Abstract
Chronic thromboembolic pulmonary disease (CTEPD) is characterized by unresolved clot burden in large pulmonary arteries, obstructive disease in smaller arteries, and increased downstream clot burden. This occurs in the setting of abnormal fibrinolysis or hematological disorders. Up to 50% of patients in some studies are unaware of a self-history of a deep venous thrombosis or pulmonary embolism. Ultimately, they present with symptoms of pulmonary hypertension (PH), which can result in right heart failure (RHF). Pulmonary endarterectomy (PEA) is curative, though many patients have prohibitive surgical risk or surgically inaccessible disease, warranting other interventions such as balloon pulmonary angioplasty (BPA) and medical therapy. Rarely, other treatment options may be implemented. We focus this review on PEA and BPA, with an overview of the history of CTEPD and the evolution of these procedures. We will briefly discuss other treatment modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic thromboembolic disease (CTEPD) is due to unresolved pulmonary emboli and can lead to devastating right heart failure. |
Advances in the surgical and medical fields have led to a more sophisticated approach in the treatment of chronic thromboembolic pulmonary hypertension (CTEPH). |
Pulmonary endarterectomy (PEA) is a complex procedure that can lead to complete resolution of CTEPH, and balloon pulmonary angioplasty (BPA) is reserved for those with either surgically inaccessible disease or with residual PH post PEA. |
Pharmacological options for the treatment of CTEPH are available and indicated in those with residual PH post PEA, or in conjunction with BPA. Some studies indicate a possible role for pre-BPA treatment with drugs, emphasizing the importance of a hybrid approach in those with surgically inaccessible disease or prohibitive risk. |
Alternative procedures such as Potts Shunt and pulmonary artery denervation are potential options in patients with distal disease but warrant further exploration. |
Introduction
Pulmonary hypertension (PH) is a chronic disease process that can result in devastating right heart failure (RHF), and is categorized into five groups based on the primary cause, as determined by the World Health Organization (WHO) classification system [1]. Chronic thromboembolic pulmonary hypertension (CTEPH), or WHO group IV PH, is a disease process that results from the pathological sequelae of thromboembolic events, and results in RHF and limiting dyspnea on exertion. Case reports of CTEPH were initially published in the first half of the twentieth century, and the disease was felt to be due to unresolved thrombosis in situ [2,3,4,5,6,7,8]. At the time, there was a newly developing understanding of the impact that thromboemboli and fibrin formation could have on the pulmonary vasculature [3, 9, 10]. These cases were consistent with pathology demonstrated during autopsy or animal specimens [3, 9, 10]. However, the lack of more sophisticated technology and pharmacological options limited the frequency of successful recognition and treatment.
The medical community has significantly evolved since that time, with various interventions made possible by the tenacity, research, and serendipity of another era. We have come to find that CTEPH impacts a higher percentage of patients than was previously reported [11, 12].
The pathophysiology of CTEPH is such that surgical intervention with pulmonary endarterectomy (PEA) is mostly curative. However, for inoperable patients, other procedures and medical interventions, primarily balloon pulmonary angioplasty (BPA), were developed and are safe and successful options [11, 13, 14].
We will review the history, definition, diagnosis, and pathology of CTEPH, and explore the nature of surgical and nonsurgical interventions, with a focus on PEA and BPA. We will briefly review unconventional procedures that may impact the vasoreactivity of the pulmonary vasculature.
History
Chronic thrombotic disease resulting in cor pulmonale was first described in the medical literature in the 1920s, and this was thought to be a rare cause of PH and RHF [2, 15]. Hypotheses regarding the underlying etiology included the development of thrombosis in situ at areas of damaged endothelium, and the inability to dissolve the clot. Physicians were resigned to the fact that it was a pathological diagnosis on autopsy.
The increasing association between these cases and a history of peripheral thrombosis prompted further investigation. Barnard described animal experiments inducing pathological changes in pulmonary arterial intima, with injection of fibrin emboli prepared in vitro from the animal’s own blood, confirming that emboli may be the primary cause of chronic thrombotic disease, at which point the term chronic thromboembolic disease (CTED) appears in the literature [9, 10].
By the 1950s, larger case series confirmed that pulmonary emboli are more common than thrombosis in situ [3, 4, 7]. In one case series, up to 50% of patients who died of RHF due to what was felt to be thrombotic pulmonary disease, in fact had a reported embolic event or deep venous thrombosis (DVT) as part of their medical history [3].
The idea of earlier detection of DVTs, and prevention of RHF and death, launched studies evaluating the best modalities for earlier diagnosis of thromboses, more aggressive anticoagulation with heparin, and expedited consideration of surgical intervention with PEA [3]. Eventually, diagnostic criteria was standardized, but not until the third World Health Organization symposium on pulmonary arterial hypertension, and again with the change in hemodynamic definition of PH [1, 16]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Definition
The accepted terminology is chronic thromboembolic pulmonary disease, or CTEPD, with the term CTEPH applied to anybody with resting pulmonary hypertension due to CTEPD [13, 14]. The diagnosis of CTEPH is made with the clinical combination of pulmonary arterial hypertension on right heart catheterization (RHC), along with imaging findings consistent with chronic embolic disease on ventilation/perfusion (VQ) scan and pulmonary angiography, after 3 months or more of treatment with oral anticoagulation to ensure resolution of any acute or subacute disease [17, 18].
Pulmonary arterial hypertension has been redefined as a mean pulmonary arterial pressure of above 20 mmHg, and with a pulmonary vascular resistance (PVR) above 2 Woods Units according to the new European Society of Cardiology definition of pulmonary hypertension [1].
Diagnosis
In patients with suspected PH, it is important to identify the possibility of CTEPD and determine if it is the primary cause of the PH. It is recommended that all patients being investigated for PH, undergo a radionuclide VQ scan as the initial screening test to identify CTEPD [19]. As a screening modality, it is inexpensive, limits exposure to intravenous contrast agents as well as radiation, and does not require advanced training to interpret the probability of having a pulmonary embolism (PE) [20].
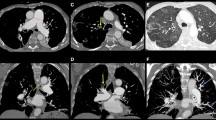
If there is a probability of PE, then pulmonary angiography is done to better classify the anatomy and distinguish between main, lobar, segmental, and subsegmental disease; this is the gold standard for determining the operability of CTEPH [19, 21]. Typically this is done with a right heart catheterization and direct injection of contrast dye to the main and subsegmental pulmonary arteries, with the use of digital extraction angiography [19] Fig. 1.
Pulmonary angiogram by right heart catheterization with digital extraction angiography. A Selective right pulmonary angiography. The main pulmonary artery and the proximal lobar arteries are significantly dilated. There is decreased perfusion noted in all the lobes. Proximal webs or bands are noted with distal pruning of the segmental vessels. B Selective left pulmonary angiography. The left main PA is significantly dilated. The left lower lobar artery was also dilated. There is decreased perfusion noted in the left lower lobe
Alternatively, computed tomography pulmonary angiogram (CTPA) can be done but only at a certified CTEPH center, as there are nuances to these modalities than can result in false-positive diagnoses, or false-negative diagnosis with a failure to recognize subtle pathophysiological changes resulting from CTEPD [19].
A multidisciplinary team including cardiac surgery, cardiology and pulmonary specialists, and radiology determine the severity of the disease, the patient’s surgical candidacy, other potential treatment options including BPA and oral pulmonary vasodilators, and the impact these interventions will have on the PH.
Risk factors for CTEPH include a history of pulmonary embolism in about 40%, deep venous thrombosis, antiphospholipid antibody syndrome, miscarriages, inflammatory bowel disease, permanent intravascular devices, and any other state promoting hypercoagulability and thrombus formation [13]. Differential diagnosis includes any tumors obstructing an artery that demonstrate similar mismatched defects on VQ imaging and findings with pulmonary angiography. Pulmonary hypertension due to other causes must be ruled out.
Clinical Pathology
It was postulated early on that fibrin deposits, formed by thromboemboli, result in neointimal remodeling and arteriopathy and this is well documented [3]. Subsequent studies have since confirmed that the difference between those patients with resolution of acute PE versus those who fail to consume the entire clot, is a disruption in endogenous fibrolysis [11]. When comparing the pathology of chronic PE with that of an acute PE, there is a noticeable absence of red cells and platelets, and instead an abundance of collagen, elastin, and sometimes calcification that results in a yellow plaque adherent to the vessel walls [17, 22]. A CTPA is highly sensitive for the visualization of bands and webs, which are the result of organization of this residual thrombotic material [11].
Demographics and Epidemiology
There is a reported incidence of about five cases per million people per year but it is thought that the true prevalence in the USA and Europe ranges between 30 and 50 cases per million [13, 23]. It has been observed in prospective studies, that at least 3.4% of patients with acute PE eventually develop CTEPH [17]. Based on the number of acute PE diagnoses, the incidence of CTEPH is likely three times more than is diagnosed, as reported in large European registries [17].
The US Registry includes about 50% female patients, a median age of 59 years, a median body mass index of 29.7 kg/m2, mostly WHO functional class III symptoms, and a median time from symptom onset to suspicion of CTEPH of 10.1 months [24]. There is a higher enrollment of Whites (65.6%) versus non-Whites (non-Hispanic Blacks 22.24%, Hispanics 5.9%, other 6.1%) in the US Registry. The rates of PEA in operable CTEPH are overall similar among the groups (Whites 75.8%; non-Hispanic Blacks 71.4%; Hispanics 90.9%; other 71.7%).
The European CTEPH Registry includes nearly 50% women, similar median ages when compared with men [62 versus 63 years], and less cardiovascular risk factors among women [25]. Women underwent PEA and other cardiovascular interventions such as coronary artery bypass grafting less often than men. The rate of refusal or surgically inaccessible disease was not statistically different between the sexes, implying a potential sex disparity in treatment. However, women have better long-term survival.
Several other registries, including the Pulmonary Hypertension Association Registry, which includes WHO group IV PH patients, exist with varying demographics depending on the country [26,27,28,29,30,31,32,33,34].
Overview of Therapy
Over the last three decades, there have been about 100 clinical trials attempting to treat CTEPH, including various drug studies, pulmonary endarterectomy, and balloon angioplasty. The first comparative study was published in 2003, reporting the surgical experience and outcomes of PEA at the University of California at San Diego, demonstrating that PEA can be curative [21]. The first drug studies were published in the early 2000s [35,36,37,38].
Currently, guidelines recommend the use of riociguat, an oral guanylate cyclase inhibitor, and treprostinil, a prostanoid, for inoperable CTEPH or residual CTEPH following PEA [13]. Subcutaneous prostanoids are preferred over intravenous as the indwelling catheters are felt to be thrombogenic, and may contribute to CTEPD. Other studies evaluating the efficacy of endothelin receptor antagonists and combination therapy are promising [39] Table 1. Additionally, all patients with CTEPH need lifelong anticoagulation with warfarin or non-vitamin K oral anticoagulants (NOAC) [13]. The standard of care for right heart failure includes the use of oral diuretics, and patients may require supplemental oxygen for hypoxemia.
Notably, with resolution of CTEPH, patients may no longer require diuretics or PH specific therapies. Discontinuation of these medications can be considered, though registry data is still unavailable for patients whose medications were discontinued following successful PEA or BPA [13].
Generally, all patients should be considered for surgical intervention with PEA, in addition to postsurgical BPA and medical management for residual CTEPD. Several factors influence the surgical candidacy of patients including their anatomy, surgical risks, and co-morbidities, thus guiding management of CTEPH.
Surgical Pulmonary Endarterectomy
History Pathologically, PEA is the only curative intervention and includes the removal of fibrous and obstructive tissue from the arterial tree. The first case series reporting pulmonary endarterectomies was published in 1965 [40]. Mortality following PEA for CTEPH was up to 50%, with significant rates of postoperative reperfusion edema and pulmonary hemorrhage, though not always associated with death [40, 41]. Since that time, technical advances and earlier identification has resulted in significantly improved outcomes, and has broadened patient selection to include those who may have once been inoperable due to the location of their obstructions or due to co-morbid diseases [21].
Indications Generally, in patients with surgically accessible disease, the gold standard treatment is PEA, with the use of BPA and oral medications for residual postoperative CTEPH. Surgically accessibly disease includes lesions in main, lobar, segmental, and subsegmental arteries. Depending on surgeon experience, more distal disease may be amenable to endarterectomy.
Every patient undergoing evaluation for CTEPH should be considered for potential PEA, even those with isolated distal disease, as some experienced centers may achieve further resolution of the disease depending on the pulmonary arterial anatomy. Importantly, a multidisciplinary team must determine the impact of the obstructive lesions on pulmonary hypertension, and in turn, the impact PEA will have on decreasing pulmonary pressures.
Contraindications Patients with contraindications or relative contraindications for cardiopulmonary bypass should be carefully considered for PEA since it requires cardiopulmonary bypass. Higher risk individuals, or those with neurological disease, recent stroke, recent myocardial infarction, bleeding diathesis, severe left heart failure, active infection, other parenchymal lung diseases, malignancy, recent trauma, and unrelated multiorgan failure may not benefit from PEA [42].
Preoperative care The goal of preoperative management in pulmonary hypertension patients is to complete surgery as quickly as possible, and to optimize RV function and fluid balance [43]. If a patient has diuretic resistance or needs inotropic support, this can be achieved with a few days of dopamine and intravenous diuretics. Interestingly, severe PH, with PVR approaching > 18 Woods Units, is associated with increased postoperative mortality, as is severe RV dysfunction. However, intravenous or inhaled prostacyclins to reduce RV afterload are not routinely used given the obstructive nature of the CTEPD [43]. Preoperative management with PH specific therapies can potentially delay the benefits offered by PEA, and there have been no studies to date that demonstrate a reduction in the potential surgical risks [43].
Anesthesia and technical overview After successful institution of cardiopulmonary bypass, the patients undergo hypothermic protocols in anticipation of circulatory arrest [21]. Following aortic cross-clamping, the first incision is made in the right pulmonary artery. Circulatory arrest is warranted due to bronchial back bleeding, which obscures visualization of the pulmonary arteries. A precise incision and the position of the patient are important for adequate visibility of the entire vascular tree. Gentle traction with forceps, while brushing away the outer vessel wall with a long miniature sucker, usually results in progressive withdrawal of the endarterectomy specimen [21].
Specimens are removed until a vessel branch is reached, at which point endarterectomy ensues on the next diseased branch. Proceeding in this order removes any potential obscuring lesions and more distal lesions remain accessible Fig. 2.
Deep hypothermic circulatory arrest is limited to 20-min intervals, with anticipation that unilateral endarterectomies are finished within this time period. Temporary neurological complications may occur with episodes of circulatory arrest lasting longer than 20 min [42].
Reperfusion can be initiated while the arteriotomy is closed, and surgery proceeds with any left-sided disease. During the rewarming period, other surgeries such as coronary artery bypass, are completed. Right ventricular remodeling occurs immediately postoperatively, with improvement in pulmonary pressures; therefore, tricuspid surgeries for tricuspid regurgitation are not routinely necessary [21].
Major adverse events These include sinus bradycardia and junctional rhythms, postoperative RV failure due to residual disease and persistent PH, reperfusion edema and lung injury, ongoing hypoxemia, and massive hemoptysis [43] Table 2. Patients have epicardial pacer wires placed intraoperatively to mitigate any postoperative arrhythmias. Reperfusion edema is described as a highly permeable pulmonary edema that occurs in the vascular territories that are being reperfused post PEA [43]. The incidence is as high as 30%, and typically occurs within the first 48 h. Preoperative pulmonary hypertension and the existence of post PEA residual disease are the two biggest risk factors in developing reperfusion edema. Avoiding higher cardiac outputs and aggressive intravenous diuresis are the mainstays of prevention and therapy for reperfusion edema [43].
Use of ECMO Veno–venous or veno–arterial extracorporeal membrane oxygenation (V–V or V–A ECMO) can be employed in instances of severe reperfusion edema and hemoptysis. Right ventricular failure and severe pulmonary hypertension postoperatively is associated with higher use of VA ECMO, and there are higher mortality rates in these patients when compared with those requiring VV ECMO support for reperfusion edema or hemoptysis in the absence of hemodynamically significant RVF [43].
Following successful PEA surgery, there are dramatic improvements in pulmonary vascular resistance, 6-min walk tests and functional status. Registry data reveals that at 1-year postoperatively there is a > 50% reduction in PVR, and > 20% improvement in walk distances [42]. Nevertheless, 30–50% of patients who undergo PEA, have residual pulmonary hypertension requiring possible BPA, and oral pulmonary vasodilators such as soluble guanylate cyclase stimulators [42].
If the endarterectomy was successful, postoperative pulmonary hypertension is attributed to secondary damage at the arteriolar and capillary levels, small vessel arteriopathy, or reversible factors such as hypercarbia and hypoxemia [21, 43]. If there was residual disease, then this too contributes to any remaining pulmonary hypertension warranting oral medical therapy [21, 43].
Recently published, single-center data analyzing the impact of socioeconomic status, race, and sex on PEA concluded that these did not adversely affect PEA outcomes and had no impact on postoperative mortality [44].
BPA Post PEA Patients may have anatomically mixed lesions, warranting both PEA and BPA. Case series have demonstrated an improvement in functional capacity and hemodynamics in those patients who underwent BPA in the post PEA time period including years later [13]. Most patients with residual CTEPH following PEA are treated with PH specific therapies in addition to possible BPA.
In those patients with surgically inaccessible disease, options for BPA and medical therapy should be offered to improve pulmonary hypertension, though there are not many studies evaluating the effects of BPA post PEA.
Balloon Pulmonary Angioplasty
History While PEA is the definitive curative therapy for CTEPH, there are up to 40% of patients with surgically inaccessible and thus inoperable disease, or who may be poor surgical candidates due to co-morbid illnesses [23]. First reported for the treatment of chronic pulmonary emboli in 1988, balloon pulmonary angioplasty developed as an alternative strategy for these patients [45]. Technical experiences gained in the congenital heart disease arena with dilatation of pulmonary stenosis, and improved guidewires and catheterization techniques allowed further refinement (Table 3) [46].
Indications Balloon pulmonary angioplasty is indicated in any patient with obstructions in the subsegmental arteries, primarily third-order branches, and with no lesions in the proximal pulmonary arteries [46]. Some subsegmental arteries are accessible with PEA so there is anatomical overlap [13]. Additionally, BPA is indicated in those patients who have a high surgical risk [46]. Pulmonary arteries are identified for BPA based on the presence of the following three features: filling defects, complete occlusions, or intravascular webs demonstrated on angiography.
Contraindications Patients who cannot undergo a RHC for any reason should be excluded. A relative contraindication is iodine contrast allergy, which would require pretreatment prior to each BPA session. Active infections, severe chronic obstructive lung disease, hypoxemia, hematological disorders prone to bleeding or clotting, and uncontrolled systemic hypertension, should all be taken into consideration prior to proceeding with a BPA.
Preoperative care Full anticoagulation must be maintained prior to the procedure as frequent interruption of anticoagulation can potentially provoke the development of pulmonary emboli. Ideally, euvolemic state for patient comfort and to optimize right ventricular hemodynamics is recommended. There is also emerging evidence for premedication with oral pulmonary vasodilators, with more significant reductions in PVR with a combination therapy versus BPA alone; see below [47, 48].
Technical overview Right internal jugular or right femoral venous access is obtained, and flexible soft tipped guidewires are directed into the pulmonary vasculature. Pig tail catheters placed into the region of interest allows for the direct delivery of contrast dye into the diseased vessel. Low-profile balloons are deployed at < 100% of the vessel diameter and < 50% of the stenotic lumen, for 1–5 s until the balloon fully expanded or until a fluoroscopic waist disappears [46, 49].
Lower lobe vessels are dilated initially, and in a sequential order, addition pulmonary arteries extending proximally, to ensure even distribution of reperfused territories. A high rate of reperfusion edema has prompted serial BPA protocols, to minimize the severity of edema, hemorrhage, and hypoxemia [46, 49,50,51].There is evidence that wire manipulation and balloon over dilatation are likely the main causes for lung injury and reperfusion edema [13]. The only clinical characteristic that is highly associated with reperfusion edema is a mean pulmonary arterial pressure of ≥ 35 mmHg, and it is not associated with the size of the vessel, age, or baseline cardiac index [46].
Other adverse events include pulmonary artery perforation, venous access site complications, and bleeding Table 3. The safety and efficacy of BPA improves over time with center and operator experience, underscoring the learning curve associated with technical and anatomical aspects of the procedure [52].
Balloon angioplasty improves pulmonary blood flow distribution and pulmonary vascular capacitance, and decreases right ventricular afterload [46]. Further, when compared with a control group of patients with distal CTEPH, Sugimura et al. demonstrated that mortality is significantly decreased following BPA when compared with those with distal CTEPH who did not undergo BPA [49] Table 4.
Kawakami et al. demonstrated that mean pulmonary arterial pressures significantly improved when compared with riociguat alone in inoperable CTEPH [47]. Similarly, the RACE trial randomized patients to medical therapy with riociguat versus BPA, and there were significant reductions in the BPA group compared with the medical therapy group, though adverse events were higher in those undergoing BPA [48]. In a 26-week ancillary follow-up, patients who remained symptomatic and with elevated PVR, underwent either BPA or medical therapy [48]. Those who were pretreated with riociguat and subsequently underwent BPA, compared with those who underwent BPA as first-line therapy, had statistically significant reductions in BPA-related complications, emphasizing the importance of a hybrid approach [48] Table 5.
Ultimately, the goal is to complete enough BPA procedures safely and effectively to achieve hemodynamic improvement and clinical improvement consistent with study data (Table 4). Additionally, BPA has resulted in the improvement in metabolic and systemic derangements associated with molecular pathophysiological cascades associated with RV dysfunction, mostly based on laboratory analysis preceding and following BPA [51, 53]. Patients who undergo BPA, in addition to a course of cardiac rehabilitation following their last BPA session, have statistically significant improvements in peak VO2, peak workload, quadriceps strength, and WHO functional class, and a tendency toward improved mental health-related quality of life (QOL) [54].
Pulmonary Artery Denervation
Pulmonary artery denervation (PADN) has been proposed as a potential treatment option in patients with residual pulmonary hypertension following PEA. Pulmonary arterial hypertension is characterized by vasoconstriction, and vascular remodeling modulated by baroreflexes [1]. Denervation occurs via radiofrequency ablation of the stretch receptors located at the bifurcation of the pulmonary arteries [1].
The TROPHY1 trial demonstrated a reduction in PVR, and an increase in 6 MWD and functional capacity in patients with WHO group 1 PAH on guideline-directed therapy [55]. Zhang et al. recently demonstrated similar results in both WHO groups 1 and 2 PH, with improvement in 6 MWD, reductions in PVR, improved RV metrics on transthoracic imaging, and decreased pro-brain natriuretic peptide (proBNP) [56, 57].
Before 2020, PADN had not been applied to CTEPH with residual disease. Romanov et al. demonstrated hemodynamic improvement following PADN in CTEPH patients. Twenty-five patients underwent PADN and another 25 were randomized to the SHAM MED group [58]. In the PADN group versus the MED SHAM group, there was a mean PVR reduction of 258 ± 135 dynes/cm5/s versus 149 ± 73 dynes/cm5/s, and 6 MWD was 470 ± 84 m versus 399 ± 116 m, respectively [58].
Potts Shunt in CTEPH
Similar to advancements made with BPA in the congenital heart arena, advancements have been made with the Potts procedure, a left pulmonary artery to descending aorta shunt that is now rarely implemented in the adult PH population, though increasingly recognized as a potential therapeutic option for PH [59,60,61].
Modified Potts Shunt is typically considered in patients with retractable idiopathic PAH, but has been applied to other forms of PH [61]. Patients with severe, refractory PH despite intensive management, RVF, and anatomic suitability, should be considered [59,60,61]. To our knowledge, there are no reports of Potts Shunt as a palliative measure in patients with CTEPH, though it would be a reasonable option in those with surgically inaccessible disease or prohibitive surgical risk. Notably, there have been no trials comparing the effectiveness of oral pulmonary vasodilators with palliative procedures such as TPS in the CTEPH population.
CTEPH Without PH
Finally, we must discuss those patients with known thromboembolic disease in the absence of pulmonary hypertension. There are no long-term data specifying the burden of disease that ultimately leads to the development of pulmonary hypertension, though some studies suggest a lower risk of progression in those with lower pulmonary pressures at the time of diagnosis [13]. In patients with CTEPD who have undergone PEA for extensive disease, the pathology was similar between those with and without CTEPH [13]. These patients, similar to those with CTEPH, should remain on lifelong anticoagulation with non-vitamin K anticoagulation or with warfarin.
Conclusions
Chronic thromboembolic pulmonary disease is a condition resulting in severe pulmonary hypertension, limiting symptoms, and can progress to right ventricular failure and death. Surgical PEA has been refined over the last several decades and is now the standard of care for surgically accessible disease in the right candidates, but BPA has too evolved in the last couple of decades as a nonsurgical strategy in addition to oral therapies in patients who cannot undergo surgery. Alternative procedures such as Potts Shunt and pulmonary artery denervation are potential options in patients with distal disease but warrant further exploration.
References
Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–731.
Davison PH, Armitage GH, McIlveen DJ. Chronic cor pulmonale due to silent pulmonary embolism. Lancet. 1956;271:224–6.
Barnard PJ. The pathology of cor pulmonale, pulmonary arteriosclerosis and pulmonary hypertension secondary to thromboembolism. Prog Cardiovasc Dis. 1959;1:371–9.
Hamer NA. Cor pulmonale from repeated pulmonary embolism. Postgrad Med J. 1958;34:19–23.
Durham JR, Ashley PF, Dorencamp D. Cor pulmonale due to tumor emboli; review of literature and report of a case. JAMA. 1961;175:757–60.
Lenegre J, Gerbaux A. Chronic cor pulmonale caused by thrombosis of the pulmonary arteries. Arch Mal Coeur Vaiss. 1952;45:289–314.
Lenegre J, Gerbaux A, Scebat L, Leconte Des Floris R. Four new cases of chronic pulmonary heart disease due to thrombosis of the pulmonary artery; clinical and hemodynamic study. Arch Mal Coeur Vaiss. 1955;48:1132–48.
WEEKLY clinopathological exercises. multiple tumor emboli of pulmonary arteries from carcinomatous mural thrombi of right side of heart with chronic cor pulmonale. N Engl J Med. 1953;248:777–84.
Barnard PJ. Pulmonary arteriosclerosis and cor pulmonale due to recurrent thromboembolism. Circulation. 1954;10:343–61.
Barnard PJ. Thrombo-embolic primary pulmonary arteriosclerosis. Br Heart J. 1954;16:93–100.
Simonneau G, Dorfmüller P, Guignabert C, Mercier O, Humbert M. Chronic thromboembolic pulmonary hypertension: the magic of pathophysiology. Ann Cardiothorac Surg. 2022;11:106–19.
Korkmaz A, Ozlu T, Ozsu S, Kazaz Z, Bulbul Y. Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost. 2012;18:281–8.
Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57:2002828.
Lang IM. Update on balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Curr Opin Pulm Med. 2022;28:369–74.
Castleman B, Bland EF. Organized emboli of the tertiary pulmonary arteries; an unusual cause of cor pulmonale. Arch Pathol (Chic). 1946;42:581–9.
Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913.
Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160112.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed). 2016;69:177.
Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62:D92–9.
Freeman LM, Stein EG, Sprayregen S, Chamarthy M, Haramati LB. The current and continuing important role of ventilation-perfusion scintigraphy in evaluating patients with suspected pulmonary embolism. Semin Nucl Med. 2008;38:432–40.
Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1500 cases. Ann Thorac Surg. 2003;76:1457–62 (discussion 1462-4).
Banks DA, Pretorius GV, Kerr KM, Manecke GR. Pulmonary endarterectomy: part I. Pathophysiology, clinical manifestations, and diagnostic evaluation of chronic thromboembolic pulmonary hypertension. Semin Cardiothorac Vasc Anesth. 2014;18:319–30.
Delcroix M, Kerr K, Fedullo P. Chronic thromboembolic pulmonary hypertension. Epidemiology and risk factors. Ann Am Thorac Soc. 2016;13(Suppl 3):S201–6.
Kerr KM, Elliott CG, Chin K, et al. Results from the united states chronic thromboembolic pulmonary hypertension registry: enrollment characteristics and 1-year follow-up. Chest. 2021;160:1822–31.
Barco S, Klok FA, Konstantinides SV, et al. Sex-specific differences in chronic thromboembolic pulmonary hypertension. Results from the European CTEPH registry. J Thromb Haemost. 2020;18:151–61.
Liu HY, Lu TP, Tao CW, et al. Incidence of chronic thromboembolic pulmonary hypertension in Taiwan. J Formos Med Assoc. 2021;120:1740–8.
Kearney K, Gold J, Corrigan C, et al. Chronic thromboembolic pulmonary hypertension in Australia and New Zealand: an analysis of the PHSANZ registry. Respirology. 2021;26:1171–80.
Kopeć G, Dzikowska-Diduch O, Mroczek E, et al. Characteristics and outcomes of patients with chronic thromboembolic pulmonary hypertension in the era of modern therapeutic approaches: data from the Polish multicenter registry (BNP-PL). Ther Adv Chronic Dis. 2021;12:20406223211002960.
Aldalaan AM, Saleemi SA, Weheba I et al. Chronic thromboembolic pulmonary hypertension in Saudi Arabia: preliminary results from the SAUDIPH registry. ERJ Open Res 2020;6:00218–02019.
Appenzeller P, Lichtblau M, Berlier C, et al. Disease characteristics and clinical outcome over 2 decades from the Swiss pulmonary hypertension registry. Pulm Circ. 2022;12: e12001.
Jansa P, Ambrož D, Kuhn M, et al. Epidemiology of chronic thromboembolic pulmonary hypertension (CTEPH) in the Czech Republic. Pulm Circ. 2022;12: e12038.
Valieva ZS, Martynyuk TV, Nakonechnikov SN, Chazova IE. Characteristics of patients with chronic thromboembolic pulmonary hypertension according to the Russian National Registry. Ter Arkh. 2021;93:1058–65.
Escribano-Subias P, Blanco I, López-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40:596–603.
Kozu K, Sugimura K, Ito M, et al. Current status of long-term prognosis among all subtypes of pulmonary hypertension in Japan. Int J Cardiol. 2020;300:228–35.
Alfieri O, Torracca L, Pardini A, et al. Pulmonary thromboendarterectomy in the surgical treatment of chronic thromboembolic pulmonary hypertension. First Italian experience. Cardiologia. 1995;40:561–5.
Kramm T, Mayer E, Dahm M, et al. Long-term results after thromboendarterectomy for chronic pulmonary embolism. Eur J Cardiothorac Surg. 1999;15:579–83 (discussion 583-4).
Ghofrani HA, Wiedemann R, Rose F, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136:515–22.
Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9.
Ghofrani HA, Simonneau G, D’Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5:785–94.
Moser KM, Houk VN, Jones RC, Hufnagel CC. Chronic, massive thrombotic obstruction of the pulmonary arteries. Analysis of four operated cases. Circulation. 1965;32:377–85.
Garvey JW, Wisoff G, Voletti C, Hartstein M. Haemorrhagic pulmonary oedema: post-pulmonary embolectomy. Thorax. 1976;31:605–9.
Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–10.
McGlothlin DP, Granton J, Klepetko W, et al. ISHLT consensus statement: Perioperative management of patients with pulmonary hypertension and right heart failure undergoing surgery. J Heart Lung Transplant. 2022;41:1135–94.
Su AY, Vinogradsky A, Wang AS et al. Impact of sex, race and socioeconomic status on survival after pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Eur J Cardiothorac Surg 2022;62:ezac364.
Voorburg JA, Cats VM, Buis B, Bruschke AV. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest. 1988;94:1249–53.
Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:10–3.
Kawakami T, Matsubara H, Shinke T, et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med. 2022;10:949–60.
Jaïs X, Brenot P, Bouvaist H, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med. 2022;10:961–71.
Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76:485–8.
Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99:1415–20.
Tatebe S, Sugimura K, Aoki T, et al. Multiple beneficial effects of balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2016;80:980–8.
Brenot P, Jaïs X, Taniguchi Y, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1802095.
Isobe S, Kataoka M, Kawakami T, Fukuda K. Adiponectin in chronic thromboembolic pulmonary hypertension. Circ J. 2018;82:1466–8.
Fukui S, Ogo T, Takaki H, et al. Efficacy of cardiac rehabilitation after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Heart. 2016;102:1403–9.
Rothman AMK, Vachiery JL, Howard LS, et al. Intravascular ultrasound pulmonary artery denervation to treat pulmonary arterial hypertension (TROPHY1): multicenter, early feasibility study. JACC Cardiovasc Interv. 2020;13:989–99.
Zhang H, Zhang J, Chen M, et al. Pulmonary artery denervation significantly increases 6-min walk distance for patients with combined pre- and post-capillary pulmonary hypertension associated with left heart failure: the PADN-5 study. JACC Cardiovasc Interv. 2019;12:274–84.
Zhang H, Wei Y, Zhang C, et al. Pulmonary artery denervation for pulmonary arterial hypertension: a sham-controlled randomized trial. JACC Cardiovasc Interv. 2022;15(23):2412–23.
Romanov A, Cherniavskiy A, Novikova N, et al. Pulmonary artery denervation for patients with residual pulmonary hypertension after pulmonary endarterectomy. J Am Coll Cardiol. 2020;76:916–26.
Esch JJ, Shah PB, Cockrill BA, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32:381–7.
Baruteau AE, Belli E, Boudjemline Y, et al. Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: updated data from the first 24 patients. Eur J Cardiothorac Surg. 2015;47:e105–10.
Grady RM, Eghtesady P. Potts shunt and pediatric pulmonary hypertension: what we have learned. Ann Thorac Surg. 2016;101:1539–43.
Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127–34.
Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29.
Sadushi-Kolici R, Jansa P, Kopec G, et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med. 2019;7:239–48.
Ogo T, Shimokawahara H, Kinoshita H et al. Selexipag for the treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J 2022;60:2101694.
Aoki T, Sugimura K, Nochioka K, et al. Effects of balloon pulmonary angioplasty on oxygenation in patients with chronic thromboembolic pulmonary hypertension—importance of intrapulmonary shunt. Circ J. 2016;80:2227–34.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author contributions
Hayah Kassis-George—writing original draft, review and editing, visualization, supervision, project administration. Candice Lee—review and editing. Mithun Chakravarthy—writing original draft, review and editing. Manreet Kanwar—visualization, supervision, project administration.
Disclosures
Dr. Hayah Kassis-George is a speaker for MERCK. Dr. Candice Lee—no disclosures. Dr. Mithun Chakravarthy—no disclosures related to this paper. Dr. Manreet Kanwar—no disclosures related to this paper.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kassis-George, H., Lee, C., Chakravarthy, M. et al. Surgical and Device Interventions in the Treatment of Chronic Thromboembolic Disease. Pulm Ther 9, 207–221 (2023). https://doi.org/10.1007/s41030-023-00217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-023-00217-z