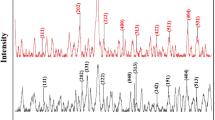
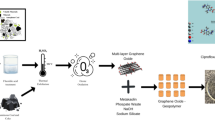
Abstract
The decontamination of excess nutrients by polysaccharide-graphene oxide composites has gained much research attention. However, the usage of synthetic polymers and toxic crosslinking agents affects the environment. This investigation is, therefore, aimed at achieving a simple, effective and nontoxic technique of fabricating nanocomposites by ionotropic gelation of chitosan and tripolyphosphate crosslinking agent. The nanochitosan-graphene oxide composite (NCS@GO) was synthesized and investigated for its potential to remove phosphate (P) and nitrate (N) from aqueous solutions. High and low amounts of zirconium (Zr) were loaded in NCT@GO composite to make it selective for the adsorbate anions. The developed nanocomposites were comparatively explored by N2 isotherms, FTIR, XRD, TGA, DTA, FESEM, EDS with mapping analysis and water regain property. Experimental design was conducted by the five-factorial central composite design-as a branch of response surface methodology (RSM). According to the design of RSM, NCS@GO/H-Zr demonstrated an excellent P and N uptake of 172.41 mgP/g and 138.88 mgN/g, reasonable pH-compatibility from 3 to 11, suitable selectivity for both adsorbates among competitor anions, desired recyclability and desorption efficiency for P and N, and retained 76% and 85% for P and N adsorption ability after ten recycles. The removal capacity of P and N anions were also assessed in bi-component systems. Thermodynamic data were considered, in which it was found that the adsorptive removal of the both anions was endothermic and spontaneous in nature. The adsorption isotherm of P and N on the surface of the NCS@GO/H-Zr was suitable for the Freundlich isotherm model, suggesting the multilayer adsorption. On the basis of kinetic studies, specific rate constants involved in the processes were calculated and the obtained result indicates that the pseudo second order kinetics was found to be a better fit. Real samples analysis indicated that the NCS@GO/H-Zr works well for removal of P and N from contaminated waters.

















Similar content being viewed by others
References
Agarwal S, Rajoria P, Rani A (2018) Adsorption of tannic acid from aqueous solution onto chitosan/NaOH/fly ash composites: Equilibrium, kinetics, thermodynamics and modeling. J Environ Chem Eng 6:1486–1499
Ali I (2014) Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev 43:175–205
Ali I, Gupta V (2006) Advances in water treatment by adsorption technology. Nat Protoc 1:2661
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183
Ali I, Alharbi OM, Tkachev A, Galunin E, Burakov A, Grachev VA (2018) Water treatment by new-generation graphene materials: hope for bright future. nviron. Sci Pollut Res 25:7315–7329
Anbia M, Salehi S (2016) Synthesis of polyaniline/mesoporous carbon nanocomposites and their application for CO2 sorption. J Polym Res 23:124
Antunes E, Jacob MV, Brodie G, Schneider P (2018) Isotherms, kinetics and mechanism analysis of phosphorus recovery from aqueous solution by calcium-rich biochar produced from biosolids via microwave pyrolysis. J Environ Chem Eng 6:395–403
Bagheri M, Younesi H, Hajati S, Borghei SM (2015) Application of chitosan-citric acid nanoparticles for removal of chromium (VI). Int J Biol Macromol 80:431–444
Banu HT, Meenakshi S (2017) One pot synthesis of chitosan grafted quaternized resin for the removal of nitrate and phosphate from aqueous solution. Int J Biol Macromol 104:1517–1527
Banu HT, Karthikeyan P, Meenakshi S (2018) Lanthanum (III) encapsulated chitosan-montmorillonite composite for the adsorptive removal of phosphate ions from aqueous solution. Int J Biol Macromol 112:284–293
Banu HT, Karthikeyan P, Meenakshi S (2019) Zr4+ ions embedded chitosan-soya bean husk activated bio-char composite beads for the recovery of nitrate and phosphate ions from aqueous solution. Int J Biol Macromol 130:573–583
Boeykens SP, Piol MN, Samudio Legal L, Saralegui AB, Vázquez C (2017) Eutrophication decrease: phosphate adsorption processes in presence of nitrates. J Environ Manag 203:888–895
Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164:1012–1018
Chatterjee S, Lee DS, Lee MW, Woo SH (2009) Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate. J Hazard Mater 166:508–513
Cui X, Dai X, Khan KY, Li T, Yang X, He Z (2016) Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour Technol 218:1123–1132
Cui X, Li H, Yao Z, Shen Y, He Z, Yang X, Ng HY, Wang C-H (2019) Removal of nitrate and phosphate by chitosan composited beads derived from crude oil refinery waste: sorption and cost-benefit analysis. J Clean Prod 207:846–856
Dewage NB, Liyanage AS, Pittman CU Jr, Mohan D, Mlsna T (2018) Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour Technol 263:258–265
Dharupaneedi SP, Anjanapura RV, Han JM, Aminabhavi TM (2014) Functionalized graphene sheets embedded in chitosan nanocomposite membranes for ethanol and isopropanol dehydration via pervaporation. Ind Eng Chem Res 53:14474–14484
Fan Y, Li Y, Wu D, Li C, Kong H (2017) Application of zeolite/hydrous zirconia composite as a novel sediment capping material to immobilize phosphorus. Water Res 123:1–11
Federation WE, Association APH (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Hamoudi S, Belkacemi K (2013) Adsorption of nitrate and phosphate ions from aqueous solutions using organically-functionalized silica materials: kinetic modeling. Fuel 110:107–113
Hu Q, Zhang Z (2019) Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: a theoretical analysis. J Mol Liq 277:646–648
Huang X, Liao X, Shi B (2009) Adsorption removal of phosphate in industrial wastewater by using metal-loaded skin split waste. J Hazard Mater 166:1261–1265
Huang W-Y, Li D, Liu Z-Q, Tao Q, Zhu Y, Yang J, Zhang Y-M (2014) Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La (OH) 3-modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem Eng J 236:191–201
Islam M, Patel R (2010) Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency. Desalination 256:120–128
Iyer K, Kunju A (1992) Extension of Harkins—Jura adsorption isotherm to solute adsorption. Colloids Surf 63:235–240
Jiang H, Chen P, Luo S, Tu X, Cao Q, Shu M (2013) Synthesis of novel nanocomposite Fe3O4/ZrO2/chitosan and its application for removal of nitrate and phosphate. Appl Surf Sci 284:942–949
Jovanovic D (1969) Physical adsorption of gases, I, Isotherms for monolayer and multilayer adsorption. Kolloid Z Z Polym 235:1203
Jóźwiak T, Filipkowska U, Szymczyk P, Kuczajowska-Zadrożna M, Mielcarek A (2017) The use of cross-linked chitosan beads for nutrients (nitrate and orthophosphate) removal from a mixture of P-PO4, N-NO2 and N-NO3. Int J Biol Macromol 104:1280–1293
Jóźwiak T, Filipkowska U, Szymczyk P, Mielcarek A (2019) Sorption of nutrients (orthophosphate, nitrate III and V) in an equimolar mixture of P-PO4, N–NO2 and N–NO3 using chitosan. Arab J Chem 12:4104–4117
Karthikeyan P, Banu HAT, Meenakshi S (2019) Synthesis and characterization of metal loaded chitosan-alginate biopolymeric hybrid beads for the efficient removal of phosphate and nitrate ions from aqueous solution. Int J Biol Macromol 130:407–418
Keshvardoostchokami M, Babaei S, Piri F, Zamani A (2017) Nitrate removal from aqueous solutions by ZnO nanoparticles and chitosan-polystyrene–Zn nanocomposite: kinetic, isotherm, batch and fixed-bed studies. Int J Biol Macromol 101:922–930
Kumar IA, Viswanathan N (2017) Fabrication of metal ions cross-linked alginate assisted biocomposite beads for selective phosphate removal. J Environ Chem Eng 5:1438–1446
Kumar IA, Viswanathan N (2019) Micro-encapsulation and hydrothermal tuning of amine decorated magnetic alginate hybrid beads for nitrate and phosphate remediation. J Taiwan Inst Chem Eng 102:283–296
Kumar IA, Jeyaprabha C, Meenakshi S, Viswanathan N (2019) Hydrothermal encapsulation of lanthanum oxide derived Aegle marmelos admixed chitosan bead system for nitrate and phosphate retention. Int J Biol Macromol 130:527–535
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li R, Wang JJ, Zhou B, Awasthi MK, Ali A, Zhang Z, Gaston LA, Lahori AH, Mahar A (2016) Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci Total Environ 559:121–129
Lin J, He S, Wang X, Zhang H, Zhan Y (2019) Removal of phosphate from aqueous solution by a novel Mg(OH)2/ZrO2 composite: adsorption behavior and mechanism. Colloids Surf A 561:301–314
Luo W, Huang Q, Zhang X, Antwi P, Mu Y, Zhang M, Xing J, Chen H, Ren S (2020) Lanthanum/Gemini surfactant-modified montmorillonite for simultaneous removal of phosphate and nitrate from aqueous solution. J Water Process Eng 33:101036
Mallakpour S, Hatami M (2019) Fabrication and characterization of pH-sensitive bio-nanocomposite beads havening folic acid intercalated LDH and chitosan: drug release and mechanism evaluation. Int J Biol Macromol 122:157–167
Mirhosseinian NS, Anbia M, Salehi S (2020) Preparation and characterization of superhydrophobic melamine and melamine-derived carbon sponges modified with reduced graphene oxide–TiO2 nanocomposite as oil absorbent materials. J Mater Sci 55:1536–1552
Negm NA, Hefni HHH, Abd-Elaal AAA, Badr EA, Abou Kana MTH (2020) Advancement on modification of chitosan biopolymer and its potential applications. Int J Biol Macromol 152:681–702
Orlando U, Baes A, Nishijima W, Okada M (2002) Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 48:1041–1046
Pan J, Gao B, Song W, Xu X, Yue Q (2020) Modified biogas residues as an eco-friendly and easily-recoverable biosorbent for nitrate and phosphate removals from surface water. J Hazard Mater 382:121073
Pooja D, Kumar P, Singh P, Patil S (2020) Sensors in water pollutants monitoring: role of material. Springer, New York
Qiang GYXJB, Yewei D (2013) Adsorption of sulfate onto Zr (IV) loaded cross-linked chitosan. Chin J Environ Eng 5:2019–2024
Rahmi L, Nurfatimah R (2018) Preparation of polyethylene glycol diglycidyl ether (PEDGE) crosslinked chitosan/activated carbon composite film for Cd2+ removal. Carbohydr Polym 199:499–505
Rajeswari A, Amalraj A, Pius A (2015) Removal of phosphate using chitosan-polymer composites. J. Environ Chem Eng 3:2331–2341
Rajeswari A, Amalraj A, Pius A (2016) Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J Water Process Eng 9:123–134
Rathod M, Mody K, Basha S (2014) Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii. J Clean Prod 84:484–493
Rodrigues LA, da Silva MLCP (2010) Thermodynamic and kinetic investigations of phosphate adsorption onto hydrous niobium oxide prepared by homogeneous solution method. Desalination 263:29–35
Salehi S, Anbia M (2019) Performance comparison of chitosan–clinoptilolite nanocomposites as adsorbents for vanadium in aqueous media. Cellulose 26:5321–5345
Salehi S, Hosseinifard M (2020) Highly efficient removal of phosphate by lanthanum modified nanochitosan-hierarchical ZSM-5 zeolite nanocomposite: characteristics and mechanism. Cellulose 5:6141–6152
Salehi S, Anbia M, Hosseiny AH, Sepehrian M (2018) Enhancement of CO2 adsorption on polyethylenimine functionalized multiwalled carbon nanotubes/Cd-nanozeolite composites. J Mol Struct 1173:792–800
Salehi S, Mandegarzad S, Anbia M (2020) Preparation and characterization of metal organic framework-derived nanoporous carbons for highly efficient removal of vanadium from aqueous solution. J Alloys Compd 812:152051
Saxena S, Tyson TA, Shukla S, Negusse E, Chen H, Bai J (2011) Investigation of structural and electronic properties of graphene oxide. Appl Phys Lett 99:013104
Shan W, Zhang D, Wang X, Wang D, Xing Z, Xiong Y, Fan Y, Yang Y (2019) One-pot synthesis of mesoporous chitosan-silica composite from sodium silicate for application in Rhenium(VII) adsorption. Microporous Mesoporous Mater 278:44–53
Shen H, Wang Z, Zhou A, Chen J, Hu M, Dong X, Xia Q (2015) Adsorption of phosphate onto amine functionalized nano-sized magnetic polymer adsorbents: mechanism and magnetic effects. RSC Adv 5:22080–22090
Shrock DL, Krasowski MD (2020) Chapter 23.5—Methemoglobinemia due to dietary nitrate. In: Ketha H, Garg U (eds) Toxicology cases for the clinical and forensic laboratory. Academic Press, Boca Raton, pp 469–472
Sowmya A, Meenakshi S (2014) A novel quaternized chitosan–melamine–glutaraldehyde resin for the removal of nitrate and phosphate anions. Int J Biol Macromol 64:224–232
Swain S, Dey R, Islam M, Patel R, Jha U, Patnaik T, Airoldi C (2009) Removal of fluoride from aqueous solution using aluminum-impregnated chitosan biopolymer. Sep Sci Technol 44:2096–2116
Tang T, Cao S, Xi C, Li X, Zhang L, Wang G, Chen Z (2020) Chitosan functionalized magnetic graphene oxide nanocomposite for the sensitive and effective determination of alkaloids in hotpot. Int J Biol Macromol 146:343–352
Teimouri A, Nasab SG, Vahdatpoor N, Habibollahi S, Salavati H, Chermahini AN (2016) Chitosan/Zeolite Y/Nano ZrO2 nanocomposite as an adsorbent for the removal of nitrate from the aqueous solution. Int J Biol Macromol 93:254–266
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Wang Z, Guo H, Shen F, Yang G, Zhang Y, Zeng Y, Wang L, Xiao H, Deng S (2015) Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4 +), nitrate (NO3−), and phosphate (PO43−). Chemosphere 119:646–653
Wang S, Ma X, Zheng P (2019) Sulfo-functional 3D porous cellulose/graphene oxide composites for highly efficient removal of methylene blue and tetracycline from water. Int J Biol Macromol 140:119–128
Wu F-C, Tseng R-L, Juang R-S (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35:613–618
Wu B, Fang L, Fortner JD, Guan X, Lo IM (2017) Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res 126:179–188
Xi Y, Mallavarapu M, Naidu R (2010) Preparation, characterization of surfactants modified clay minerals and nitrate adsorption. Appl Clay Sci 48:92–96
Xiong W, Peng J (2008) Development and characterization of ferrihydrite-modified diatomite as a phosphorus adsorbent. Water Res 42:869–4877
Yadav M, Ahmad S (2015) Montmorillonite/graphene oxide/chitosan composite: synthesis, characterization and properties. Int J Biol Macromol 79:923–933
Yan B, Zeng C, Yu L, Wang C, Zhang L (2018) Preparation of hollow zeolite NaA/chitosan composite microspheres via in situ hydrolysis-gelation-hydrothermal synthesis of TEOS. Microporous Mesoporous Mater 257:262–271
Yang L, Yang M, Xu P, Zhao X, Bai H, Li H (2017) Characteristics of nitrate removal from aqueous solution by modified steel slag. Water 9:757
Yazdi F, Anbia M, Salehi S (2019) Characterization of functionalized chitosan-clinoptilolite nanocomposites for nitrate removal from aqueous media. Int J Biol Macromol 130:545–555
Yin Q, Wang R, Zhao Z (2018) Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J Clean Prod 176:230–240
Zamparas M, Drosos M, Georgiou Y, Deligiannakis Y, Zacharias I (2013) A novel bentonite-humic acid composite material Bephos™ for removal of phosphate and ammonium from eutrophic waters. Chem Eng J 225:43–51
Zhang L, Liu X, Xia W, Zhang W (2014) Preparation and characterization of chitosan-zirconium (IV) composite for adsorption of vanadium (V). Int J Biol Macromol 64:155–161
Zhang J, Chen N, Tang Z, Yu Y, Hu Q, Feng C (2015) A study of the mechanism of fluoride adsorption from aqueous solutions onto Fe-impregnated chitosan. Phys Chem Chem Phys 17:12041–12050
Acknowledgments
The authors would like to thank the Materials and Energy Research Center (Grant No. 9911940) for the financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salehi, S., Hosseinifard, M. Optimized removal of phosphate and nitrate from aqueous media using zirconium functionalized nanochitosan-graphene oxide composite. Cellulose 27, 8859–8883 (2020). https://doi.org/10.1007/s10570-020-03382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03382-5