Abstract
Elevated arsenic levels in soil and water resources due to improper waste management by industry cause detrimental impacts on the environment and human health due to its carcinogenicity and high toxicity. This work demonstrated a facile and scalable method for the synthesis of γ-Fe2O3-PEG-PPG-PEG composites for practical arsenic adsorption. The synthesis of γ-Fe2O3-PEG-PPG-PEG composites resulted in a high percentage yield of 91.4%. Morphological analyses confirmed the microstructures and crystallinity of γ-Fe2O3-PEG-PPG-PEG composites. Besides, HR-TEM showed a homogeneous distribution of γ-Fe2O3 nanoparticles with an average particle size of 25 nm. The peak at 452 cm−1 in FT-IR spectra corresponds to the Fe–O stretching vibration, thus confirming the presence of γ-Fe2O3 nanoparticles. The synthesized composites showed increasing surface area (31–117 m2/g) and thermal stability with increasing γ-Fe2O3 nanoparticle compositions. The As(V) batch adsorption study revealed efficient As(V) removal up to 56.5 µg/g determined by the Langmuir adsorption isotherm. Furthermore, the equilibrium adsorptions were attained between 30 and 120 min. Notably, the adsorption capacity of the γ-Fe2O3-PEG-PPG-PEG composites increased significantly as the As(V) initial concentration increases. The research findings showed that the γ-Fe2O3-PEG-PPG-PEG composites could be potential adsorbents to treat arsenic-contaminated wastewater. Besides, the synthesized composites were proven to be economical and practical for scaling up due to their high product yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic is a highly toxic metalloid element, and it has been identified to be carcinogenic to humans. In natural water environments, arsenic predominantly exists in trivalent and pentavalent oxidation states, as As(III) and As(V) oxides. As(III) is the most toxic arsenic species; it predominates in an anoxic environment such as groundwater [1,2,3]. At pH 6.5 to 8.5, As(V) species are present in oxidizing waters as HAsO42− (aq) and H2AsO4− (aq), while As(III) existed as uncharged H3AsO3 (arsenious acid). In this regard, the soluble form of arsenic compounds can easily contaminate the natural water environments, thus inflicting a direct danger to public health [4]. Exposure to large doses of arsenic could severely affect the neurological, gastrointestinal, cardiovascular, and immunological systems as well as premature death [5, 6]. Therefore, health organizations such as the World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA) have reduced the maximum permissible arsenic content in drinking water from 50 to 10 µg/l [1, 2].
The removal of As(V) in waters, involving technologies such as precipitation, adsorption, membrane filtration, ion exchange, and coagulation-flocculation, is reported in numerous publications [7,8,9,10,11]. However, the adsorption technique is considered advantageous for water treatment as it is economical and results in high removal efficiency. Heavy metal adsorption refers to the bonding of heavy metal ions onto the surface of solid materials known as adsorbents [12]. Adsorbents such as carbon nanotubes, zeolite, natural iron oxides, and activated carbon have shown promising As(V) adsorptive properties in numerous studies [2, 13, 14]. However, high production costs and non-reusable property limit these materials’ continued use at an industrial scale [2].
Interestingly, nanoparticles have shown promising potential as adsorbents for the removal of heavy metals. Nanoparticle research has been rapidly progressed over the years with excellent removal efficiency due to rapid adsorption kinetics, high surface area to mass ratio, and high surface reactivity [7]. Furthermore, nanoparticles such as zero-valent iron or other functionalized iron oxide nanoparticles, titanium oxides, and copper oxides showed promising properties as adsorbents for As(V) adsorption [7, 15,16,17]. However, nanoparticles tend to cause a high-pressure drop gradient in fixed-bed, and continuous flow adsorption systems resulted from the small particle size and aggregation effect [7]. To counter these problems, support materials such as polymers, graphene, and montmorillonite were used to synthesize nanoparticle-based composites, and it was proven to be a practical approach to maintain the properties of the adsorbent, mechanical strength, and porous structure [5, 18,19,20].
Using porous materials as a support matrix to immobilize nanoparticles is a promising approach to reduce the agglomeration problem. Macroporous siliceous foam (MOSF) as the support matrix of iron oxide nanoparticles has attracted much attention due to tunable pore property, large surface area, and pore size for enhanced adsorptive performance [21]. For instance, Zhao et al. [21] observed a homogenous distribution of zero-valent iron nanoparticles (NZVI) (~ 10 nm) inside the pores of MOSF with size distribution between 20 and 120 nm. They further reported that the MOSF-NZVI has excellent adsorptive properties to remove Cr(VI). Another study reported the synthesis of MOSF via supra-assembly approach of non-ionic block copolymers. The MOSF served as a sacrificial template and silicon source. The release of silicate ions reacted with magnesium ions triggered magnesium silicate nanoparticles’ formation around the MOSF’s structure forming macroporous-mesoporous magnesium silicate (HMMgS) adsorbents. The HMMgS adsorbents demonstrated excellent adsorption capacities for methylene blue (MB) adsorbing 602 mg/g and lead (Pb2+) adsorbing 253 mg/g [22].
This study aims to develop an arsenic adsorbent composed of γ-Fe2O3-PEG-PPG-PEG composites with poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG-PPG-PEG) followed by dispersion of the nanoparticles within the porous structure. The adsorbent was characterized by the presence of significant functional groups, surface morphology through field emission electron microscope (FESEM) and high-resolution transmission electron microscope (HR-TEM), crystallinity, and thermal behavior. Furthermore, the effects of contact time, initial As(V) concentration, adsorption isotherms, and adsorption kinetics are studied for As(V) adsorption.
Materials and Method
Materials
All chemicals used in this study were of analytical grade and used without further purification. Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Mn ~ 5800) and sodium arsenate dibasic heptahydrate (Na2HAsO4·7H2O) were purchased from Sigma-Aldrich Co., Ltd., Germany. Potassium iodide (KI), ascorbic acid, and nitric acid were obtained from Fischer Scientific, USA. Iron chloride tetrahydrate (FeCl2·4H2O), iron (III) chloride hexahydrate (FeCl3·6H2O), and tetraethyl orthosilicate (TEOS) were purchased from Nacalai Tesque, Inc., Japan. Friedemann Schmidt, Australia, supplied sodium acetate anhydrous (CH3COONa) and glacial acetic acid, while sodium sulfate (Na2SO4) was purchased from Ajax FineChem Pty Ltd. Acetone and 30% ammonia solution were purchased from R&M Chemical, UK, and Macron Fine Chemicals, Belgium, respectively. Lastly, Kofa Chemical (M) Sdn Bhd supplied ethanol.
Method
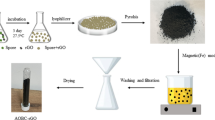
The γ-Fe2O3-PEG-PPG-PEG composites were prepared according to Zhao et al. [21] with modifications. The synthesis processes are illustrated in Fig. 1.
Synthesis of Macroporous Siliceous Foam
At 35 °C, poly(ethylene glycol)d-block-poly(propylene glycol)-block-poly(ethylene glycol) (1 g) and Na2SO4 (0.40 M) (1.7 g) were dissolved in pH 5 sodium acetate (NaAc-HAc) buffer solution (30 ml) (Ct = 0.02 M, where Ct = CNaAc + CHAc) to form a homogenous solution under stirring. To this mixture solution, tetraethyl orthosilicate (TEOS) (2.2 ml) was added under stirring. After 5 min, the stirring was stopped. The resultant mixture was kept in a static condition for 24 h and treated at 100 °C in a furnace for 24 h. The white precipitates were filtered, repeatedly washed with water to remove the inorganic salts, and dried at room temperature. The final MOSF product, abbreviated as PEG-PPG-PEG, was obtained by calcination at 550 °C in the furnace for 5 h.
Preparation of γ-Fe2O3 Dispersed in Macroporous Siliceous Foam
First, Fe3O4 nanoparticles were prepared by dissolving iron (III) hexahydrate (FeCl3·6H2O) (2.7 g) and iron chloride tetrahydrate (FeCl2·4H2O) (1 g) in distilled water (130 ml). The reaction mixture’s pH was adjusted to pH 9.5 using 30% ammonia solution under rapid mechanical stirring. The black solution was then heated at 60 °C and vigorously stirred for 10 min. The resultant nanoparticles were collected using a magnet and washed several times with acetone and distilled water, followed by drying in an oven at 60 °C. The dried Fe3O4 nanoparticles were dispersed in distilled water (50 ml) by ultrasonic treatment. The dispersed nanoparticles were washed with distilled water and later precipitated with acetone. Thereafter, the precipitated nanoparticles (0.5 g) were dispersed in distilled water (25 ml), forming 20 wt% ferrofluids. The ferrofluids (2 ml) were diluted in distilled water (40 ml), followed by the addition of 30% ammonia solution (10 ml). Afterwards, the nanoparticle mixture was heated up to 40 °C. Finally, ethanol (140 ml) was added, producing 20 wt% ethanolic solution containing Fe(NO3)3·9H2O.
At room temperature, MOSF (1 g) was homogeneously dispersed in ethanol (20 ml) to obtain solution (a) (Fig. 1c). Then, both solution (a) and 20 wt% ethanolic solution were mixed. The solvents were evaporated by continuous stirring at room temperature. The obtained wet powder (referred to as PEG-PPG-PEG-Fe) was transferred into a glass bottle, followed by the addition of acetic acid (20 ml). Subsequently, the mixture was heated in an oven at 80 °C for 3 h. After cooling, the solid powder (named as PEG-PPG-PEG-Fe-HAc) was dried at 80 °C for 15 min to remove acetic acid remnants. The final γ-Fe2O3-PEG-PPG-PEG composites were obtained via pyrolysis at 400 °C in a furnace for 30 min and 150 °C for 2 h. Different weight percentages of γ-Fe2O3 in the final composites (denoted FexPEG-PPG-PEG) were prepared as follows: 11.8, 21.0, and 34.8 wt% (x = 1, 2, and 3, respectively).
Material Characterization
The surface morphology of the γ-Fe2O3-PEG-PPG-PEG composites was characterized using the Hitachi SU8010 field emission scanning electron microscope (FESEM). The samples were coated with platinum. The Tecnai G2 20 S-TWIN high-resolution transmission electron microscope (HR-TEM) was used to study the microscopic structure of γ-Fe2O3-PEG-PPG-PEG composites. The synthesized composites’ functional groups were analyzed using Fourier transform infrared (FTIR) between 400 and 4000 cm−1. Powder X-ray diffraction (XRD) patterns of γ-Fe2O3-PEG-PPG-PEG composites were recorded in the range of 5–90° at ambient temperature using Bruker D8 Discover XRD. The surface area, average pore size, and average pore volume were analyzed using Micromeritics ASAP 2020 surface area and porosity analyzer. The samples were degassed for 4 h at 250 °C before the analysis under an N2 atmosphere followed by N2 adsorption at − 196 °C. The thermograms of γ-Fe2O3-PEG-PPG-PEG composites were obtained using TGA Q50 V20.10 Build 36 with temperatures ranging between 25 and 900 °C under nitrogen.
Arsenic Adsorption Study
The stock solution of sodium arsenate (Na2HAsO4·7H2O) with a concentration of 10,000 µg/l was prepared in 2% nitric acid solution (100 ml). The concentration of As(V) was measured using a flow injection analysis system-atomic absorption spectrophotometer (FIAS-AAS) at 193.7-nm wavelength (FIAS-AAS, PinAAcle 900F series, PerkinElmer, Germany) after conversion of arsenic to volatile arsenic hydride. To reduce As(V) to As(III), 5% (w/v) of potassium iodide (KI) and 5% (w/v) of ascorbic acid were added to the aliquot sample 1 h before analysis. The amount of adsorbed metal ions (qe, µg/g) was calculated by Eq. 1, where C0 and Ce were the initial and equilibrium metal ion concentration (µg/l), respectively. Meanwhile, V and m were the volume of metal solution (L) and mass of the FexPEG-PPG-PEG (g), respectively.
The adsorption capacity of as-prepared FexPEG-PPG-PEG composites was determined by investigating several experimental parameters, including contact time (0–300 min) and initial As(V) concentration (50–1000 µg/l). In both studies, FexPEG-PPG-PEG (300 mg) was packed in a glass column, supported on cotton wools. Subsequently, 250 µg/l of As(V) (30 ml) was poured into the column. The effect of contact time was studied by measuring the As(V) residual concentration in 1-ml aliquot at a different time interval until it reached equilibrium. The analysis of As(V) residual concentration to investigate the effect of As(V) initial concentration was performed at the respective FexPEG-PPG-PEG composites’ equilibrium time.
Adsorption Isotherm
An equilibrium study was conducted by adding FexPEG-PPG-PEG composites (300 mg) with varying γ-Fe2O3 contents of 11.8, 21, and 34.8 wt% into As(V) solution with concentration ranging from 50 to 1000 µg/l. The Langmuir and Freundlich linear isotherm models (Eqs. 2 and 3, respectively) were used to describe the experimental sorption data.
Here, qe means the amount of As(V) adsorbed (µg/g), qm is the maximum adsorption capacity of As(V) (µg/g), and Ce is defined as the As(V) concentration at equilibrium (µg/l). In addition, KL and KF are the Langmuir equilibrium (l/µg) and Freundlich equilibrium (l/g) constant, respectively. The value of nF is the heterogeneity factor of the adsorption sites (dimensionless).
Adsorption Kinetics
Two kinetic models, namely pseudo-first-order and pseudo-second-order, were used to determine the rate constant for As(V) adsorption onto the FexPEG-PPG-PEG composites.
Statistical Analysis
The experimental data were analyzed statistically using the IBM SPSS Statistics software. The statistical methods include one-way ANOVA, Tukey test, and p ˂ 0.05.
Results and Discussion
The percentage yield of the final product (γ-Fe2O3-PEG-PPG-PEG composites) in Fig. 1 was recorded to be 91.4%.
Field Emission Scanning Electron Microscope (FESEM)-Energy-Dispersive X-Ray (EDX) Analysis
FESEM analysis was conducted to study the surface morphology of γ-Fe2O3-PEG-PPG-PEG composites at × 80.0 k magnification as shown in Fig. 2a–c. The γ-Fe2O3-PEG-PPG-PEG composites exhibited morphology of microstructures and highly porous. The composites exhibited uniform hexagonally arrayed pores. Due to the unique pore structure, γ-Fe2O3 nanoparticles were able to attach on the surfaces of MOSF and diffused within the porous structures. Therefore, aggregates of γ-Fe2O3 nanoparticles were seen embedded within the porous structures of MOSF. Figure 3 a and b show the EDX microanalysis of Fe3PEG-PPG-PEG composite. Carbon (C) and oxygen (O) elements correlate to the chemical compositions of MOSF formed via supra-assembly of non-ionic PEG-PPG-PEG block copolymers. Silica (Si) was also detected due to the precursor used during the MOSF synthesis. Most importantly, iron (Fe) element confirms the presence of iron oxide nanoparticles immobilized within the MOSF. Based on Fig. 3a, the presence of Fe is evident in the scanned region, indicating the promising use of Fe3PEG-PPG-PEG composite as arsenic adsorbents.
High-Resolution Transmission Electron Microscope (HR-TEM)
The structure of FexPEG-PPG-PEG composites was further investigated using HR-TEM analysis. Non-uniform deposition of irregular shaped γ-Fe2O3 nanoparticles was observed under HR-TEM observations, as shown in Fig. 4a. At a higher γ-Fe2O3 weight percentage embedded within the MOSF, abundant black particles originated from γ-Fe2O3 nanoparticles were observed in Fig. 4c. The size distribution of γ-Fe2O3 nanoparticles ranges between 10 and 60 nm, with an average particle size of 25 nm.
Fourier Transform Infrared (FT-IR)
Figure 5a presents the FT-IR spectra for PEG-PPG-PEG’s starting material and the final product of γ-Fe2O3-PEG-PPG-PEG composites. The FTIR spectrum of PEG-PPG-PEG exhibited a weak broad peak at 3544 cm−1 corresponded to the –OH group. Absorption bands at 2870 and 1104 cm−1 were attributed to –CH and –CO stretching vibrations. The prominent functional groups of PEG-PPG-PEG in Fig. 5b were identified by FTIR analysis. Compared with the PEG-PPG-PEG spectrum, a new absorption band at 452 cm−1 corresponded to Fe–O stretching vibration was identified. This finding confirmed the successful preparation of γ-Fe2O3-PEG-PPG-PEG composites [23]. Besides, absorption bands at 3400 and 1078 cm−1 can be indexed to –OH and –CO stretching vibrations of PEG-PPG-PEG.
X-Ray Diffraction (XRD)
The diffraction profiles of the synthesized FexPEG-PPG-PEG composites are presented in Fig. 5c. Strong crystallization was observed on the FexPEG-PPG-PEG composites spectra. According to literature, the diffraction peaks that indicate the presence of γ-Fe2O3 are located at 2θ = 30.26° (200), 35.64° (311), 43.34° (400), 53.84° (422), 57.36° (511), and 62.96° (440) [24]. Diffraction peaks around 30° corresponding to (200) plane confirmed the incorporation of γ-Fe2O3 in the composites. Moreover, typical diffraction peaks for γ-Fe2O3 were also observed at 35.54° and 35.69°, corresponding to (311) plane [6].
Thermogravimetric Analysis (TGA)
Figure 5d shows the TGA/DTG curves of FexPEG-PPG-PEG composites. The TGA curve of Fe1PEG-PPG-PEG composite exhibited three stages of weight loss between 25 and 900 °C. The first two peaks observed from the DTG curve of Fe1PEG-PPG-PEG composite at around 41 and 76 °C (~ 15% weight loss) were due to the evaporation of loosely bound water from the polymer network structure. A minor weight loss of 5% at 291 °C was due to the evaporation of tightly attached water molecules on the polymeric structure. A noticeable gradual weight loss was observed for Fe1PEG-PPG-PEG between 510 and 894 °C with approximately 24% weight loss. The significant weight loss indicates the decomposition of PEG-PPG-PEG triblock polymers [25]. It was observed that 11% of solid content was gradually decomposed between 587 and 895 °C. Similarly, three-step thermal decomposition was observed on Fe2PEG-PPG-PEG composite at about 33–58 °C, 600–790 °C, and 840–900 °C. On the other hand, Fe3PEG-PPG-PEG composite exhibited two-step thermal decomposition at around 25–80 °C and 620–900 °C. The decomposition stage of Fe3PEG-PPG-PEG composite started around 620 °C with 9% weight loss, which is lower than Fe1PEG-PPG-PEG and Fe2PEG-PPG-PEG composites. It can be concluded that a higher weight percentage of γ-Fe2O3 resulted in improved thermal stability.
Specific Surface Area and Pore Characteristics by N2 Adsorption–Desorption Study
N2 adsorption–desorption was conducted to calculate the specific surface area, pore size, and pore volume of FexPEG-PPG-PEG composites. The N2 adsorption–desorption isotherms of FexPEG-PPG-PEG composites are shown in Fig. 6a–c. The detailed analysis of the specific surface area and pore characteristics is tabulated in Table 1. As stated by IUPAC classification, the FexPEG-PPG-PEG composites samples represent type III isotherm with an intermediate flat region of the isotherm indicating monolayer formation and mesoporous characteristics [26]. Fe1PEG-PPG-PEG composite in Fig. 6a revealed the lowest specific surface area of 31.0 m2/g, corresponding to average pore size and pore volume of 20.6 nm and 0.217 cm3/g, respectively. As the weight percentage of γ-Fe2O3 increases to 21.0 and 34.8 wt%, the specific surface area drastically increased by 2–4 times than that of 11.8 wt%. In addition to that, it was observed that the average pore size reduces from 20.6 to 17.1 nm with the increase in the weight percentage of γ-Fe2O3, thus explaining the increase in specific surface area.
Arsenic Adsorption Study
Contact Time
Figure 7a presents the effect of contact time on arsenic adsorption capacity of FexPEG-PPG-PEG composites with iron oxide content adjusted to 11.8, 21.0, and 34.8 wt% at an initial As(V) concentration of 250 µg/l. The presence of an abundant number of vacant adsorption sites on the surface of FexPEG-PPG-PEG composites resulted in rapid adsorption in the initial minutes. Based on the statistical analysis, the equilibrium contact times for FexPEG-PPG-PEG composites with varying iron oxide contents of 11.8, 21, and 34.8 wt% were 30, 120, and 90 min, respectively. After the equilibrium contact time, the adsorption capacity increased at a slower rate due to the exhaustion of adsorption sites [27]. Furthermore, Fe2PEG-PPG-PEG composite showed the highest adsorption capacity of 26.358 ± 0.763 µg/g followed by Fe3PEG-PPG-PEG and Fe1PEG-PPG-PEG composites with 19.386 ± 2.318 µg/g and 15.612 ± 2.215 µg/g. Although Fe3PEG-PPG-PEG composite recorded the highest surface area, it was found that the adsorption capacity was lower than the Fe2PEG-PPG-PEG composite. This is attributed to the reduced interaction between active adsorption sites and metal ions. Literature reported that iron oxide nanoparticles have a high tendency to cause agglomeration. An excessive amount of γ-Fe2O3 nanoparticles incorporated into the MOSF could trigger the nanoparticles’ agglomeration, leading to reduced active sites for efficient adsorption [28]. This could explain the decreased adsorption capacity for Fe3PEG-PPG-PEG composite.
Initial As(V) Concentration
The effect of initial As(V) concentration on the adsorption capacity of FexPEG-PPG-PEG composites was investigated. The obtained are shown in Fig. 7b. The adsorbents showed increased adsorption capacity with increased As(V) initial concentrations from 50 to 1000 µg/l. Fe2PEG-PPG-PEG composites showed the most pronounced effect with a significant increase from 7.028 ± 0.593 to 52.076 ± 3.354 µg/g. Meanwhile, As(V) adsorption onto Fe1PEG-PPG-PEG and Fe3PEG-PPG-PEG composites increased from 5.196 ± 0.262 to 34.815 ± 4.927 µg/g and 3.823 ± 0.351 to 32.005 ± 3.140 µg/g, respectively. The observed pattern was similar to the literature due to the greater driving force at a higher As(V) concentration to transfer the As(V) molecules from aqueous state to solid state. Subsequently, this resulted in greater interaction between As(V) molecules with the adsorbents, thus increasing the adsorption uptake [29, 30].
Adsorption Isotherm
Adsorption isotherm studies are important to elucidate the interaction between As(V) ions and FexPEG-PPG-PEG composites and investigate the maximum metal ion sorption capacities of FexPEG-PPG-PEG composites. In this study, Langmuir and Freundlich isotherm models were used to test the equilibrium data obtained for As(V) removal as shown in Fig. 8a and b. Furthermore, the R2 values of both linear plots were used to evaluate the adsorption data and determine the best-fitted model for the adsorption of As(V) onto FexPEG-PPG-PEG composites. The R2 values and corresponding correlation coefficients for both isotherms are listed in Table 2.
The Langmuir isotherm is applicable for the homogenous adsorption system as it is based on the assumptions of monolayer surface coverage and no interactions between adsorbed ions. Unlike Langmuir isotherm, Freundlich isotherm assumes that a multi-layer of adsorption process occurs on the heterogeneous surface of FexPEG-PPG-PEG composites. This isotherm model further suggests that the metal ion’s adsorption energy depends on the occupation of the adjacent active sites. By comparing the R2 values of all FexPEG-PPG-PEG composites with varying iron oxide contents, the Langmuir isotherm model is shown in Fig. 8a fitted well into the experimental data rather than the Freundlich isotherm model with R2 > 0.90. This suggests the homogeneous distribution of vacant adsorption sites on the surface FexPEG-PPG-PEG composites resulting in monolayer adsorption. The dimensionless factor (RL) was calculated using Eq. 4 to confirm the favorability of the adsorption process.
where KL (l/µg) is the Langmuir constant related to the adsorption energy, and C0 is the initial As(V) concentration. The value of RL indicates the shape of the isotherms to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0). Table 2 tabulates the calculated RL values of FexPEG-PPG-PEG composites ranging from 0 to 1, indicating the favorable uptake of As(V). In comparison, it was found that the Fe2PEG-PPG-PEG composite has the highest qm of 56.497 µg/g, followed by Fe3PEG-PPG-PEG and Fe1PEG-PPG-PEG composites with 49.020 µg/g and 43.668 µg/g, respectively. The adsorption capacity of the best FexPEG-PPG-PEG composites was comparable with reported arsenic adsorbents; for instance, the adsorption performance of iron arsenate coated with activated alumina was recorded to be 89.7 µg/g [31]. In another study by Li et al. [32], the column adsorption test by iron-exchanged zeolite (Fe-eZ) adsorbents recorded an adsorption capacity of 50 µg/g for As(V).
Adsorption Kinetics
Kinetic models are introduced to describe the adsorption process and predict the time needed for adsorption and the rate of target sorbate uptake [33]. The pseudo-first-order and pseudo-second-order models were studied to understand better As(V)’s adsorption behavior onto FexPEG-PPG-PEG composites. The pseudo-first-order kinetic model is described in Eq. 5.
where qe (µg/g) and qt (µg/g) are the corresponding amounts of adsorbed As(V) at equilibrium and at time t (min), respectively. Meanwhile, k1 is defined as the rate constant.
On the other hand, the pseudo-second-order kinetic model is based on equilibrium Eq. 6 as follows:
where k2 is the rate constant for the pseudo-second-order model.
From these plots in Fig. 8c and d, it can be concluded that the adsorption of As(V) onto Fe2PEG-PPG-PEG composites and Fe3PEG-PPG-PEG composites was found to be a pseudo-second-order kinetic model. The fitting of both adsorbents to pseudo-second-order does not merely imply that the adsorption process proceeds more likely through electrostatic interaction between As(V) molecules and the surface of adsorbents. Rather, this fitting serves as a valuable insight for future investigations aimed at determining the adsorption mechanisms, whether they are of physical or chemical nature. Analytical techniques such as Raman spectroscopy, solid-state 13C nuclear magnetic resonance (NMR), X-ray diffraction (XRD), X-ray photoelectron (XPS), and thermal analysis can be employed to identify the formation of specific chemical bonds, allowing for a more comprehensive understanding of the adsorption mechanism [34, 35]. The R2 value of Fe1PEG-PPG-PEG composites fitted well into the pseudo-second-order model; the calculated qe was not in agreement with the experimental qe. Therefore, it is concluded that Fe1PEG-PPG-PEG composites fitted well into pseudo-first-order kinetic model with an R2 value of 0.9328 and a calculated qe of 12.871 µg/g, which is closer to the experimental qe of 15.612 µg/g (Table 2).
Conclusion
In conclusion, the synthesized γ-Fe2O3-PEG-PPG-PEG composites have shown favorable adsorptive properties for efficient removal of As(V). FESEM-EDX and HR-TEM studies showed the surface morphology of the composites, and γ-Fe2O3 with an average size of 26 nm can be seen embedded within their porous structures. FT-IR analysis confirmed the presence of γ-Fe2O3 nanoparticles at 452 cm−1, responsible for Fe–O stretching vibration, whereas XRD confirmed the crystallinity of γ-Fe2O3-PEG-PPG-PEG composites. The specific surface area for the γ-Fe2O3-PEG-PPG-PEG ranges between 31 and 117 m2/g as the weight percentage of γ-Fe2O3 nanoparticles increases. Thermal analysis revealed that the synthesized γ-Fe2O3-PEG-PPG-PEG composites were thermally stable, with Fe3PEG-PPG-PEG composite exhibited the highest thermal stability. Fe2PEG-PPG-PEG composite recorded the highest adsorption capacity of 56.5 µg/g. The adsorption of As(V) was saturated within 30–120 min. Additionally, the adsorption capacities at different As(V) initial concentrations showed a significant increase from 50 to 1000 µg/l. The experimental data from As(V) adsorption isotherms fitted well into Langmuir isotherm. Therefore, γ-Fe2O3-PEG-PPG-PEG composites were proven to be efficient, practical, and promising adsorbents for removing As(V) from aqueous solutions. This study served as a proof-of-concept for future fixed-bed column adsorption studies to explore the potential of continuous operation at an industrial scale, aiming for longer service times, cost-effectiveness, and sustainability in water treatment applications.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because they have not been published yet but are available from the corresponding author upon reasonable request.
References
Yin Y, Zhou T, Luo H, Geng J, Yu W, Jiang Z (2019) Adsorption of arsenic by activated charcoal coated zirconium-manganese nanocomposite: performance and mechanism. Colloids Surf A: Physicochem Eng Asp 575:318–328
Sahu UK, Mahapatra SS, Patel RK (2017) Synthesis and characterization of an eco-friendly composite of jute fiber and Fe2O3 nanoparticles and its application as an adsorbent for removal of As (V) from water. J Mol Liq 237:313–321
Leus K, Folens K, Nicomel NR, Perez JPH, Filippousi M, Meledina M et al (2018) Removal of arsenic and mercury species from water by covalent triazine framework encapsulated γ-Fe2O3 nanoparticles. J Hazard Mater 353:312–319
Haldar D, Duarah P, Purkait MK (2020) MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: a review. Chemosphere 251:126388
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As (III) and As (V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Siddiqui SI, Chaudhry SA (2019) Nanohybrid composite Fe2O3-ZrO2/BC for inhibiting the growth of bacteria and adsorptive removal of arsenic and dyes from water. J Clean Prod 223:849–868
Contreras S, Henríquez-Vargas L, Álvarez PI (2017) Arsenic transport and adsorption modeling in columns using a copper nanoparticles composite. J Water Process Eng 19:212–219
Greenstein KE, Myung NV, Parkin GF, Cwiertny DM (2019) Performance comparison of hematite (α-Fe2O3)-polymer composite and core-shell nanofibers as point-of-use filtration platforms for metal sequestration. Water Res 148:492–503
Lee C-G, Alvarez PJ, Nam A, Park S-J, Do T, Choi U-S et al (2017) Arsenic (V) removal using an amine-doped acrylic ion exchange fiber: kinetic, equilibrium, and regeneration studies. J Hazard Mater 325:223–229
Feistel U, Otter P, Kunz S, Grischek T, Feller J (2016) Field tests of a small pilot plant for the removal of arsenic in groundwater using coagulation and filtering. J Water Process Eng 14:77–85
Guzmán A, Nava JL, Coreño O, Rodríguez I, Gutiérrez S (2016) Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor. Chemosphere 144:2113–2120
Asere TG, Stevens CV, Du Laing G (2019) Use of (modified) natural adsorbents for arsenic remediation: a review. Sci Total Environ 676:706–720
Li Z, Wu L, Sun S, Gao J, Zhang H, Zhang Z et al (2019) Disinfection and removal performance for Escherichia coli, toxic heavy metals and arsenic by wood vinegar-modified zeolite. Ecotoxicol Environ Saf 174:129–136
Cashion J, Khan S, Patti A, Adeloju S, Gates W (2017) Mechanism of groundwater arsenic removal by goethite-coated mineral sand. Hyperfine Interact 238(1):101
Nithya K, Sathish A, Kumar PS, Ramachandran T (2018) Fast kinetics and high adsorption capacity of green extract capped superparamagnetic iron oxide nanoparticles for the adsorption of Ni (II) ions. J Ind Eng Chem 59:230–241
Wagle D, Shipley HJ (2016) Adsorption of arsenic (V) to titanium dioxide nanoparticles: effect of particle size, solid concentration, and other metals. Environ Eng Sci 33(5):299–305
Lingamdinne LP, Chang Y-Y, Yang J-K, Singh J, Choi E-H, Shiratani M et al (2017) Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem Eng J 307:74–84
Zhu H, Jia Y, Wu X, Wang H (2009) Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J Hazard Mater 172(2–3):1591–1596
Bhowmick S, Chakraborty S, Mondal P, Van Renterghem W, Van den Berghe S, Roman-Ross G et al (2014) Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: kinetics and mechanism. Chem Eng J 243:14–23
Cumbal L, Greenleaf J, Leun D, SenGupta AK (2003) Polymer supported inorganic nanoparticles: characterization and environmental applications. React Funct Polym 54(1–3):167–180
Zhao C, Yang J, Wang Y, Jiang B (2017) Well-dispersed nanoscale zero-valent iron supported in macroporous silica foams: synthesis, characterization, and performance in Cr (VI) removal. J Mater 2017:1–13
Zhao C, Yang J, Jiang B (2017) Rational design of hierarchical macroporous–mesoporous magnesium silicate for highly efficient removal of organic dye and Pb 2+. RSC Adv 7(75):47225–47234
Ahangaran F, Hassanzadeh A, Nouri S (2013) Surface modification of Fe 3 O 4@ SiO 2 microsphere by silane coupling agent. Int Nano Lett 3(1):23
Zhu H, Jiang R, Xiao L, Zeng G (2010) Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Biores Technol 101(14):5063–5069
Wang X, Miao X, Li Z, Deng W (2010) Fabrication of mesoporous silica hollow spheres using triblock copolymer PEG–PPG–PEG as template. J Non-Cryst Solids 356(18):898–905
Rouquerol J, Rouquerol F, Llewellyn P, Maurin G, Sing KS (2013) Adsorption by powders and porous solids: principles, methodology and applications. Academic press
Gokila S, Gomathi T, Sudha P, Anil S (2017) Removal of the heavy metal ion chromiuim (VI) using Chitosan and Alginate nanocomposites. Int J Biol Macromol 104:1459–1468
Rahimi S, Moattari RM, Rajabi L, Derakhshan AA, Keyhani M (2015) Iron oxide/hydroxide (α, γ-FeOOH) nanoparticles as high potential adsorbents for lead removal from polluted aquatic media. J Ind Eng Chem 23:33–43
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G (2017) Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J Hazard Mater 336:146–157
Lin L, Xu X, Papelis C, Xu P (2017) Innovative use of drinking water treatment solids for heavy metals removal from desalination concentrate: synergistic effect of salts and natural organic matter. Chem Eng Res Des 120:231–239
Das B, Devi RR, Umlong IM, Borah K, Banerjee S, Talukdar AK (2013) Arsenic (III) adsorption on iron acetate coated activated alumina: thermodynamic, kinetics and equilibrium approach. J Environ Health Sci Eng 11:1–10
Li Z, Jean J-S, Jiang W-T, Chang P-H, Chen C-J, Liao L (2011) Removal of arsenic from water using Fe-exchanged natural zeolite. J Hazard Mater 187(1):318–323
Wang B, Bai Z, Jiang H, Prinsen P, Luque R, Zhao S et al (2019) Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: characteristics, modeling and application. J Hazard Mater 364:192–205
Lima ÉC, Adebayo MA, Machado FM (2015) Kinetic and Equilibrium Models of Adsorption. In: Bergmann CP, Machado FM (eds) Carbon nanomaterials as adsorbents for environmental and biological applications. Springer International Publishing, Cham, pp 33–69
Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Thanks for the financial support from the Faculty of Science, Monash University Australia, under the Cross-Campus Initiative Fund numbered SCI/MUA/10–2017/006.
Author information
Authors and Affiliations
Contributions
Nuraina Anisa Dahlan: methodology, investigation, formal analysis, writing—original draft. Anand Kumar Veeramachineni: conceptualization, methodology, investigation. Ragul Paramasivam: Investigation Antonio Patti: supervision, funding acquisition, validation, writing—review and editing. Janarthanan Pushpamalar: supervision, funding acquisition, validation, writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dahlan, N.A., Veeramachineni, A.K., Paramasivam, R. et al. Harnessing Magnetite Poly(ethylene Glycol)-Block-Poly(propylene Glycol)-Block-Poly(ethylene Glycol) (PEG-PPG-PEG) Composites for Efficient Arsenic Removal in Water Treatment. Water Conserv Sci Eng 8, 29 (2023). https://doi.org/10.1007/s41101-023-00205-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41101-023-00205-z