Abstract
Worldwide, over 2 billion children under the age of 5 experience stunting, wasting, or are underweight. Malnutrition contributes to 45% of all deaths in this age group (approximately 3.1 million deaths) [1]. Poverty, food insecurity, suboptimal feeding practices, climate change, and conflict are all contributing factors. Malnutrition causes significant respiratory problems, including increased risk of respiratory infections, impaired lung function, and increased risk of subsequent adult respiratory disease, including asthma, COPD, and lung cancer. Childhood malnutrition not only has serious consequences for children's health but it also has numerous consequences on wellbeing and educational attainment. Childhood malnutrition is a complex and multifaceted problem. However, by understanding and addressing the underlying causes, and investing in prevention and treatment programs, it is possible to maximize children's health and wellbeing on a global scale. This narrative review will focus on the impact of childhood malnutrition on lung development, the consequent respiratory disease, and what actions can be taken to reduce the burden of malnutrition on lung health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Malnutrition—comprised of undernutrition, overnutrition, and micronutrient deficiency—affects up to half of children worldwide. Malnutrition can be fatal, and children who survive often suffer lifelong health and economic consequences. |
The root causes of childhood malnutrition are inextricably linked to socioeconomic inequality at an individual and national level. The current agro-food system favors the production of cheap, high-calorie, low-nutrition foods, and families are priced out of a varied nutritious diet. Acute shocks such as climate crises, COVID-19, and conflict worsen these inequalities. |
Malnutrition impacts healthy lung development from conception through adulthood. This can occur through direct mechanisms including reducing surfactant production, decreased alveolarization, slow lung growth, and airway inflammation, and through indirect mechanisms including increasing the risk of prematurity, IUGR, and respiratory tract infections. |
Malnutrition both increases the risk of developing common childhood respiratory diseases, including asthma and respiratory tract infections, and worsens disease outcomes. Malnutrition, particularly undernutrition, is a significant predictor of morbidity and mortality in cystic fibrosis and bronchiectasis. |
Mitigation strategies, including antenatal nutritional supplementation and free school meals, can improve morbidity and mortality associated with childhood malnutrition. To address the root causes of childhood malnutrition, however, a child’s rights approach is required ensuring accountability at the corporate, governmental, and societal levels. |
Introduction
There continues to be a global crisis of childhood malnutrition. In 2019, one in three preschool children worldwide were either under or overweight, and one in two children suffer some form of nutritional deficiency [2]. Malnutrition can be fatal, and contributes to approximately 45% (3.1 million) of all deaths for children under the age of 5 through direct and indirect causal pathways [2].
The landscape of global childhood malnutrition is changing. While slow progress is being made to address life-threatening severe undernutrition in lower- and middle-income countries (LMICs), families in these regions now also face the chronic double-burden of obesity and stunting [3]. Children in high-income countries (HICs) also face food insecurity, driving high rates of childhood obesity and increasing rates of hospitalizations for hidden hunger and nutritional deficiencies. Underlying this change is a food system whereby processed, high-calorie, low-nutrient food is widely advertised, more readily available, and cheaper in comparison to fresh produce [4]. Food production and access to a healthy diet is then further impacted by ‘shocks to the system’ such as the COVID-19 pandemic and subsequent economic recession, conflicts, and climate change events. These issues surrounding the food system are compounded by increasingly sedentary lifestyles.
Lung growth and function is influenced by nutritional status throughout the life course, starting with prenatal maternal nutrition. Malnutrition negatively impacts lung function, increases the risk of infective and non-communicable child and adult respiratory diseases, and drives poor disease outcomes [5]. This narrative review will outline what is meant by malnutrition, the key drivers of childhood malnutrition, and how this affects healthy lung growth and respiratory disease physiology and outcomes. In undertaking this review, we conducted a search of global peer-reviewed literature from online databases, and governmental and third-sector white papers, up to December 2023. Key search terms used were “malnutrition”, “pulmonary”, “development”, “children”, and “pediatric”. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Malnutrition
What is malnutrition?
Malnutrition is defined by the World Health Organization (WHO) as a “deficiency or excess in nutrient intake, imbalance of essential nutrients or impaired nutrient utilization”, [6] which encompasses undernutrition, micro-nutrient related malnutrition, overweight, and obesity [7].
Undernutrition is malnutrition-caused inadequate calorie intake. It is historically the most common form of malnutrition, particularly in LMICs, [8] and despite being the focus of many global health campaigns including the Sustainable Development Goals (SDG) Goal 2 “to end hunger”, still poses a significant threat to children’s lives [9]. Acute episodes of undernutrition drive weight loss, and severe undernutrition causes wasting (low weight for height) [6] and kwashiorkor (acute edematous malnutrition, driven by a high-cereal, protein-deficient diet in children under the age of 5). Acute severe undernutrition is immediately life-threatening, and children who survive the acute illness are at increased risk of metabolic disorders and increased morbidity and mortality throughout their life-course [10].
Chronic undernutrition can also cause stunting (low height for age) [6]. Being shorter is not always a health concern at an individual level, but a comparatively shorter pediatric population reflects systemic problems with chronic childhood undernutrition and is a robust indicator for overall child wellbeing and inequalities within a society [11]. Stunting is associated with poor neurocognitive outcomes and decreased economic prospects, as well as endocrinopathies and metabolic consequences which confer increased risks of adult obesity and cardiovascular disease [12]. Stunting is often used as a marker for progress for LMICs but often underused to assess wellbeing in HICs [11].
Micronutrient deficiency (iron, folate, iodine, vitamin A, and zinc being the most prevalent) [13] can happen with or without calorie deficiency, leading children to be “over fed but undernourished” [14]. Lack of diversity in a diet increases the risk of micronutrient deficiency, particularly for families who rely on low-cost food staples to keep their children full. UNICEF’s State of the World’s Children report in 2019 estimated that one in two children world-wide suffer from hidden hunger and micronutrient deficiency [2].
Obesity is an excessive accumulation of fat that is detrimental to health and is measured as a body mass index (BMI) greater than 30 [7]. It is the fastest-growing type of malnutrition both in HICs and LMICs [15]. Increasing obesity rates are now driven by socioeconomic inequality and poverty – changing food landscapes, particularly in urban areas, drive cheap, calorie-dense diets with reduced physical activity and access to green/blue spaces for exercise [16].
The social and environmental causes of malnutrition
While malnutrition can be a consequence of underlying pathology affecting intake and absorption, as well as causing excessive losses or increased requirements, this review focuses on the social and environmental drivers of poor dietary intake.
Childhood malnutrition is inextricably linked to poverty at both individual household and overall national levels. Every country in the world is affected by a degree of malnutrition, [17] but countries in the poorest quartile for gross domestic product (GDP) per capita are significantly more likely to face ‘double burden malnutrition’ of wasting/stunting and obesity, with the poorest families within individual countries often carrying this burden [3]. In 2020, 94% of young children with stunting, 97% of children with wasting, and 75% of overweight children were living in Africa and Asia [18]. While some progress has been made in reducing the number of undernourished children on these continents, it has been slow, unequal, and likely reversed during the COVID-19 pandemic. Levels of overweight children in these regions have remained static and have increased in many Asian and African countries [18]. Despite ‘secure’ food supplies with sufficient production and import in many HICs including Europe and North America, many children grow up in food-insecure households and are also at risk of malnutrition, in particular obesity and hidden hunger. In England, almost a quarter of 10 to 11-year-olds are obese. The disparity in obesity prevalence has been gradually widening over 15 years, and children from the most deprived decile are now over twice as likely to be obese as those in the least deprived decile [2, 19]. Simultaneously, rates of undernutrition are rising. During the COVID-19 pandemic in 2020, UNICEF launched a campaign to distribute food parcels to hungry children in the UK [20], the first time in its history [19]. The number of children and adults admitted to hospital with malnutrition has doubled since 2008 [21]. Between 2022 and 2023, the prevalence of underweight 10 to 11-year-olds in England was 1.6%, the highest recorded since 2009 [22].
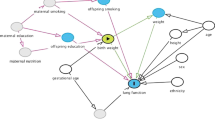
Socioeconomic deprivation creates barriers to a healthy nutritious diet at every step of the process of providing food for children (Fig. 1) [19]. Inequality at the individual/household level is exacerbated by a food system that is flawed by societal, governmental, and corporate factors, in which healthy food has become more costly, and highly processed food, high in refined sugars and fats and low in nutrients, is widely advertised and easily available [3, 4].
A ‘clock/capacity/cost analysis of limitations of a healthy diet’ demonstrating a the steps required in providing a healthy diet and b the time, resources, and financial constraints driven by economic deprivation [19]
Global warming driven climate changes also impact the food environment from production to distribution. A comparison of recorded temperature anomalies and international data on Food Insecurity Experience Scale found that for every 1 ℃ temperature anomaly, severe global food insecurity increases by 1.4% [23], and a recent systematic review found a significant correlation between increasing extreme weather events from climate change including droughts and flooding and childhood undernutrition in Africa and Asia [24].
Nutrition and healthy lung development
Antenatal exposure to nutrition
The impact of malnutrition on respiratory health starts with antenatal and maternal nutrition. Lung development is a multistage process that begins as early as 4 weeks gestation and continues into early adulthood. Suboptimal maternal nutrition, whether insufficient or excessive, has significant respiratory consequences for offspring [25].
Intrauterine growth restriction (IUGR) in undernourished mothers is one well-identified mechanism behind this relationship. IUGR affects 10–15% of pregnancies worldwide, and maternal undernutrition prior to and during pregnancy represents the most common maternal etiology of IUGR [25,26,27]. Numerous animal studies show that prolonged fetal undernutrition contributes to restriction of lung growth, impaired alveolar function, and reduced pulmonary vascular growth [5, 27]. There is longstanding evidence of a correlation between lower birthweight and reduced lung function in later life, initially theorized by the Barker hypothesis [28, 29]. More recently, both Dekker et al. and Suresh et al. conducted studies that associated lower birthweight with poor lung function in children and young adults, respectively [30, 31]. Chronic antenatal undernutrition associated with IUGR also causes epigenetic changes that impair subsequent lung development through alterations in specific signaling pathways—including transforming growth factor beta and the peroxisome proliferator-activated receptor pathways. These changes may be combatted by increased maternal docosahexaenoic acid (DHA) intake and breastfeeding [25, 27].
Maternal obesity also poses a risk to infant respiratory health, and significantly increases risk of preterm birth, itself a separate risk factor for poor respiratory health [32]. Liu et al. have demonstrated a significant association between childhood asthma and pre-pregnancy obesity or overweight mothers [33]. Some animal studies have also implicated maternal obesity in the impairment of fetal lung maturation and surfactant production [34, 35]. This is particularly pertinent due to a steep increase in maternal obesity, particularly in high- and middle-income countries [36]. For example, maternal obesity rates have increased from 7.6 to 22.3% between 1989 and 2019 in the UK [37].
Maternal micronutrient intake is also important for childhood respiratory health. The most common ‘hidden hunger’ of pregnancy is iron deficiency anemia, which affects nearly 50% of pregnancies worldwide [38]. It is the most common nutrient deficiency globally and is exacerbated during pregnancy [39]. There are a multitude of poor outcomes that can occur secondary to maternal anemia, including pre-term delivery, neonatal anemia, low birth weight, and neonatal stunting [40]. Stunting is associated with various respiratory health consequences for children, including poor lung function, asthma, and poorer outcomes for pneumonia [41,42,43]. Maternal vitamin E deficiency is also associated with an increased risk of asthma and atopy [44]. Bedard et al. found that children of mothers who adopted a “Mediterranean diet” during pregnancy were found to have higher small airway function at 8–9 years old compared to those that did not [45]. Maternal consumption of n3 (DHA and eicosapentaenoic acid—EPA) and n6 fatty acids, particularly during the second trimester, is also associated with reduced risk of asthma and allergic sensitization [46].
Breast feeding and lung growth
The nutritional, immune, cognitive, and economic benefits of breastfeeding are well established, with the WHO and UNICEF recommendation that children are exclusively breastfed until 6 months of age [47]. Newer studies have shown potential benefits of breastfeeding on children’s respiratory health as well as those previously demonstrated [48, 49].
Several observational studies have concluded that breastfeeding for at least 4 months results in improved lung function at school age, compared to children who are not breastfed [48]. Dogaru et al. noted that breastfed children of asthmatic mothers have higher forced vital capacity (FVC) and forced expiratory volume (FEV1) measurements in a dose–responsive relationship with duration of breastfeeding compared to those who are not breastfed [50]. Various potential mechanisms for this have been hypothesized, from a direct effect on lung growth to the suggestion that the physical act of suckling itself encourages structural changes leading to improved lung volumes [49].
The relationship between breastfeeding and childhood respiratory disease is more conflicting, specifically around asthma. Lodge et al. found there was an association between longer breastfeeding duration and a reduced risk of asthma in 5 to 18-year-olds [51] whereas other studies have previously found no significant association [48, 52].
Despite the WHO and UNICEF recommendation and the recognized benefits of breastfeeding, rates of breastfeeding in HICs such as the UK consistently fall from birth to 6 months. A study conducted in England demonstrated that in 2020, while breastfeeding was initiated in almost 85% of newborns, by 6 weeks only 38% were exclusively breast fed and by 6 months this figure dropped to 18% [53].
Childhood malnutrition and lung health
Undernutrition represents a significant respiratory health risk for children and has significant consequences throughout childhood [54]. Being underweight is associated with slow lung growth and reduced alveolarization, resulting in reduced surface area for gas exchange as well as reduced lung function. Undernutrition also compromises immune cell function and mucociliary clearance, which leads to increased risk of developing respiratory tract infections (RTIs), and worse clinical outcomes [55].
Obesity is also known to have cardiovascular, metabolic, and respiratory consequences, as well as an overall increased risk of mortality. Children who are overweight or obese have an increased risk of asthma and sleep-disordered breathing [15]. During the COVID-19 pandemic, the most common comorbidity of children admitted to Paediatric Intensive Care Units in the US and Canada was obesity [56], suggesting that childhood obesity is also associated with worse clinical outcomes in RTIs. In adults, there is a recognized inverse relationship between body mass index (BMI) and FEV1 [57], however this finding is not always present in children. Dysanapsis—the discrepancy between lung growth and airway caliber defined by reduced FEV1/FVC with normal FEV1 and FVC measurements—is more common in childhood obesity and asthma [58]. Forno et al. found that airway dysanapsis is more common in overweight and obese children and is associated with more severe disease for children with asthma [59]. Obesity in children is also associated with sleep-disordered breathing, particularly obstructive sleep apnea syndrome (OSAS). Some studies have reported a prevalence of OSAS from 13 to 59% in obese children compared to 1–2% in children of average-weight children [56, 60]. Besides the physical deposits of adipose tissue around the upper airway, another proposed causality of OSAS is leptin resistance. Leptin, released by adipose tissue, is involved in triggering ventilation, and leptin resistance is known to be more present in obesity [5, 61, 62].
Pathophysiological explanations for obesity-induced reduced lung function and respiratory illness include both mechanical processes, such as reduced chest wall compliance, increased airway resistance and low volume breathing resulting in airway hyper-responsiveness, as well as inflammatory processes. Inflammatory cytokines, which are associated with severe asthma symptoms such as interleukin-6 (IL-6), are released from adipose tissue, encourage neutrophilic airway inflammation, and impair immune function [56, 63].
The micronutrient deficiency of hidden hunger, either with or without undernutrition, can also impact childhood respiratory health. Vitamin E and A deficiencies, as well as iron, zinc, and selenium seem to have the most specific respiratory consequences [44, 54, 64]. Both antenatal and childhood deficiency of vitamin E and selenium are associated with increased risk of asthma and atopy, whereas optimal intake of dietary carotenoids is linked to a reduced risk of developing asthma and improved ventilatory function [44]. Zinc, vitamin A, and iron deficiencies are all associated with increased risk with RTIs. Zinc deficiency is common in LMICs, and a 2004 randomized clinical trial performed in India demonstrated that zinc treatment in boys with acute RTIs reduced the severity and duration of their illness [64]. Vitamin A plays a pivotal role in immune function and in hemoglobin production, and vitamin A deficiency correlates with increased risk of morbidity and mortality from RTIs, in childhood [65]. Iron-deficient anemia in childhood also increases a child’s risk of RTIs—studies from Nepal, Egypt, Israel, Lebanon, Romania, and India all identify that children with anemia are at 2–4 times higher risk of developing RTIs [66]. A retrospective cohort study in Ecuador found that children with anemia were more susceptible to the impacts of air pollution, with increased hospitalization for pneumonia [67].
Another important consideration in malnutrition is its effect on the gut microbiome. The concept of a gut–lung axis has been proposed as an internal communication that has microbial and immune implications and as a result, the gut–lung axis could affect the course of respiratory disease [63, 68]. If a healthy gut microbiome is crucial to establishing and maintaining good respiratory health, unhealthy diets and malnutrition can significantly compromise this. A study performed in Bangladesh observed that the gut microbiome of malnourished infants had increased pathogenic species and minimal organism diversity when compared to the microbiome of healthy infants [54]. It then follows that microbiome changes due to poor nutrition could compromise the gut–lung axis and have respiratory health implications.
Long-term impacts and adult disease
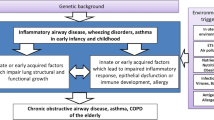
Lung development is an ongoing process which continues into young adulthood with maximum lung function achieved by approximately 22 years old. Lung function subsequently decreases, and the rate of this decline is based on a number of factors, including lifestyle, environment, and smoking [69]. Therefore, if optimum lung function is not achieved in utero or childhood, adults are more vulnerable to developing respiratory disease at a younger age as any decline will have a significant clinical impact [70] (see Fig. 2).
Lung trajectories in health and disease [71]
Lopuhaä et al. found that children born during the Dutch famine (1944–1945), whose mothers were exposed to famine in early to mid-gestation, experienced a higher probability of chronic obstructive pulmonary disease (COPD) in adulthood [72]. A large Australian study demonstrated that RTIs and poorly controlled asthma in childhood, which can be exacerbated by poor nutrition and being underweight, are associated with decline in adult FEV1 and increased risk of COPD [73]. In addition to increased risk of COPD, more than one meta-analysis has associated reduced lung function with an increased risk of lung cancer [74, 75], and a longitudinal study in the UK demonstrated that RTIs under 2 years of age significantly increased risk of early death from respiratory disease, even when adjusted for other respiratory risk factors—demonstrating the extensive impact of childhood exposures on adult lung health [76].
Malnutrition and respiratory disease outcomes
Asthma
As previously described, both undernutrition and obesity increase the risk of childhood asthma. Childhood undernutrition is associated with vitamin D deficiency, leptin deficiency, poor lung growth, poor alveolarization, reduced lung function, and increased IL-4 and CD23 + levels. These factors are all posited to be relevant in the development of childhood asthma [41]. A study based on the European Prospective Investigation into Cancer and nutrition (EPIC) cohort, who were exposed to the Dutch famine during childhood, showed a dose-dependent relationship between exposure to famine and risk of hospitalization with asthma later in life [77]. A Chinese study also demonstrated that exposure to famine, antenatally and during childhood, increased the risk of asthma and reduced lung function in adulthood, with the highest risk associated with antenatal exposure [78].
Current evidence suggests that obesity affects both allergic and non-allergic asthma. The provocation of a pro-inflammatory state appears to exaggerate the allergic inflammatory response and the mechanical consequences of an obese habitus are also likely to exacerbate symptoms [63]. There is also evidence that the presence of insulin resistance alongside obesity is more strongly associated with asthma [79]. While there are some nutritional factors that are protective from asthma, including longer duration of breastfeeding and adopting a Mediterranean diet [63, 80], this protection does not supersede the risk posed by obesity. A 2023 study [81] showed that exclusively breastfed children who were overweight remained more prone to developing asthma, compared to children who were exclusively breastfed but not overweight.
Respiratory tract infections
Every year, RTIs cause 4.3 million deaths of children under 5 years old, making it the most common cause of childhood mortality in the world [82]. Malnutrition is proven to increase rates of child mortality from respiratory infection—a 2021 meta-analysis demonstrated that severely underweight children hospitalized with pneumonia were 4.5 times more likely to die than those of healthy weight [83].
Poor nutrition, particularly poor protein intake, can compromise the innate immune system and impair cytokine release, due to reduced synthetic capacity. Acute illness is often accompanied by reduced food intake, with a negative nitrogen balance (where excreted nitrogen exceeds protein intake) driven by pyrexia. As such, children can become trapped in a vicious cycle, whereby malnutrition drives infection, and infections can perpetuate malnutrition [84].
Micronutrient deficiency is associated with increased risk of RTIs in children. As well as zinc, vitamin A, and iron deficiencies, several studies have implicated vitamin D deficiency in increased risk of RTIs in children [85]. Another consideration in underweight children is leptin deficiency. Leptin is produced by adipose tissue and has been identified as an important pro-inflammatory hormone within the immune system. Leptin deficiency has been associated with reduced circulating CD4 + T cells, impaired T cell proliferation, and reduced cytokine release, which can contribute to increased susceptibility to infection [86].
Cystic fibrosis (CF) and bronchiectatic conditions
Malnutrition, particularly undernutrition and micronutrient deficiency, has traditionally been one of the common consequences of cystic fibrosis (CF), due to high energy loss, recurrent infections and malabsorption, and is a significant risk factors for poorer clinical outcomes, with stunting being a significant independent risk factor for mortality in CF [87, 88]. Intense nutritional support (enteral or parenteral) is therefore an important management strategy, as optimal nutritional status is associated with improved lung function and reduced mortality [89]. However, with the introduction of cystic fibrosis transmembrane conductance regulator (CFTR) modulators, and the ability to correct the defective CFTR, the landscape of CF management has shifted. When CFTR modulators are started early, they have the potential to reduce the risk of undernutrition in children [90, 91], and a 2023 study found that the prevalence of overweight and obesity among people with CF had increased from 15% in 2001 to 36–40% in 2021 [92]. So far, there is no evidence that obesity is related to negative outcomes in CF [93]. Given this rise in obesity and potential impact of CFTR modulators on nutritional status, as well as the role of undernutrition on clinical outcomes (especially considering that not all children with CF will have access to modulators), it is important for pediatricians to consider all forms of malnutrition in the management of their CF patients [90, 94].
For patients with bronchiectasis and childhood interstitial lung diseases, optimizing nutritional status also remains a management priority. The prevalence of malnutrition in patients with interstitial lung disease has been reported to be between 9 and 55%, with higher body mass index shown to predict better outcomes [95]. Fouda et al. demonstrated that a 9-month nutritional intervention program for malnourished patients improved body composition and respiratory symptoms, reduced the number of exacerbations, and need for hospital admission [96].
Actions to address the socioenvironmental causes of malnutrition
Ending global childhood malnutrition due to socioenvironmental causes is achievable. It would take an estimated $5 billion USD per year to meet global targets for stunting for under 5s [97], less than the annual marketing budget for some food and beverage companies [98]. The drivers for poor nutrition are so widespread and deeply engrained in society that upscale large changes are required [99]. To achieve this, children’s rights to a nutritious diet must be at the forefront of policy, law, and health and social programs to address corporate, governmental, and societal vectors of malnutrition, including policy failings. This includes enshrining the right to food in national laws. The United Nations Convention on the Rights of the Child (UNCRC) comprehensively lays out global child rights, including the importance of combatting malnutrition, protecting every child’s right to access adequate nutritious foods, and protecting them against unhealthy food environments [100]. Enshrining the UNCRC in domestic laws, and taking a child rights-based approach to malnutrition would ensure accountability at national and international levels.
Interventions targeting childhood malnutrition
Addressing early-years malnutrition starts with maternal health. Bhutta et al. demonstrated that interventions to combat the health effects of malnutrition are effective and can improve morbidity and mortality rates significantly. With effective maternal supplementation of folate and micronutrients, there is a 16% reduction in low birth weight at term. When strategies are implemented to address complementary feeding, food supplementation, micronutrient supplementation, and educational strategies, stunting and mortality by 36 months of age are reduced by 36% and 25%, respectively, with significant economic payoff [101].
For older children, schools provide an opportunity to address dietary needs. Free school meals are a commonly implemented social safety net and have been proven to improve nutrition. Kristjansson et al.’s meta-analysis of global school meal programs showed that school children receiving a standard meal for 200 days per year gained an additional 0.37 kg, and pre-school children gained 0.54 cm per year [102]. Abizari et al. also demonstrated that children who participated in a school meals program in Ghana had higher levels of micronutrient adequacy and a 10% reduction in the incidence of anemia [103].
Food system changes
The need for increased food production itself also presents challenges. It is estimated that food production will have to increase by 50% by 2050 to meet the anticipated demands of a growing population [104]. Current intensive agricultural practices have been shown to both contribute to climate change and detrimentally affect food production. Implementing agro-food systems that implement sustainable methods, reduce environmental impact, promote inclusive small-scale farming, and utilize technology to improve efficiency would contribute to achieving the SDG aim of eliminating malnutrition by 2030, and will require global and national policy change [105, 106].
Despite agreement across various global bodies that tackling childhood malnutrition is a priority, there is a lack of data on several relevant factors, including age-specific dietary intake, feeding challenges, as well as parental perceptions of nutrition. Dietary surveillance on a global scale would allow a comprehensive understanding of varying needs and enable the implementation of targeted interventions. Micha et al. proposed that a central surveillance unit to monitor global dietary trends could present population-specific recommendations as well as support existing regional initiatives [107]. Existing data sets would lend themselves to this and can be upscaled to optimize data collection. For example, pregnant mothers and children attending health care services typically have regular anthropometrical examinations, but these data are not necessarily widely available, or of consistent quality [108]. Collecting individual-level quantitative dietary data using Individual Food Consumption Surveys (IFCS) is an effective method of dietary surveillance. Traditionally, IFCS have been collected in HICs, but over the last 20 years, their use has become more common in LMICs. The Food and Agriculture Organization of the United Nations and World Health Organization Global Individual Food consumption data Tool (FAO/WHO GIFT) is a catalogue of these data [109]. However, while the availability of these data is increasing, it is rarely integrated into healthcare systems. Huybrechts et al. showed that not only were integrated IFCS associated with better response rates and more representative results, they also encouraged evaluation of healthcare policy, guidelines, and public health priorities [110]. In summary, investments in nutrition data systems would support policy and strategy, as well as monitoring progress of interventions, to address malnutrition on a large scale [108].
Conclusions
The triple burden of malnutrition—underweight, overweight, and hidden hunger of micronutrient deficiencies—risks healthy lung development, worsens respiratory disease outcomes, and are a significant threat to children’s lives. The consequences are wide-reaching, and include pre-term birth due to maternal malnutrition, a higher risk of mortality from childhood respiratory infections, as well as increased risk of asthma, COPD, and malignancy in adulthood. Most socioeconomically deprived children across the world are disproportionately affected by malnutrition, as families are priced out of nutritious diets in an increasingly unhealthy global agro-food system.
Only by addressing these issues with meaningful structural changes, using a child-rights approach at the corporate, governmental, and societal levels, can children’s nutritional status, and subsequent respiratory health in both childhood and adulthood, be optimized.
Data Availability
All data used are available in the references provided.
References
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. Available from: https://pubmed.ncbi.nlm.nih.gov/23746772/. Cited 3 Dec 2023.
Aguayo VM, Branca F, Demaio S, Fanzo J, Haddad L, Menon P, et al. The state of the world’s children.: 2019 :: children, food and nutrition : growing well in a changing world. UNICEF. 2019;1–258. Available from: https://digitallibrary.un.org/record/3896035. Cited 27 Nov 2023
Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. The Lancet. 2020;395(10217):65–74. Available from: http://www.thelancet.com/article/S0140673619324973/fulltext. Cited 27 Nov 2023
The Broken Plate 2023 | Food Foundation. Available from: https://foodfoundation.org.uk/publication/broken-plate-2023. Cited 3 Dec 2023
Kuiper-Makris C, Selle J, Nüsken E, Dötsch J, Alejandre Alcazar MA. Perinatal Nutritional and Metabolic Pathways: Early Origins of Chronic Lung Diseases. Front Med. Front Media S.A. 2021;8
Malnutrition. Available from: https://www.who.int/health-topics/malnutrition#tab=tab_1. Cited 27 Nov 2023
Fact sheets—Malnutrition. Available from: https://www.who.int/news-room/fact-sheets/detail/malnutrition. Cited 8 Feb 2024
Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, et al. The global syndemic of obesity, undernutrition, and climate change: the lancet commission report. The Lancet. 2019;393(10173):791–846.
Goal 2: Zero Hunger | United Nations Development Programme [Internet]. Available from: https://www.undp.org/sustainable-development-goals/zero-hunger. Cited 3 Dec 2023
Child alert: Severe wasting | UNICEF. Available from: https://www.unicef.org/child-alert/severe-wasting. Cited 27 Nov 2023
de Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. 2016;12 Suppl 1(Suppl 1):12–26. Available from: https://pubmed.ncbi.nlm.nih.gov/27187907/. Cited 3 Dec 2023
Soliman A, De Sanctis V, Alaaraj N, Ahmed S, Alyafei F, Hamed N, et al. Early and Long-term Consequences of Nutritional Stunting: From Childhood to Adulthood. Acta Bio Medica : Atenei Parmensis. 2021;92(1):2021168. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7975963/. Cited 27 Nov 2023
Lowe NM. The global challenge of hidden hunger: perspectives from the field. Proceedings of the Nutrition Society. 2021;80(3):283–9. Available from: https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/global-challenge-of-hidden-hunger-perspectives-from-the-field/62BD2454B25499EFC54713E86C5D7BAA. Cited 27 Nov 2023
Kumar A, Kerketta A, Dewali S, Sharma N, Kumari Panda A, Singh Bisht S. Tackling Hidden Hunger: Understanding Micronutrient Deficiency and Effective Mitigation Strategies. Emerging Solutions in Sustainable Food and Nutrition Security. 2023;305–19. Available from: https://link.springer.com/chapter/https://doi.org/10.1007/978-3-031-40908-0_12. Cited 27 Nov 2023
McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1 · Epidemiology. Thorax. 2008;63(7):649–54. Available from: https://thorax.bmj.com/content/63/7/649. Cited 12 Nov 2023
Obesity Profile - Data - OHID. Available from: https://fingertips.phe.org.uk/profile/national-child-measurement-programme/data#page/13/. Cited 27 Nov 2023
Fact sheets - Malnutrition. Available from: https://www.who.int/news-room/fact-sheets/detail/malnutrition. Cited 27 Nov 2023
Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: key findings of the 2021 edition.. Available from: https://www.who.int/publications/i/item/9789240025257. Cited 27 Nov 2023
Lee AR, Kingdon CC, Davie M, Hawcutt D, Sinha IP. Child poverty and health inequalities in the UK: a guide for paediatricians. Arch Dis Child. 2023;108(2):94–101. Available from: https://pubmed.ncbi.nlm.nih.gov/35680401/. Cited 27 Nov 2023
UNICEF Launches First Ever Domestic Emergency Response Programme To Provide Food Support For Vulnerable Children Across The UK—UNICEF UK. Available from: https://www.unicef.org.uk/press-releases/unicef-launches-first-ever-domestic-emergency-response-programme-to-provide-food-support-for-vulnerable-children-across-the-uk/. Cited 3 Dec 2023
Hospital admissions for malnutrition - NHS Digital. Available from: https://digital.nhs.uk/supplementary-information/2020/hospital-admissions-for-malnutrition. Cited 3 Dec 2023
National Child Measurement Programme, England, 2022/23 School Year - NHS Digital.. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-child-measurement-programme/2022-23-school-year. Cited 12 Nov 2023
Dasgupta S, Robinson EJZ. Attributing changes in food insecurity to a changing climate. Sci Rep. 2022;12(1):1–11. Available from: https://www.nature.com/articles/s41598-022-08696-x. Cited 27 Nov 2023
Lieber M, Chin-Hong P, Kelly K, Dandu M, Weiser SD. A systematic review and meta-analysis assessing the impact of droughts, flooding, and climate variability on malnutrition. Glob Public Health. 2022;17(1):68–82. Available from: https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/17441692.2020.1860247. Cited 27 Nov 2023
Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and lung growth. Nutrients. 2018;10:919.
Armengaud JB, Yzydorczyk C, Siddeek B, Peyter AC, Simeoni U. Intrauterine growth restriction: clinical consequences on health and disease at adulthood. Reprod Toxicol. 2021;1(99):168–76.
Pike K, Jane Pillow J, Lucas JS. Long term respiratory consequences of intrauterine growth restriction. In: Seminars in fetal and neonatal medicine. W.B. Saunders Ltd; 2012. p. 92–8.
Barker DJP, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–5. Available from: https://pubmed.ncbi.nlm.nih.gov/1912913/. Cited 7 Oct 2023
Hoy WE, Nicol JL. The Barker hypothesis confirmed: association of low birth weight with all-cause natural deaths in young adult life in a remote Australian Aboriginal community. J Dev Orig Health Dis. 2019;10(1):55–62. Available from: https://www.cambridge.org/core/journals/journal-of-developmental-origins-of-health-and-disease/article/barker-hypothesis-confirmed-association-of-low-birth-weight-with-allcause-natural-deaths-in-young-adult-life-in-a-remote-australian-aboriginal-community/2875FDC3D72987F944F4476FF73650D3. Cited 10 Oct 2023
Den Dekker HT, Sonnenschein-Van Der Voort AMM, De Jongste JC, Anessi-Maesano I, Arshad SH, Barros H, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta-analysis of 25,000 children. J Allergy Clin Immunol. 2016;137(4):1026–35.
Suresh S, Mamun AA, O’Callaghan M, Sly PD. The impact of birth weight on peak lung function in young adults. Chest. 2012;142(6):1603–10. Available from: https://pubmed.ncbi.nlm.nih.gov/22539648/. Cited 12 Apr 2023
Leddy MA, Power ML, Schulkin J. the impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170. Available from: /pmc/articles/PMC2621047/. Cited 1 Mar 2023
Liu S, Zhou B, Wang Y, Wang K, Zhang Z, Niu W. Pre-pregnancy maternal weight and gestational weight gain increase the risk for childhood asthma and wheeze: an updated meta-analysis. Front Pediatr. 2020;3(8):521327.
Mayor RS, Finch KE, Zehr J, Morselli E, Neinast MD, Frank AP, et al. Biomarkers in Lung Diseases: from Pathogenesis to Prediction to New Therapies: Maternal high-fat diet is associated with impaired fetal lung development. Am J Physiol Lung Cell Mol Physiol. 2015;309(4):L360. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4538234/. Cited 23 Nov 2023
Lock MC, McGillick EV, Orgeig S, McMillen IC, Mühlhäusler BS, Zhang S, et al. Differential effects of late gestation maternal overnutrition on the regulation of surfactant maturation in fetal and postnatal life. J Physiol [Internet]. 2017;595(21):6635. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5663831/. Cited 23 Nov 2023
Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS One. 2018;13(8). Available from: /pmc/articles/PMC6084991/. Cited 7 Nov 2023
Ziauddeen N, Huang JY, Taylor E, Roderick PJ, Godfrey KM, Alwan NA. Interpregnancy weight gain and childhood obesity: analysis of a UK population-based cohort. Int J Obes 2021 46:1. 2021;46(1):211–9. Available from: https://www.nature.com/articles/s41366-021-00979-z. Cited 7 Nov 2023
Shand AW, Kidson-Gerber GL. Anaemia in pregnancy: a major global health problem. The Lancet. 2023;401(10388):1550–1. Available from: http://www.thelancet.com/article/S0140673623003963/fulltext. Cited 16 Nov 2023
Gosdin L, Martorell R, Bartolini RM, Mehta R, Srikantiah S, Young MF. The co-occurrence of anaemia and stunting in young children. Matern Child Nutr. 2018;14(3):e12597. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/mcn.12597. Cited 16 Nov 2023
Nadhiroh SR, Micheala F, Tung SEH, Kustiawan TC. Association between maternal anemia and stunting in infants and children aged 0–60 months: a systematic literature review. Nutrition. 2023;1(115):112094.
Sapartini G, Wong GWK, Indrati AR, Kartasasmita CB, Setiabudiawan B. Stunting as a Risk Factor for Asthma: The Role of Vitamin D, Leptin, IL-4, and CD23+. Medicina. 2022;58(9):1236. Available from: https://www.mdpi.com/1648-9144/58/9/1236/htm. Cited 16 Nov 2023
Moschovis PP, Addo-Yobo EOD, Banajeh S, Chisaka N, Christiani DC, Hayden D, et al. Stunting is associated with poor outcomes in childhood pneumonia. Tropical Medicine & International Health. 2015;20(10):1320–8. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/tmi.12557. Cited 16 Nov 2023
Agrawal A, Mishra N, Salvi S, Lyngdoh T. Low lung function in the developing world is analogous to stunting: a review of the evidence. Wellcome Open Res. 2020;5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7745193/. Cited 16 Nov 2023
Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–17.
Bédard A, Northstone K, John Henderson A, Shaheen SO. Mediterranean diet during pregnancy and childhood respiratory and atopic outcomes: birth cohort study. Eur Respir J. 2020;55(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7066469/. Cited 7 Nov 2023
Maslova E, Rifas-Shiman SL, Oken E, Platts-Mills TAE, Gold DR. Fatty acids in pregnancy and risk of allergic sensitization and respiratory outcomes in childhood. Ann Allergy Asthma Immunol. 2019;122(1):120-122.e3.
World Health Organization. UNICEF. Global strategy for infant and young child feeding. 2003;30.
Di Filippo P, Lizzi M, Raso M, Di Pillo S, Chiarelli F, Attanasi M. The role of breastfeeding on respiratory outcomes later in childhood. Front Pediatr. 2022;28(10):829414.
Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax. 2009;64(1):62. Available from: /pmc/articles/PMC2630423/. Cited 8 Nov 2023
Dogaru CM, Strippoli MPF, Spycher BD, Frey U, Beardsmore CS, Silverman M, et al. Breastfeeding and Lung Function at School Age. 101164/rccm201108-1490OC. 2012;185(8):874–80. Available from: www.atsjournals.org. Cited 8 Nov 2023
Lodge C, Tan D, Lau M, Dai X, Tham R, Lowe A, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr [Internet]. 2015;104(467):38–53. Available from: https://pubmed.ncbi.nlm.nih.gov/26192405/. Cited 9 Nov 2023
Burgess SW, Dakin CJ, O’Callaghan MJ. Breastfeeding does not increase the risk of asthma at 14 years. Pediatrics. 2006;117(4). Available from: https://pubmed.ncbi.nlm.nih.gov/16585289/. Cited 11 Nov 2023
Quigley MA, Harrison S, Levene I, McLeish J, Buchanan P, Alderdice F. Breastfeeding rates in England during the Covid-19 pandemic and the previous decade: Analysis of national surveys and routine data. PLoS One. 2023;18(10). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10566678/. Cited 12 Nov 2023
Fontaine F, Turjeman S, Callens K, Koren O. The intersection of undernutrition, microbiome, and child development in the first years of life. Nat Commun 2023 14:1. 2023;14(1):1–9. Available from: https://www.nature.com/articles/s41467-023-39285-9. Cited 12 Nov 2023
Harding R, Snibson K, O’Reilly M, Maritz G. Early environmental influences on lung development: implications for lung function and respiratory health throughout life. In: Newnham JP, Ross MG editors. Early life origins of human health and disease. Switzerland: Karger; 2009. pp. 78–88.
Di Palmo E, Filice E, Cavallo A, Caffarelli C, Maltoni G, Miniaci A, et al. Childhood obesity and respiratory diseases: which link?. Children 2021. 2021;8(3):177. Available from: https://www.mdpi.com/2227-9067/8/3/177/htm. Cited 12 Nov 2023
Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318(5):293. Available from: https://pubmed.ncbi.nlm.nih.gov/10555090/. Cited 12 Nov 2023
Leger MCGD, Vocos M, Kruger A, Primrose DS, Roque M, Andrada G, et al. Small airways dysanapsis in overweight/obese asthmatic children. Eur Respir J. 2022;60(suppl 66):3696. Available from: https://erj.ersjournals.com/content/60/suppl_66/3696. Cited 12 Nov 2023
Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, et al. Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med. 2017;195(3):314–23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5328183/. Cited 12 Nov 2023
Kang M, Mo F, Witmans M, Santiago V, Tablizo MA. Trends in Diagnosing Obstructive Sleep Apnea in Pediatrics. Children 2022;9(3):306. Available from: https://www.mdpi.com/2227-9067/9/3/306/htm. Cited 12 Nov 2023
Shimura R, Tatsumi K, Nakamura A, Kasahara Y, Tanabe N, Takiguchi Y, et al. Fat Accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127(2):543–9.
Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev. 2006;7(4):247–59.
Meslamani AZ Al. Insights into the immunological links between dietary habits and asthma. Expert Rev Clin Immunol. 2023;1–4. Available from: https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/1744666X.2023.2277864. Cited 31 Oct 2023
Mahalanabis D, Lahiri M, Paul D, Gupta S, Gupta A, Wahed MA, et al. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr. 2004;79(3):430–6.
Tian W, Yi W, Zhang J, Sun M, Sun R, Yan Z. The correlation between the vitamin A, D, and E levels and recurrent respiratory tract infections in children of different ages. Am J Transl Res. 2021;13(5):5665. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8205845/. Cited 27 Nov 2023
Jayamanna U, Jayaweera JAAS. Childhood anemia and risk for acute respiratory infection, gastroenteritis, and urinary tract infection: a systematic review. J Pediatr Infect Dis. 2023;18(2):61–70. Available from: http://www.thieme-connect.com/products/ejournals/html/https://doi.org/10.1055/s-0042-1760237. Cited 27 Nov 2023
Harris AM, Sempértegui F, Estrella B, Narváez X, Egas J, Woodin M, et al. Air pollution and anemia as risk factors for pneumonia in Ecuadorian children: a retrospective cohort analysis. Environ Health. 2011;10(1):1–8. Available from: https://ehjournal.biomedcentral.com/articles/https://doi.org/10.1186/1476-069X-10-93. Cited 27 Nov 2023
Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut–lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7042389/. Cited 12 Nov 2023
Stocks J, Hislop A, Sonnappa S. Early lung development: Lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–42.
Zhai T, Li S, Hu W, Li D, Leng S. Potential micronutrients and phytochemicals against the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nutrients. 2018;10(7):813. Available from: https://www.mdpi.com/2072-6643/10/7/813/htm. Cited 16 Nov 2023
Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7(4):358–64. Available from: http://www.thelancet.com/article/S2213260018305290/fulltext. Cited 27 Nov 2023
Lopuhaä CE, Roseboom TJ, Osmond C, Barker DJP, Ravelli ACJ, Bleker OP, et al. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax. 2000;55(7):555–61.
Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6(7):535–44. Available from: http://www.thelancet.com/article/S2213260018301000/fulltext. Cited 16 Nov 2023
Wasswa-Kintu S, Gan WQ, Man SFP, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60(7):570–5. Available from: https://pubmed.ncbi.nlm.nih.gov/15994265/. Cited 16 Nov 2023
Fry JS, Hamling JS, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating FEV1 decline to lung cancer risk. BMC Cancer [Internet]. 2012;12. Available from: https://pubmed.ncbi.nlm.nih.gov/23101666/. Cited 16 Nov 2023
Allinson JP, Chaturvedi N, Wong A, Shah I, Donaldson GC, Wedzicha JA, et al. Early childhood lower respiratory tract infection and premature adult death from respiratory disease in Great Britain: a national birth cohort study. The Lancet. 2023;401(10383):1183–93. Available from: http://www.thelancet.com/article/S0140673623001319/fulltext. Cited 27 Nov 2023
Van Abeelen AFM, Elias SG, De Jong PA, Grobbee DE, Bossuyt PMM, Van Der Schouw YT, et al. Famine in the Young and Risk of Later Hospitalization for COPD and Asthma. PLoS One [Internet]. 2013 Feb 23 [cited 2023 Nov 16];8(12):e82636. Available from: https://journals.plos.org/plosone/article?id=https://doi.org/10.1371/journal.pone.0082636
Jin C, Zhang T, Li Y, Shi W. Early-life exposure to malnutrition from the Chinese famine on risk of asthma and chronic obstructive pulmonary disease in adulthood. Front Nutr. 2022;31(9):848108.
Allinson JP, Patel PH, Donaldson GC. Obesity, insulin resistance, and asthma. Am J Respir Crit Care Med. 2022;206(9):1057–8. Available from: www.atsjournals.org. Cited 12 Nov 2023
Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179(10):1153–67. Available from: https://doi.org/10.1093/aje/kwu072. Cited 16 Nov 2023
Kian N, Bagheri A, Salmanpour F, Soltani A, Mohajer Z, Samieefar N, et al. Breast feeding, obesity, and asthma association: clinical and molecular views. Clinical and Molecular Allergy 2023 21:1. 2023;21(1):1–14. Available from: https://clinicalmolecularallergy.biomedcentral.com/articles/https://doi.org/10.1186/s12948-023-00189-0. Cited 16 Nov 2023
Vinod A, Kaimal RS. Study on acute respiratory infection in children aged 1 year to 5 years-A hospital-based cross-sectional study. J Family Med Prim Care. 2023;12(4):666. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10259544/. Cited 16 Nov 2023
Kirolos A, Blacow RM, Parajuli A, Welton NJ, Khanna A, Allen SJ, et al. The impact of childhood malnutrition on mortality from pneumonia: a systematic review and network meta-analysis. BMJ Glob Health. 2021;6(11):e007411. Available from: https://gh.bmj.com/content/6/11/e007411. Cited 16 Nov 2023
Rodríguez L, Cervantes E, Ortiz R. Malnutrition and Gastrointestinal and Respiratory Infections in Children: A Public Health Problem. Int J Environ Res Public Health. 2011;8(4):1174. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3118884/. Cited 16 Nov 2023
Taylor CE, Camargo CA. Impact of micronutrients on respiratory infections. Nutr Rev. 2011;69(5):259–69. Available from: https://doi.org/10.1111/j.1753-4887.2011.00386.x. Cited 16 Nov 2023
Bernotiene E, Palmer G, Gabay C. The role of leptin in innate and adaptive immune responses. Arthritis Res Ther. 2006;8(5):1–10. Available from: https://arthritis-research.biomedcentral.com/articles/https://doi.org/10.1186/ar2004. Cited 16 Nov 2023
Culhane S, George C, Pearo B, Spoede E. Malnutrition in cystic fibrosis. nutrition in clinical practice. 2013;28(6):676–83. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1177/0884533613507086. Cited 17 Nov 2023
Pencharz PB, Durie PR. Pathogenesis of malnutrition in cystic fibrosis, and its treatment. Clin Nutr. 2000;19(6):387–94.
Panagopoulou P, Fotoulaki M, Nikolaou A, Nousia-Arvanitakis S. Prevalence of malnutrition and obesity among cystic fibrosis patients. Pediatrics International. 2014;56(1):89–94. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/ped.12214. Cited 17 Nov 2023
Mailhot G, Denis MH, Beauchamp-Parent C, Jomphe V. Nutritional management of people living with cystic fibrosis throughout life and disease continuum: Changing times, new challenges. Journal of Human Nutrition and Dietetics. 2023;36(5):1675–91. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/jhn.13214. Cited 17 Nov 2023
Isa HM, Al-Ali LF, Mohamed AM. Growth assessment and risk factors of malnutrition in children with cystic fibrosis. Saudi Med J. 2016;37(3):293. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4800894/. Cited 17 Nov 2023
Snowball JE, Flight WG, Heath L, Koutoukidis DA. A paradigm shift in cystic fibrosis nutritional care: clinicians' views on the management of patients with overweight and obesity. J Cyst Fibros. 2023;22(5):836–42. https://doi.org/10.1016/j.jcf.2023.03.011.
Nagy R, Gede N, Ocskay K, Dobai BM, Abada A, Vereczkei Z, et al. Association of body mass index with clinical outcomes in patients with cystic fibrosis: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(3):e220740–e220740. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2789687. Cited 17 Nov 2023
Vieni G, Faraci S, Collura M, Lombardo M, Traverso G, Cristadoro S, et al. Stunting is an independent predictor of mortality in patients with cystic fibrosis. Clin Nutr. 2013;32(3):382–5.
Rinaldi S, Balsillie C, Truchon C, AL-Mubarak A, Mura M, Madill J. Nutrition implications of intrinsic restrictive lung disease. Nutrition in Clinical Practice. 2022;37(2):239–55. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/ncp.10849. Cited 17 Nov 2023
Fouda E, Alhusseiny A, Gamal Y, Mujahed A, Elbeblawy N, Toaima D, et al. Nutritional assessment and nutritional rehabilitation in children with bronchiectasis and childhood interstitial lung diseases (ChILD): effects on pulmonary functions and clinical severity. QJM: An International J Med. 2018;111(suppl_1). Available from: https://doi.org/10.1093/qjmed/hcy200.129. Cited 17 Nov 2023
Understanding the Cost of Achieving the Sustainable Development Goals by Dana Lauren Vorisek, Shu Yu :: SSRN. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3545657. Cited 27 Nov 2023
Nestlé Group marketing spend 2022 | Statista. Available from: https://www.statista.com/statistics/685708/nestle-group-marketing-spend/.Cited 1 Mar 2024
Annual Reports & Proxy Information. Available from: https://www.pepsico.com/investors/financial-information/annual-reports-proxy-information. Cited 29 Nov 2023
UN Convention on the Rights of the Child - UNICEF UK. Available from: https://www.unicef.org.uk/what-we-do/un-convention-child-rights/. Cited 27 Nov 2023.
Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. The Lancet. 2008;371(9610):417–40. Available from: http://www.thelancet.com/article/S0140673607616936/fulltext. Cited 18 Nov 2023 Nov 18
Kristjansson EA, Gelli A, Welch V, Greenhalgh T, Liberato S, Francis D, et al. Costs, and cost-outcome of school feeding programmes and feeding programmes for young children. Evidence and recommendations. Int J Educ Dev. 2016;48:79–83.
Abizari AR, Buxton C, Kwara L, Mensah-Homiah J, Armar-Klemesu M, Brouwer ID. School feeding contributes to micronutrient adequacy of Ghanaian schoolchildren. Br J Nutr. 2014;112(6):1019–33. Available from: https://pubmed.ncbi.nlm.nih.gov/24990068/. Cited 20 Nov 2023
Megatrends in the agri-food sector: global overview and possible policy response from an EU perspective | Think Tank | European Parliament. Available from: https://www.europarl.europa.eu/thinktank/en/document/IPOL_STU(2019)629205. Cited 20 Nov 2023
Wijerathna-Yapa A, Pathirana R. Sustainable agro-food systems for addressing climate change and food security. Agriculture 2022;12(10):1554. Available from: https://www.mdpi.com/2077-0472/12/10/1554/htm. Cited 20 Nov 2023
Agostoni C, Baglioni M, La Vecchia A, Molari G, Berti C. Interlinkages between climate change and food systems: the impact on child malnutrition—narrative review. Nutrients 2023;15(2):416. Available from: https://www.mdpi.com/2072-6643/15/2/416/htm. Cited 20 Nov 2023
Micha R, Coates J, Leclercq C, Charrondiere UR, Mozaffarian D. Global Dietary Surveillance: Data Gaps and Challenges. [Internet]. 2018 Feb 25 [cited 2023 Nov 18];39(2):175–205. Available from: https://journals.sagepub.com/doi/full/https://doi.org/10.1177/0379572117752986
Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397(10282):1388. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7613170/. Cited 18 Nov 2023
de Quadros VP, Balcerzak A, Allemand P, de Sousa RF, Bevere T, Arsenault J, et al. Global trends in the availability of dietary data in low and middle-income countries. Nutrients. 2022;14(14). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9324857/. Cited 20 Nov 2023
Huybrechts I, Aglago EK, Mullee A, De Keyzer W, Leclercq C, Allemand P, et al. Global comparison of national individual food consumption surveys as a basis for health research and integration in national health surveillance programmes. Proc Nutr Soc. 2017;76(4):549–67. Available from: https://pubmed.ncbi.nlm.nih.gov/28803558/. Cited 20 Nov 2023
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Ramiyya Tharumakunarajah and Alice Lee wrote the original manuscript and updates, Ian P Sinha conceptualized the review and supervised writing, Daniel B Hawcutt and Nicola L Harman reviewed and edited the draft manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Ramiyya Tharumakunarajah, Alice Lee, Ian P Sinha, Daniel B Hawcutt, and Nicola L Harman have no conflicts of interest to declare.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tharumakunarajah, R., Lee, A., Hawcutt, D.B. et al. The Impact of Malnutrition on the Developing Lung and Long-Term Lung Health: A Narrative Review of Global Literature. Pulm Ther 10, 155–170 (2024). https://doi.org/10.1007/s41030-024-00257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-024-00257-z