honeder, J., Raschl, P., Schinnerl, M.U., Tangemann, R.: Diesel-Emissionstechniken von BMW für künftige weltweite Abgasnormen. MTZ Motortechnische Zeitschrift 70(3), S210–S216 (2009)
Steinparzer, F., Nefischer, P., Stütz, W., Hiemesch, D. U., Kaufmann, M.: Die neue BMW Sechszylinder Spitzenmotorisierung mit innovativem Aufladekonzept. In: 37. Internationales Wiener Motorensymposium 2016. Wien (2016)
Steinparzer, F., Nefischer, P., Hiemesch, D., Kaufmann, M. U., Steinmayr, T.: The New Six-Cylinder Diesel Engines from the BMW In-Line Engine Module. In: 24. Aachener Kolloquium Fahrzeug- und Motorentechnik 2015. Aachen (2015)
Epling, W.S., Campbell, L.E., Yezerets, A., Currier, N.W.U., Parks, J.E.: Overview of the fundamental reactions and degradation mechanisms of NO x storage/reduction catalysts. Catal. Rev. 46(2), S163–S245 (2004)
Takahashi, N., Shinjoh, H., Iijima, T., Suzuki, T., Yamazaki, K., Yokota, K., Suzuki, H., Miyoshi, N., Matsumoto, S.I., Tanizawa, T., Tanaka, T., Tateishi, S.S.U., Kasahara, K.: The new concept 3-way catalyst for automotive lean-burn engine. NO x storage and reduction catalyst. Catal. Today 27(1–2), S63–S69 (1996)
Matsumoto, S. U., Shinjoh, H.: Dynamic behavior and characterization of automobile catalysts. In: Marin, G. B. (ed.): Advances in chemical engineering. advances in chemical engineering, vol. 33, pp. S1–S279. s.l.: Elsevier textbooks (2008)
Engström, P., Amberntsson, A., Skoglundh, M., Fridell, E.U., Smedler, G.: Sulphur dioxide interaction with NO x storage catalysts. Appl. Catal. B Environ. 22(4), L241–L248 (1999)
Matsumoto, S.: Recent advances in automobile exhaust catalysts. Catal. Today 90(3–4), 183–190 (2004)
Choi, J.S., Partridge, W.P.U., Daw, C.S.: Sulfur impact on NO x storage, oxygen storage, and ammonia breakthrough during cyclic lean/rich operation of a commercial lean NO x trap. Appl. Catal. B Environ. 77(1–2), S145–S156 (2007)
Hadl, K., Ratzberger, R., Eichlseder, H., Linares, W., Schüßler, M. U., Bürgler, L.: Grundlegende Betrachtungen zu Ver- und Entschwefelungsmechanismen an NO x -Speicherkatalysatoren (NSK). In: 9. Internationales Forum Abgas- und Partikel-Emissionen. Ludwigsburg, Deutschland 2016, pp S185–S202
Kim, D.H., Yezerets, A., Li, J., Currier, N., Chen, H.-Y., Hess, H., Engelhard, M.H., Muntean, G.G.U., Peden, C.H.: Effect of sulfur loading on the desulfation chemistry of a commercial lean NO x trap catalyst. Catal. Today 197(1), S3–S8 (2012)
Breitbach, H., Schommers, J., Binz, R., Lindemann, B., Lingens, A.U., Reichel, S.: Brennverfahren und Abgasnachbehandlung im Mercedes-Benz-Bluetec-Konzept. MTZ Motortechnische Zeitschrift 68(6), S432–S439 (2007)
Maroteaux, D., Beaulieu, J.U., D’Oria, S.: Entwicklung der NO x -Nachbehandlung für Renault-Dieselmotoren. MTZ Motortechnische Zeitschrift 71(3), S184–S189 (2010)
Rohr, F., Grißtede, I., Göbel, U.U., Müller, W.: Dauerhaltbarkeit von NO x -Nachbehandlungssystemen für Dieselmotoren. MTZ Motortechnische Zeitschrift 69(3), S212–S219 (2008)
Cabello Galisteo, F., López Granados, M., Martín Alonso, D., Mariscal, R.U., Fierro, J.: Loss of NO x storage capacity of Pt–Ba/Al2O3 catalysts due to incorporation of phosphorous. Catal. Commun. 9(3), S327–S332 (2008)
Li, Y., Roth, S., Dettling, J.U., Beutel, T.: Effects of lean/rich timing and nature of reductant on the performance of a NO x trap catalyst. Top. Catal. 16/17, S139–S144 (2001)
DiGiulio, C.D., Pihl, J.A., Choi, J.-S., Parks, J.E., Lance, M.J., Toops, T.J.U., Amiridis, M.D.: NH3 formation over a lean NO x trap (LNT) system. Effects of lean/rich cycle timing and temperature. Appl. Catal. B Environ. 147, S698–S710 (2014)
Abdulhamid, H., Fridell, E.U., Skoglundh, M.: Influence of the type of reducing agent (H2, CO, C3H6 and C3H8) on the reduction of stored NO x in a Pt/BaO/Al2O3 model catalyst. Top. Catal. 30/31, S161–S168 (2004)
AL-Harbi, M.U., Epling, W.S.: The effects of regeneration-phase CO and/or H2 amount on the performance of a NO x storage/reduction catalyst. Appl. Catal. B Environ. 89(3–4), S315–S325 (2009)
Dasari, P., Muncrief, R.U., Harold, M.P.: Cyclic lean reduction of NO by CO in excess H2O on Pt–Rh/Ba/Al2O3. Elucidating mechanistic features and catalyst performance. Top. Catal. 56(18–20), 1922–1936 (2013)
Limousy, L.: A study of the regeneration of fresh and aged SO x adsorbers under reducing conditions. Appl. Catal. B 45(3), 169–179 (2003)
Li, J., Theis, J., Chun, W., Goralski, C., Kudla, R., Ura, J., Watkins, W., Chattha, M. U. Hurley, R.: Sulfur poisoning and desulfation of the lean NO x trap. SAE Technical Paper Series. SAE International 400 Commonwealth Drive, Warrendale, PA, United States (2001)
Bunluesin, T., Gorte, R.J.U., Graham, G.W.: Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh. Implications for oxygen-storage properties. Appl. Catal. B Environ. 15(1–2), S107–S114 (1998)
Jacobs, G.: Water-gas shift. Comparative screening of metal promoters for metal/ceria systems and role of the metal. Appl. Catal. A 258(2), 203–214 (2004)
Jain, R., Poyraz, A.S., Gamliel, D.P., Valla, J., Suib, S.L.U., Maric, R.: Comparative study for low temperature water-gas shift reaction on Pt/ceria catalysts. Role of different ceria supports. Appl. Catal. A Gener. 507, S1–S13 (2015)
Baunach, T., Schänzlin, K.U., Diehl, L.: Sauberes Abgas durch Keramiksensoren. Physik J. 5, S33–S38 (2006)
Acknowledgements
Open access funding provided by Graz University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maurer, M., Fortner, T., Holler, P. et al. Impact of cyclic lean–rich aging under DeSO x condition on the lean-gas light-off and hydrogen formation ability of a lean NO x trap (LNT). Automot. Engine Technol. 2, 63–77 (2017). https://doi.org/10.1007/s41104-017-0019-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41104-017-0019-3