Abstract
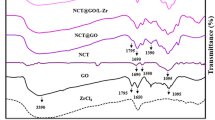
In this study, the synthesis of nanocomposite using graphene oxide and chitosan and its application for the removal of Cr (VI) and Ni (II) ions from wastewater was explored. The characterization of the synthesized graphene oxide-chitosan nanocomposite (GO-CNC) has been investigated with XRD, FT-IR, and SEM. The effect of pH, adsorbent dosage, effluent concentration, and contact time on Cr(VI) and Ni(II) removal was studied. It was found that for Cr (VI) maximum of 90.74% removal was observed at pH 5, 105 min, initial metal ion concentration 10 mg L−1, and adsorbent dosage of 20 g L−1. Ni(II) removal of 58.56% occurred at pH of 8, 90 min, initial metal ion concentration of 10 mg L−1, and adsorbent dosage of 20 g L−1. Adsorption isotherms Langmuir, Freundlich, and Temkin were used. Best fit for Cr(VI) was Freundlich isotherm ions and Langmuir isotherm for Ni(II) with correlation coefficient values greater than 0.9. The Freundlich isotherm value 1/n less than 1 implies the favorability of the adsorption onto the GO-CNC. Our experimental data was described by the pseudo-second order kinetic model for both the metal ions. To forecast the best model, error functions, the sum of squared errors (SSE), the average relative error (ARE), the sum of absolute errors (EABS), and Marquardt’s percent standard deviation (MPSD) were used to predict the single parameters by using non-linear regression analysis to reduce the bias of linear correlation coefficient. The regeneration of GO-CNC nanocomposite showed significant removal efficiencies up to four cycles.









Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Verma R, Dwivedi P (2013) Heavy metal water pollution — a case study. Recent Res Sci Technol 5:98–99
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F et al (2021) Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504. https://doi.org/10.1016/J.ETI.2021.101504
Fiyadh SS, Alardhi SM, Al Omar M et al (2023) A comprehensive review on modelling the adsorption process for heavy metal removal from waste water using artificial neural network technique. Heliyon 9:e15455. https://doi.org/10.1016/J.HELIYON.2023.E15455
Parvin F, Rikta SY, Tareq SM (2019) Application of nanomaterials for the removal of heavy metal from wastewater. In: Nanotechnology in water and wastewater treatment. Elsevier, pp 137–157
Lingamdinne LP, Koduru JR, Karri RR (2019) A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J Environ Manag 231:622–634. https://doi.org/10.1016/J.JENVMAN.2018.10.063
Sabzevari M, Duncan Cree E, Lee Wilson D (2018) Graphene oxide–chitosan composite material for treatment of a model dye effluent. ACS Omega 3:13045–13054
Udayakumar GP, Muthusamy S, Selvaganesh B et al (2021) Biopolymers and composites: properties, characterization and their applications in food, medical and pharmaceutical industries. J Environ Chem Eng 9:105322. https://doi.org/10.1016/J.JECE.2021.105322
Khan Z, AL-Thabaiti SA (2022) Chitosan capped silver nanoparticles: adsorption and photochemical activities. Arab J Chem 15:104154. https://doi.org/10.1016/J.ARABJC.2022.104154
Gonnelli C, Renella G (2013) Chromium and nickel. In: Alloway B (eds) Heavy Metals in Soils. Environmental Pollution, vol 22. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4470-7_11
Sharma V, Yan R, Feng X, Xu J, Pan M, Kong L, Li L (2023) Removal of toxic metals using iron sulfide particles: a brief overview of modifications and mechanisms. Chemosphere 346:140631
Chandra V, Park J, Chun Y, Lee JW, Hwang IC, Kim KS (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS nano 4(7):3979–3986
Liu X, Ma R, Wang X et al (2019) Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environ Pollut 252:62–73. https://doi.org/10.1016/J.ENVPOL.2019.05.050
Paulchamy B, Arthi G, Lignesh BD (2015) A simple approach to stepwise synthesis of graphene oxide nanomaterial. J Nanomed Nanotechnol 6:1000253. https://doi.org/10.4172/2157-7439.1000253
Rasoulzadehzali M, Namazi H (2018) Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int J Biol Macromol 116:54–63. https://doi.org/10.1016/j.ijbiomac.2018.04.140
Mohammadi A, Doctorsafaei AH, Zia KM (2018) Alginate/calix[4]arenes modified graphene oxide nanocomposite beads: preparation, characterization, and dye adsorption studies. Int J Biol Macromol 120:1353–1361. https://doi.org/10.1016/J.IJBIOMAC.2018.09.136
Lace A, Ryan D, Bowkett M, Cleary J (2019) Chromium monitoring in water by colorimetry using optimised 1,5-diphenylcarbazide method. Int J Environ Res Public Health 16:1803. https://doi.org/10.3390/ijerph16101803
Kekes T, Kolliopoulos G, Tzia C (2021) Hexavalent chromium adsorption onto crosslinked chitosan and chitosan/β-cyclodextrin beads: novel materials for water decontamination. J Environ Chem Eng 9:105581. https://doi.org/10.1016/J.JECE.2021.105581
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
Pan B, Xing B (2010) Adsorption kinetics of 17α-ethinyl estradiol and bisphenol A on carbon nanomaterials. I. Several concerns regarding pseudo-first order and pseudo-second order models. J Soils Sediments 10:838–844
Ersan G, Kaya Y, Ersan MS, Apul OG, Karanfil T (2019) Adsorption kinetics and aggregation for three classes of carbonaceous adsorbents in the presence of natural organic matter. Chemosphere 229:515–524
Chithra K, Akshayaraj RT, Pandian K (2018) Polypyrrole-protected magnetic nanoparticles as an excellent sorbent for effective removal of Cr(VI) and Ni(II) from effluent water: kinetic studies and error analysis. Arab J Sci Eng 43:6219–6228. https://doi.org/10.1007/s13369-018-3421-x
Chithra K, Lakshmi S, Jain A (2014) Carica papaya seed as a biosorbent for removal of Cr (VI) and Ni (II) ions from aqueous solution: thermodynamics and kinetic analysis of experimental data. Int J Chem React Eng 12:91–102. https://doi.org/10.1515/ijcre-2013-0096
Allen SJ, Gan Q, Matthews R, Johnson PA (2003) Comparison of optimised isotherm models for basic dye adsorption by kudzu. Bioresour Technol 88:143–152. https://doi.org/10.1016/S0960-8524(02)00281-X
Demirbas E, Kobya M, Konukman AES (2008) Error analysis of equilibrium studies for the almond shell activated carbon adsorption of Cr(VI) from aqueous solutions. J Hazard Mater 154:787–794. https://doi.org/10.1016/j.jhazmat.2007.10.094
Ganesan S, Halliah GP (2019) Synergetic performance of graphene oxide and chitosan on the removal of direct red 7. Orient J Chem 35:1789–1798. https://doi.org/10.13005/ojc/350623
Zuo P-P, Feng H-F, Xu Z-Z et al (2013) Fabrication of biocompatible and mechanically reinforced graphene oxide-chitosan nanocomposite films. Chem Cent J 7:39. https://doi.org/10.1186/1752-153X-7-39
Gong Y, Yu Y, Kang H et al (2019) Synthesis and characterization of graphene oxide/chitosan composite aerogels with high mechanical performance. Polymers (Basel) 11:777. https://doi.org/10.3390/polym11050777
El Rouby WMA, Farghali AA, Sadek MA, Khalil WF (2018) Fast removal of Sr(II) from water by graphene oxide and chitosan modified graphene oxide. J Inorg Organomet Polym Mater 28:2336–2349. https://doi.org/10.1007/s10904-018-0885-9
Kumar ASK, Jiang SJ (2016) Chitosan-functionalized graphene oxide: a novel adsorbent an efficient adsorption of arsenic from aqueous solution. J Environ Chem Eng 4:1698–1713. https://doi.org/10.1016/J.JECE.2016.02.035
Nayl AA, Abd-Elhamid AI, Arafa WAA et al (2022) Chitosan-functionalized-graphene oxide (GO@CS) beads as an effective adsorbent to remove cationic dye from wastewater. Polymers (Basel) 14:4236. https://doi.org/10.3390/polym14194236
Nitsae M, Madjid A, Hakim L, Sabarudin A (2016) Preparation of chitosan beads using tripolyphosphate and ethylene glycol diglycidyl ether as crosslinker for Cr(VI) adsorption. Chem Chem Technol 10:105–113. https://doi.org/10.23939/chcht10.01.105
Piccin JS, Dotto GL, Pinto LAA (2011) Adsorption isotherms and thermochemical data of FD & C Red no 40 binding by chitosan. Braz J Chem Eng 28:295–304. https://doi.org/10.1590/S0104-66322011000200014
Neolaka YAB, Lawa Y, Naat JN et al (2020) The adsorption of Cr(VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): Study of kinetics, isotherms and thermodynamics. J Mater Res Technol 9:6544–6556. https://doi.org/10.1016/j.jmrt.2020.04.040
Deveci H, Kar Y (2013) Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J Ind Eng Chem 19:190–196. https://doi.org/10.1016/j.jiec.2012.08.001
Lei C, Wang C, Chen W et al (2020) Polyaniline@magnetic chitosan nanomaterials for highly efficient simultaneous adsorption and in-situ chemical reduction of hexavalent chromium: removal efficacy and mechanisms. Sci Total Environ 733:139316. https://doi.org/10.1016/j.scitotenv.2020.139316
Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A (2008) Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr Polym 72:261–271. https://doi.org/10.1016/J.CARBPOL.2007.08.010
Jeon S, Jung H, Kim SH, Lee KB (2018) Double-layer structured CO2 adsorbent functionalized with modified polyethyleneimine for high physical and chemical stability. ACS Appl Mater Interfaces 10(25):21213–21223
Author information
Authors and Affiliations
Contributions
NSE, & SMJ: Conducted experimental works, Methodology, Manuscript draft & graph plotting; CK: Supervision, Review and Editing, Approval of final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elumalai, N.S., Jaisankar, S.M. & Kumaran, C. Utilization of Graphene Oxide-Chitosan Nanocomposite for the Removal of Heavy Metals: Kinetics, Isotherm, and Error Analysis. Water Conserv Sci Eng 9, 9 (2024). https://doi.org/10.1007/s41101-024-00241-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41101-024-00241-3