Abstract
Introduction
The objective of this Delphi study was to understand and assess the level of consensus among respiratory experts on the clinical application of GOLD 2023 recommendations in management of patients with chronic obstructive pulmonary disease (COPD).
Methods
The study comprised two online surveys and a participant meeting with 34 respiratory experts from 16 countries. Responses of 73 questions were recorded using a Likert scale ranging from 0 (disagreement) to 9 (agreement). The consensus threshold was 75%.
Results
Survey 1 and survey 2 had 34 and 32 participants, respectively; and 25 attended the participant meeting. Consensus was reached on survey 1: 28/42; survey 2: 18/30 close-ended questions. A consensus was reached on the clinical relevance of most updates in definitions and diagnosis of COPD. Mixed results for the treatment recommendations by GOLD were noted: 74% agreed with the recommendation to initiate treatment with dual bronchodilators for group E patients; 63% agreed for including inhaled corticosteroids (ICS)/long-acting β2 agonist(LABA)/ Long-acting muscarinic receptor antagonists (LAMA) as a treatment option for GOLD B patients. Also, consensus lacked on removing ICS + LABA as an initial therapeutic option, in countries with challenges in access to other treatment option;. 88% agreed that they use GOLD recommendations in their daily clinical practice.
Conclusions
This Delphi study demonstrated a high level of consensus regarding key concepts of GOLD 2023 report, with most participants favoring recent updates in definitions, diagnosis, management, and prevention of COPD. More evidence on the etiotype based management and treatment options for group B and E are required which could further strengthen clinical application of the GOLD report.
Plain Language Summary
The goal of this Delphi study was to understand and assess the level of alignment among the respiratory experts on the application of key changes and recommendations proposed by the GOLD 2023 report in their routine clinical practice for the management of patients with chronic obstructive pulmonary disease (COPD). There were two online surveys in this study, and experts from 16 countries (primarily focused on developing countries) were invited to participate. Using the Delphi method, expert representatives shared their insights with the aim of optimizing patient care. The alignment was assessed in six well-defined themes: 1) Overall view on GOLD/other recommendations; 2) Assessing patients with COPD; 3) Initial pharmacological treatment in patients with COPD; 4) Vaccination for patients with COPD; 5) Follow-up pharmacological treatment in patients with COPD; and 6) Survival evidence in patients with COPD. Participants expressed a high level of agreement regarding key concepts of the GOLD 2023 report, with most of them agreeing with recent updates in definitions, diagnosis, management, and prevention of COPD. The results also highlighted the need to publish GOLD reports in multiple languages and in a shorter, pocket-sized format to increase awareness and adaptation among healthcare providers.
Similar content being viewed by others
Why carry out this study? |
This Delphi study was conducted to understand and assess the level of consensus among respiratory experts on the clinical application of GOLD 2023 recommendations in management of patients with chronic obstructive pulmonary disease (COPD). |
The study aimed to understand the alignment of GOLD recommendations with the current clinical practice. |
What was learned from the study? |
Agreement was reached on the clinical relevance of most updates in definitions and diagnosis of COPD. Mixed opinions regarding the treatment recommendations by GOLD were noted. |
No agreement was reached removing inhaled corticosteroids with long-acting β2 agonist as an initial therapeutic option, in countries with challenges in access to other treatment options. Participants agreed that they use GOLD recommendations in their daily clinical practice. |
In this Delphi study, a high agreement level regarding key concepts of GOLD 2023 report was observed, with most experts agreeing with recent updates in definitions, diagnosis, management, and prevention of COPD. |
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of mortality and morbidity, accounting for 3.3 million deaths and 74.4 million disability-adjusted life years (DALYs) [1, 2]. While it was the sixth most common cause of death in the 1990s [3], it became the third major cause of death by 2019 [1]. Although COPD has received its due attention as a major public health problem since the late 1990s, a majority of health care providers believed that COPD was a self-inflicted disease, and were nihilistic about treating the patients who smoked [4].
To explore possible solutions, experts in COPD from across the globe met in Brussels in January 1997, including representatives of the National Heart, Lung and Blood Institute, USA (NHLBI) and World Health Organization (WHO). The idea of Global Initiative for Chronic Lung Disease (GOLD) was conceived in 1998 [5]. Since inception, GOLD strived to provide updated information on management and prevention of COPD [6]. The first GOLD report was published in 2001 and has undergone major revisions and multiple smaller annual updates [7]. In the latest 2023 report, GOLD updated the definitions of COPD, exacerbations, taxonomy, disease assessment, and classification [8]. The report described COPD as a heterogenous lung condition with different manifestations, etiopathology, and structural irregularities leading to airflow obstruction [8]. Two new terminologies, “pre-COPD” and preserved ratio impaired spirometry (PRISm), were introduced for patients not meeting spirometric criteria for COPD, but carrying higher risk of developing COPD [8].
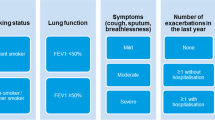
The recent update also refined the ABCD assessment tool, which was introduced in 2011 and modified in 2017 to exclude spirometry from severity grading system. To reflect the clinical implications of exacerbations independent of symptoms, former C and D groups were merged into a single group ‘E’ [8]. Thus, the initial and follow-up algorithms were simplified for early treatment optimization to reduce the risk of future exacerbations [8]. Triple therapy with long-acting beta (β2) agonist (LABA) + long-acting muscarinic antagonist (LAMA) + inhaled corticosteroid (ICS) has been recommended for patients with exacerbations and circulating blood eosinophil count (BEC) ≥ 300 cells/µl. Single inhaler triple therapy (SITT) is recommended to reduce mortality in patients at risk of exacerbations [8]. Finally, vaccination recommendations according to Centers for Disease Control and Prevention (CDC) guidelines, USA have also been included [8, 9].
Despite the high enthusiasm associated with the new report, there could be challenges in implementing these updates in clinical practice as noted previously [10]. Currently, there are limited data to demonstrate the extent to which pulmonologists apply GOLD 2023 recommendations in their day-to-day clinical practice. This Delphi study was conducted to understand and assess the level of consensus among respiratory experts about applying the key changes and recommendations by GOLD 2023 in real-life clinical practice, which would demonstrate the alignment between recommendations and clinical practice.
Methods
Study Design
The Delphi study included two rounds of cross-sectional surveys and an online participant meeting. The research team developed the survey questionnaire, which was administered by an independent vendor using Decipher software (version Compact = 153). Responses were analyzed after each round of survey using Microsoft Excel. Participants’ information was kept confidential and anonymous. The research complied with UK Data Protection law (GDPR), and the British Healthcare Business Intelligence Association’s (BHBIA) Legal and Ethical Guidelines [11]. Ethical committee approval was not required since this was a non-interventional physician survey.
Participants
Thirty-four respiratory experts were invited to participate from 16 countries representing different regional, geographic, sociocultural, and health system conditions (Argentina, Brazil, Chile, Colombia, Ecuador, Egypt, Guatemala, India, Indonesia, Mexico, Philippines, Saudi Arabia, South Africa, Thailand, Turkey, and Vietnam). Experts from developing countries were invited considering a lack of available data regarding the applicability of GOLD 2023 report in these countries. Countries were selected based on the availability of SITT and/or fixed dose LABA + LAMA combination, and the treatments recommended by GOLD 2023. Participants were identified in a non-random manner based on their expertise in COPD management, and were invited if they fulfilled four or more of the following criteria:
-
(i)
Key opinion leaders in COPD with > 10 years of clinical experience in the field;
-
(ii)
Researcher in COPD;
-
(iii)
Involved in diverse scientific activities related to COPD including speaker in national/international congresses;
-
(iv)
Membership in a respiratory society;
-
(v)
Member of an international and/or national COPD guidelines committee;
-
(vi)
Interested in improving COPD patient care.
Stages of the Delphi Procedure
Delphi Surveys and Participant Meeting
Survey 1 questionnaire was developed using a comprehensive literature review on the management of COPD, referring to GOLD 2023 report on COPD management. There were 14 open-ended and 42 close-ended Likert-style questions (Supplementary Material Table S3). Questions were stratified in six themes in relation to COPD management:
-
(i)
Overall view on GOLD/other recommendations;
-
(ii)
Assessing patients with COPD;
-
(iii)
Initial pharmacological treatment;
-
(iv)
Vaccination for patients with COPD;
-
(v)
Follow-up pharmacological treatment;
-
(vi)
Survival evidence in patients with COPD.
Survey 1 was conducted in May 2023; approximately six months after GOLD 2023 report was released, allowing adequate time for experts to understand and integrate the recommendations in their real-life practice. Online survey link was shared with participants via email. Respondents of survey 1 were invited to a virtual meeting to discuss the results, and to understand their opinion. The participant meeting was conducted in June 2023 and was moderated by two expert facilitators. A subsequent questionnaire was developed which contained questions that did not achieve consensus in survey 1 and those that were modified or added based on feedback from the participant meeting. Survey 2 was conducted in June 2023 and contained 31 questions (30 closed-ended, one open-ended, Supplementary Material Table S4). Questionnaires were validated by a non-participating respiratory expert.
Development of Consensus
A 0–9-point Likert scale was used to frame the questions and measure responses, ranging from 0 (strongly disagree) to 9 (strongly agree). These scores were divided into disagree (0–3), neither agree nor disagree (4–6) and agree (7–9).
Data Analysis
Completed questionnaires were considered for descriptive analysis. Mean, standard deviation (SD), median, and range of the collected data was presented. Consensus was indicated when 75% of the respondents scored 7–9 points (agreement range) and negative consensus was indicated when respondents scored 0–3 points (disagreement range) (Supplementary Material p 16). This was consistent with previous Delphi studies [12, 13].
Results
Participants
Thirty-four participants participated in survey 1 (response rate 100%), 25 (73.5%) attended the participant meeting, and 32 (94.1%) completed survey 2. Participants had a mean age of 54 years, an average 24.7 years of experience, and treated on an average 65 patients with COPD per month. Summary of discussion of the participant meeting is described in Supplementary Material (Table S1).
Delphi Survey Results
In survey 1, consensus was reached on 28 of the 42 close-ended questions. Fourteen close-ended questions for which consensus was not reached in survey 1 were discussed in the virtual participant meeting and repeated in survey 2 along with the other questions. In survey 2, consensus was reached on 18 of the 30 close-ended questions (60%). Survey questions reaching consensus are summarized in Table 1. Open-ended questions and participants’ responses are presented in Supplementary Material (Table S2).
Overall View on GOLD/Other Recommendations
In survey 1, 88% of the participants agreed that they used earlier GOLD recommendations (GOLD 2022 or earlier) in clinical practice. Participants agreed (82%) that GOLD 2023 recommendations were mostly followed by respiratory specialists in their countries and was the participants’ main reference document (85%). Participants also followed other local and regional guidelines (Supplementary Material p 5). Those who did not agree (3%) or remained neutral (12%) stated two main reasons: GOLD report is large, less user-friendly; and their countries lacked all the interventions stated in GOLD 2023 recommendations. There was no consensus (24% agreement) on the statement that GOLD 2023 recommendations were followed by primary care physicians (PCPs). Consensus was reached on the statement that introducing ‘PRISm’, and “young COPD” would challenge physician’s perception that COPD is a disease of older patients (82% agreement for both terminologies). Further, 85% of the participants agreed that the new definition of exacerbation was more clinically appropriate since it included symptoms, signs, and a timeframe. In survey 2, 84% of the participants agreed about the lack of evidence on prevention, assessment, treatment, and rehabilitation recommendations for different etiotypes of COPD, other than smoking cessation in the smoking related COPD, in GOLD 2023. Further, 75% of the participants agreed that new definition of COPD was relevant to their clinical practice; 97% agreed with the proposal to include risk factors, other disease involving multiple systems (88%), and the word ‘spirometry’ (78% agreement), in the existing definition of COPD to make GOLD 2023 appropriate to their clinical practice.
Participants agreed (94%) that introduction of the concept “pre-COPD” in GOLD 2023 would enable healthcare providers to identify patients at risk of developing persistent airflow obstruction. Furthermore, 84% participants agreed that “pre-COPD” would increase awareness among PCPs about COPD, similar to the concepts of prediabetes and pre-hypertension for diabetes and hypertension, respectively.
No consensus was reached when 69% of the participants agreed that initial management of all COPD patients was done by PCPs in their respective countries. Additionally, only 13% agreed (survey 2) that PCPs adhered to GOLD recommendations while 50% agreed that the revised taxonomy would change their practice (survey 2; Fig. 1).
Assessing Patients with COPD
In survey 1, most participants agreed (97%) to applying ABE classification for new patients with COPD, and to the merger of group C and D into group “E” (85% agreement). Consensus was reached (76% agreed) on using BODE index (Body mass index, Obstruction, Dyspnea, Exercise capacity) as a composite grading system to predict mortality risk. Consensus was reached regarding the need of a validated user-friendly tool for early patient recognition and identification of COPD exacerbations (88% agreed). In survey 2, 81% of the participants agreed to use CT imaging for assessing disease severity, however, access to computed tomography (CT) imaging was a challenge in their respective countries (78% agreed).
No consensus was reached for not including severity of airflow obstruction (forced expiratory volume (FEV1) in pharmacological treatment decision (survey 1–38%; survey 2–41%; Fig. 2).
Initial Pharmacological Treatment
Ninety-four percent of the participants preferred SITT over multiple inhalers. However, no consensus was reached (survey 1–62%; survey 2–69%; Fig. 3) for excluding ICS + LABA combination as an option for initial treatment. Main reasons stated by the participants included: widespread use of ICS + LABA in asthma and COPD, and better accessibility and affordability of ICS + LABA. Information regarding different groups of COPD patients is presented below:
-
a)
Group A: Consensus was reached to initiate treatment with one bronchodilator in patients with modified Medical Research Council (mMRC) 0–1 or COPD assessment test (CAT) score < 10 with and 0–1 moderate exacerbation (81% agreement); there were agreements on adding dual bronchodilators as therapeutic option for patients with one moderate exacerbation (81% agreement) or those with FEV1 ≤ 50% (94% agreement). Ninety-one percent of the participants agreed with the need to have more clinical studies in this population group.
-
b)
Group B: Participants agreed (94%) with the recommendation for using fixed LABA + LAMA combination. Consensus was not reached for exclusion of triple therapy as part of initial pharmacological treatment (62% agreed, survey 1; 66% agreed, survey 2, Fig. 3). Participants mentioned that patients might be at a higher risk of exacerbation and tend to lose lung function rapidly, and triple therapy could be useful in reducing the risk of exacerbation and future loss of lung function. No consensus was reached for considering triple therapy as a treatment option for patients with either one moderate exacerbation and high symptom score, or BEC ≥ 100 cells/µl for initial treatment (63% agreed, Fig. 3).
-
c)
Group E: 97% participants agreed to include triple therapy for initial management of patients with BEC ≥ 300 cells/µl. However, no consensus was reached (74% agreed, survey 1; 63% agreed, survey 2; Fig. 3) to initiate treatment with fixed LABA + LAMA for naive patients, either with ≥ 2 moderate exacerbations or ≥ 1 exacerbation leading to hospitalization. 94% participants agreed with the recommendation to use SITT over multiple inhaler therapy.
Responses on initial pharmacological treatment. CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease, GOLD global initiative for chronic obstructive lung disease, ICS inhaled corticosteroid, LABA long-acting beta-agonist, LAMA long-acting muscarinic antagonist, mMRC modified medical research council, Min. minimum, Max. maximum, SD standard deviation
Vaccination for Patients with COPD
In survey 1, consensus was reached regarding the need to offer vaccination to COPD patients against influenza (100% agreed), SARS-CoV-2 (97%), pneumococcal (97%), and herpes zoster for patients with COPD over 50 years (79% agreement). However, consensus was not reached for pertussis vaccination as participants indicated the lack of studies demonstrating evidence of benefits in COPD (74% agreed, survey 1; 69% agreed, survey 2; Fig. 4).
Follow-Up Pharmacological Treatment in Patients with COPD
In survey 1, a consensus was reached on GOLD recommendations to assess patient’s symptoms (dyspnea, exacerbations) for the follow-up pharmacological treatment, and the treatment algorithm should be applied to any patient who was on maintenance treatment, irrespective of initial ABE classification (82% agreement for both). All participants agreed (100%) to use LABA + LAMA for patients with consistent breathlessness or exercise limitation on bronchodilator monotherapy. Eighty-eight percent of the participants agreed to escalate the treatment to fixed LABA + LAMA for patients with BEC < 300 cells/µl and developing exacerbations under mono long-acting bronchodilator treatment. Most participants (88%) agreed that patients developing exacerbations under mono long-acting bronchodilator treatment, with BEC ≥ 300 cells/µl should be escalated to fixed LABA + LAMA + ICS. Likewise, 85% participants agreed to escalate to fixed triple therapy from LABA + LAMA treatment in patients developing exacerbations and having BEC ≥ 100 cells/µl. For patients who develop further exacerbations with LABA + LAMA as their initial treatment, and have BEC < 100 cells/µl, no consensus was reached for using roflumilast (if FEV1 < 50% of predicted and chronic bronchitis, 56% agreement in surveys 1 and 2), or azithromycin (preferentially in former smokers, 68% agreement–survey 1, 72% agreement–survey 2) (Fig. 5).
In survey 2, all participants (100%) agreed for the need to confirm the diagnosis of pneumonia in patients with COPD through CT scan or X-ray. Regarding triple therapy, there was a consensus (94% agreement) on two questions. First, the benefits of exacerbation reduction outweighed incremental risk of future pneumonia in patients at high-risk of exacerbations; and second, other risk factors for pneumonia such as BMI, age, FEV1 and comorbidities should be considered before withdrawal from an ICS based triple therapy.
Interestingly, no consensus was reached (survey 1–47%; survey 2–38%; Fig. 5) for withdrawing ICS from patients on stable triple therapy, developing pneumonia. Participants identified two episodes (53%), followed by one episode (32%), ≥ 3 (12%) and no episodes (3%) of pneumonia that would prompt them to consider ICS de-escalation (Supplementary Material p 6).
Survival Evidence in Patients with COPD
All questions reached consensus during survey 1. Seventy-nine percent of the participants agreed with reduced all-cause mortality associated with triple therapy in symptomatic patients with frequent exacerbation or one severe exacerbation. Most participants agreed (94%) with GOLD 2023 recommendation about non-pharmacological interventions such as smoking cessation and pulmonary rehabilitation, for reducing all-cause mortality in patients with COPD.
Discussion
This Delphi study assessed the opinion of a panel of international experts on clinical application of GOLD 2023 recommendations in COPD management. Consensus was reached on key aspects such as revised definition, ABE classification and new terminologies (pre-COPD and PRISm). Participants had differing opinions for initial and follow-up treatment recommendations, particularly for patients with higher symptom burden or elevated risk of exacerbations.
Similar to previous studies, participants highlighted lack of awareness for the recommendations, and availability of the report only in English and Spanish languages, whereas earlier reports were translated into Vietnamese, French, German, and Mandarin [10, 14, 15]. Participants suggested that shorter and user-friendly versions in local languages could be more effective for dissemination across PCPs.
GOLD 2023 report includes previously reported non-smoking COPD etiotypes like genetic factors, long-standing asthma, air pollution, smoke exposure, diet, and early childhood respiratory infections [8, 9, 16]. Participants agreed that these etiotypes cover the heterogeneity of chronic airflow obstruction, moving away from the idea that COPD is a single disease caused by tobacco smoking and required diagnostic criterion of COPD. However, the GOLD 2023 report only included interventions for smoking-induced COPD and lacked clarity for non-smoking-etiotype treatment. Thus, participants highlighted the need for further research for the prevention and management of these etiotypes.
No consensus was reached in either survey regarding GOLD 2023 recommendation for excluding FEV1 in the pharmacological treatment decision. Abnormal spirometry results are characteristic of COPD [17, 18]. According to ECLIPSE study, FEV1 severity poorly correlated with dyspnea and quality of life, and studies using decline in FEV1 for the assessment of COPD progression typically require ≥ 3 years [19]. General physicians still rely on FEV1 values to assess airflow obstruction and classify the disease severity [18]. Since GOLD grading of patient severity is based on FEV1 and it is also component of BODE index, more clarity is needed on its role in management of COPD.
Participants agreed that future studies were required to demonstrate benefits of triple therapy as treatment option for Group B patients, and to assess the association of higher symptom burden with exacerbation risk, which may require early intervention with triple therapy. Increased symptoms and CAT scores were associated with a higher risk of exacerbations [20]. Therefore, addition of triple therapy as an option in this group would be beneficial if comorbidities are well-controlled. The DEPICT study demonstrated long-term benefits of early initiation of triple therapy at an earlier stage of the disease [21]. Further, triple therapy also reduces the risks of mortality and exacerbations [22].
No consensus was reached for removal of ICS + LABA from the initial and follow-up treatment algorithms for dyspnea and exacerbations. Main reasons according to participants’ discussion were unavailability of all options in some countries, easy accessibility, and affordability of ICS + LABA versus LAMA/LABA or triple therapy, hence, PCPs initiate ICS + LABA for both, asthma, and COPD. Despite differing views, participants agreed to consider ICS + LABA in patients with low symptoms and BEC ≥ 300 cells/µl, if LABA + LAMA or triple therapy was not accessible. Similarly, studies recommended ICS + LABA for patients with asthma-COPD overlap [23, 24].
COPD treatment with ICS can be associated with increased risk of pneumonia in elderly, patients with dementia, malnutrition, and with BEC < 100 cells/µl [25]. Participants highlighted the importance of history and co-morbidities. Triple therapy should be given to patients with clinical indication, proper vaccination protocol and better control of comorbidities, thus preventing the development of other respiratory infections. Similarly, another Delphi study reported that in addition to ICS use, a patient with COPD was more likely to develop pneumonia if they had a history of smoking, diabetes, pneumonia, or exacerbations; older age, lower BMI, and severe airway obstruction [22].
Participants agreed with GOLD 2023 recommendation for vaccinating COPD patients with all, but pertussis vaccine. Elderly patients with COPD appear to be at higher risk of severe pertussis, and there is insufficient data describing trends of hospitalizations in such patients [26]. Further, addressing factors like vaccine cost, access and awareness could improve pertussis vaccine uptake in adults [26].
Participants disagreed on the inclusion of roflumilast or azithromycin in the treatment regimen for patients with COPD treated with LABA + LAMA who develop exacerbations with BEC < 100 cells/µl. While roflumilast was recommended if FEV1 was < 50% of predicted and chronic bronchitis, administration of azithromycin was recommended in former smokers. Participants were concerned regarding the side-effects of these two drugs, especially in aged patients, or those with cardiac comorbidities. Side-effects such as gastrointestinal symptoms and weight loss with roflumilast [27]; and hearing loss, antibiotic resistance, and arrhythmia with azithromycin have been reported [28].
This study not only identified the alignment and gaps in real-life clinical application of GOLD 2023 recommendations, but also provided possible solutions regarding awareness among PCPs, new taxonomy, fixed treatment combinations for different categories of COPD patients, and vaccinations of patients with COPD. These suggestions could be evaluated for clinical applicability before being considered for future GOLD report updates.
The clinical implications of this Delphi analysis include adopting the new concepts like pre-COPD and applying ABE classification to address gaps in patient care including early diagnosis and timely management of patients with COPD. Vaccinating patients with COPD reduces serious illness requiring hospitalization and prescribing triple therapy shows an overall benefit in symptomatic patients with frequent exacerbations. Important strengths of the study include the involvement of a large participant group from diverse geographies (16 countries) with a focus on developing countries. The Delphi procedure assumes that opinions of a group are more valid than that of an individual and the use of an independent facilitator and anonymous controlled feedback ensures robustness of the process. Previous respiratory studies suggest that a panel with at least 12 experts was sufficient to reach a meaningful consensus [13, 29, 30]. Another strength of this study was enhanced participant engagement as indicated by high response rate (survey 1, 100%; participant meeting, 73%; survey 2, 94%).
There are certain limitations to this study. All participants were respiratory experts specializing in COPD and may not represent non-specialists involved in COPD management. The study does not directly represent the views of experts globally. Absence of participants from other developing countries means that an expert panel involving them may not reach similar conclusions. Difference between accessibility and affordability of diagnostic tools and treatment among developed and developing countries could possibly result in different opinions. This geographical bias might limit the ability to generalize these findings. Therefore, the data should be interpreted accordingly.
Conclusions
This Delphi study aimed to understand the clinical applications of GOLD 2023 report. Consensus was reached on various recommendations including introduction of new concepts, treatment initiation and follow-up, vaccinating high-risk patients and suggestions to reduce all-cause mortality. Shorter documents published in local language can increase awareness among PCPs. The disagreements revealed complexities of COPD management and differences in the accessibility of diagnostic tests and treatment regimens. Further research is needed to assess new evolving concepts, including identification of optimal treatments for patients with different etiotypes, cutoffs of BEC to guide treatment decisions, and adding more treatment options for Group B and E patients with COPD.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. BMJ. 2022;378:e069679.
World Health Organization. Chronic obstructive pulmonary disease (COPD). 2018. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Accessed 03 July 2023.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349(9064):1498–504.
Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD resource network needs assessment survey. Am J Med. 2005;118(12):1415.e9-e17.
Pauwels R. Global initiative for chronic obstructive lung diseases (GOLD): time to act. Eur Respir Soc. 2001;18:901–2.
Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Global initiative for chronic obstructive lung disease: the changes made. Cureus. 2019;11(6): e4985.
Vogelmeier C, Criner G, Martinez F. Global initiative for chronic obstructive lung disease (GOLD) revisions 2001–2017: historical and critical perspective. Barcelona Respir Netw. 2017;3(3):151–65.
Global strategy for prevention, diagnosis and management of COPD: 2023 Report. 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf. Accessed 28 July 2023.
Tamondong-Lachica DR, Skolnik N, Hurst JR, et al. GOLD 2023 update: implications for clinical practice. Int J Chron Obstruct Pulmon Dis. 2023;18:745–54.
Albitar HAH, Iyer VN. Adherence to global initiative for chronic obstructive lung disease guidelines in the real world: current understanding, barriers, and solutions. Curr Opin Pulm Med. 2020;26(2):149–54.
Association BHBI. BHBIA legal and ethical guidelines for healthcare market research. Hertfordshire, UK: British Healthcare Business Intelligence Association 2020.
Miravitlles M, Soler-Cataluña JJ, Alcazar B, Viejo JL, Garcia-Rio F. Factors affecting the selection of an inhaler device for COPD and the ideal device for different patient profiles. Results of EPOCA Delphi consensus. Pulm Pharmacol Ther. 2018;48:97–103.
Domingo C, Garcia G, Gemicioglu B, et al. Consensus on mild asthma management: results of a modified Delphi study. J Asthma. 2023;60(1):145–57.
Alabi FO, Alkhateeb HA, Zibanayi MT, et al. The adherence to and utility of the global initiative for chronic obstructive lung disease guidelines for treating COPD among pulmonary specialists: a retrospective analysis. BMC Pulm Med. 2023;23(1):216.
Translated gold materials - global initiative for chronic obstructive lung disease. 2023. https://goldcopd.org/translated-gold-pocket-guides/. Accessed 28 July 2023.
Zeng G, Sun B, Zhong N. Non-smoking-related chronic obstructive pulmonary disease: a neglected entity? Respirology. 2012;17(6):908–12.
McNulty W, Usmani OS. Techniques of assessing small airways dysfunction. Eur Clin Respir J. 2014;1(1):25898.
Johns DP, Walters JA, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis. 2014;6(11):1557–69.
Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–73.
Adibi A, Sin DD, Safari A, et al. The acute COPD exacerbation prediction tool (ACCEPT): a modelling study. Lancet Respir Med. 2020;8(10):1013–21.
Singh D, Litewka D, Páramo R, et al. Delaying disease progression in COPD with early initiation of dual bronchodilator or triple inhaled pharmaco therapy (DEPICT): a predictive modelling approach. Adv Ther. 2023. https://doi.org/10.1007/s12325-023-02583-1.
Miravitlles M, Acharya S, Aggarwal B, et al. Clinical concepts for triple therapy use in patients with COPD: a Delphi consensus. Int J Chron Obstruct Pulmon Dis. 2023;18:1853–66.
Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–78.
Kaplan AG. Inhaled corticosteroid treatment in chronic obstructive pulmonary disease (COPD): boon or bane? J Am Board Fam Med. 2020;33(2):289–302.
Chen B, Liu W, Chen Y, et al. Effect of poor nutritional status and comorbidities on the occurrence and outcome of pneumonia in elderly adults. Front Med. 2021;8: 719530.
Hoe Nam L, Chiu C-H, Heo JY, et al. The need for pertussis vaccination among older adults and high-risk groups: a perspective from advanced economies of the Asia Pacific region. Expert Rev Vaccines. 2021;20(12):1603–17.
Pleasants RA. Clinical pharmacology of oral maintenance therapies for obstructive lung diseases. Respir Care. 2018;63(6):671–89.
Ahmadian S, Sin DD, Lynd L, Harrison M, Sadatsafavi M. Benefit–harm analysis of azithromycin for the prevention of acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2022;77(11):1079–87.
Bousquet J, Winchester C, Papi A, et al. Inhaled corticosteroid/long-acting beta(2)-agonist combination therapy for asthma: attitudes of specialists in Europe. Int Arch Allergy Immunol. 2012;157(3):303–10.
Sriprasart T, Siasoco MB, Aggarwal B, et al. The role of modeling studies in asthma management and clinical decision-making: a Delphi survey of physician knowledge and perceptions. J Asthma. 2023;60(9):1687–701.
Acknowledgements
The authors would like to thank all the participants who completed the surveys and contributed to discussions in the participants’ meeting, including: Ahmed Shawky Mohamed, Alberto Santos, Andrea Colli, Andres Pinto, Chu Thi Hanh, Dario Londono, Deddy Herman, Diego Litewka, Efrain Sanchez, Elvis Irusen, Ingrid Nunez, Kittipong Maneechotesuwan, Le Thi Thu Huong, Le Thuong Vu, Luisa Manrique, Mohamed Abdelhakim El Nady, Rafael Hernández Zenteno, Ramón Rojas, Romulo Uy, Rosemeri Maurizi, Sarai Toral, Suzana Tanni, Tomas Realiza, Venkata Nagarjuna Maturu and Wipa Reechaipichitkul. All the participants have provided consent to participate in the study and to be acknowledged in this publication.
Medical Writing/Editorial Assistance.
Medical writing and editorial support was provided by EVERSANA and was funded by GSK.
Funding
This study was funded by GSK. Meetings, data analysis, and medical writing assistance were funded by GSK. The study sponsor also funded the journals’ Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Antonio Anzueto: conceptualization, data curation, formal analysis, methodology, supervision, validation, visualization, writing—original draft, and writing—review & editing. Mark Cohen: methodology, validation, visualization, writing—original draft, and writing—review & editing. Andres L Echazarreta: methodology, validation, visualization, writing—original draft, and writing—review & editing. Gehan Elassal: methodology, validation, visualization, writing—original draft, and writing—review & editing. Irma Godoy: methodology, validation, visualization, writing—original draft, and writing—review & editing. Rafael Paramo: methodology, validation, visualization, writing—original draft, and writing—review & editing. Abdullah Sayiner: methodology, validation, visualization, writing—original draft, and writing—review & editing. CAT-D: methodology, validation, visualization, writing—original draft, and writing—review & editing. Sudeep Acharya: conceptualization, data curation, formal analysis, funding, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, and writing– review & editing. Bhumika Aggarwal: conceptualization, data curation, formal analysis, funding, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, and writing—review & editing. Hakan Erkus: conceptualization, data curation, formal analysis, funding, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, and writing—review & editing. Gur Levy: conceptualization, data curation, formal analysis, funding, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
Antonio Anzueto received funding for this study from GSK and consulting fees in personal capacity from GSK, AstraZeneca, Verona Pharma, Sanofi, Boehringer Ingelheim, Viatrix. Mark Cohen reports travel support and payment for lectures and presentations from AstraZeneca, Boehringer Ingelheim, GSK, Luminova and Novartis; payment for participation in advisory boards of AstraZeneca, Boehringer Ingelheim; and has participated in an unpaid capacity in committees for the Asoaciación Latinoamericana del Tórax (ALAT), and the American Thoracic Society (ATS). Andres L Echazarreta received funding from GSK for this study, payment for lectures, and meeting attendance from AstraZeneca, Kamada/Tuteur, Grifols; payment for participation in advisory boards from GSK, AstraZeneca; and payment for expert testimony from AstraZeneca. Gehan Elassal reports receiving honoraria from GSK, AstraZeneca, and Sanofi for lectures. Irma Godoy received funding from Sanofi for participation in advisory board and received honoraria from MSD for lecture presentation. Rafael Paramo reports receiving financial support to attend conferences and medical events from GSK, AstraZeneca, Chiesi, Novartis and Boehringer Ingelheim; consulting fees from GSK, Chiesi; and payment for participation in advisory board of GSK; and has participated in an unpaid capacity in committees for the Sociedad Queretana de neumologia y Cirugia de Torax AC and the Sociedad Mexicana de neumologia y Cirugia de Torax AC. Abdullah Sayiner received consulting fees or honoraria (for lectures and educational events) from GSK, Abdi Ibrahim, Pfizer, and Abbott. Carlos A Torres-Duque has received payment as advisory board participant or speaker from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, and Sanofi-Aventis; has participated in clinical trials from AstraZeneca, Novartis, and Sanofi-Aventis; has received unrestricted grants for investigator-initiated studies at Fundacion Neumologica Colombiana from AstraZeneca, Boehringer Ingelheim, GSK, Grifols and Novartis. Sudeep Acharya is an employee of GSK and holds shares in GSK. Bhumika Aggarwal is an employee of GSK and holds shares in GSK. Hakan Erkus is an employee of GSK and holds shares in GSK. Gur Levy is an employee of GSK and holds shares in GSK.
Ethical Approval
As this is a non-interventional physician survey based on a Delphi process gathering expert opinions without patient participation, no ethical approval was required for this study. Rights involved in the Delphi study were explained at the survey onset, and by returning the questionnaires, the participants gave their consent to take part in the study. These measures support the ethical principles of respect and the right of self-determination and of obtaining an informed consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Anzueto, A., Cohen, M., Echazarreta, A.L. et al. Delphi Consensus on Clinical Applications of GOLD 2023 Recommendations in COPD Management: How Aligned are Recommendations with Clinical Practice?. Pulm Ther 10, 69–84 (2024). https://doi.org/10.1007/s41030-023-00248-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-023-00248-6