Abstract
Hepatic hydrothorax (HH) represents a distinct clinical entity within the broader classification of pleural effusion that is associated with significant morbidity and mortality. The median survival of patients with cirrhosis who develop HH is 8–12 months. The diagnosis is typically made in the context of advanced liver disease and ascites, in the absence of underlying cardio-pulmonary pathology. A multi-disciplinary approach to management, involving respiratory physicians, hepatologists, and palliative care specialists is crucial to ensuring optimal patient-centered care. However, the majority of accepted therapeutic options are based on expert opinion rather than large, adequately powered randomized controlled trials. In this narrative review, we discuss the epidemiology, pathophysiology, clinical characteristics, and management of HH, highlighting the use of salt restriction and diuretic therapy, porto-systemic shunts, and liver transplantation. We include specific sections focusing on the role of pleural interventions and palliative care, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatic hydrothoraces are commonly right-sided transudative effusions that can be present even in the absence of demonstrable ascites. |
The mainstay of treatment is diuretic therapy, dietary salt restriction, and management of the underlying hepatic condition. |
Pleural interventions (e.g., thoracocentesis, or indwelling pleural catheter placement) are often required but carry significant morbidity. |
Multi-disciplinary management involving respiratory medicine, hepatology, and palliative care is crucial to optimizing patient-centered care. |
Indwelling pleural catheters have an acceptable safety profile and are commonly used in the context of palliation of symptoms relating to rapid accumulation of a hepatic hydrothorax. |
Introduction
Pleural medicine is increasingly recognized as a subspecialty within respiratory medicine. Over the last decade, there has been a proliferation of guidelines and trials focused on the management of various pleural conditions, including infections of the pleural space, malignant and non-malignant pleural effusions, pneumothoraces, and pleural malignancies (e.g., mesothelioma). The evidence base has enabled development of robust, patient-centered management options in, for example, malignant pleural effusion or pleural infection [1, 2]. However, some other conditions, such as hepatic hydrothorax (HH), are relatively understudied, and reviews of the evidence base can be helpful.
HH describes the presence of fluid in the pleural space (typically greater than 500 ml) secondary to liver failure, in the absence of underlying cardiac, renal, or pulmonary disease [3]. It forms part of the spectrum of non-malignant pleural effusions, which also encompasses effusions arising from cardiac failure, renal failure, rheumatological disease (such as rheumatoid arthritis), or any condition causing a non-specific pleuritis. The wide differential associated with non-malignant pleural effusions is beyond the scope of this review.
In the majority of cases, HH is associated with significant ascites; however, as will be outlined in the following discussion, patients may occasionally present without overt evidence of ascites. In such instances, the diagnosis of HH can be easily missed if not specifically considered within the broader differential of pleural effusions. Notably, while large volumes of ascitic fluid may be reasonably well tolerated by patients, significant breathlessness and hypoxia can develop following relatively modest (e.g., 1–2 L) fluid accumulation within the pleural space [4]. This presents specific challenges for the management of patients with HH, which may necessitate a combination of interventional and non-interventional therapeutic approaches.
In this article, we provide a narrative review of the available literature concerning the epidemiology, pathophysiology, and clinical manifestations of HH, including a discussion of the current therapeutic strategies employed in the management of this condition. This review is based solely on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Epidemiology and Clinical Context
Recent studies from the United Kingdom (UK) and the United States (US) have clearly demonstrated that the incidence of pleural diseases is on the rise [1, 5]. Non-malignant (or ‘benign’) pleural effusions, in particular, carry a significant healthcare burden. In a 2016 US analysis, Mummadi et al. reported approximately 43,000 emergency room attendances relating to pleural disease, with 361,270 hospitalizations occurring. Notably, non-malignant pleural effusions, including HH, accounted for 85.5% of these visits, alongside 63.5% of hospitalizations and 66.3% of 30-day readmissions [5]. While equivalent data do not exist for the UK or Europe, there is no reason to believe that the associated costs with regards to admission and treatment are dissimilar.
Retrospective case series suggest HH occurs in 5–16% of patients with cirrhosis and portal hypertension and is associated with high mortality [6, 7]. In a well-cited longitudinal study, Badillo and Rockey (2014) analyzed all patients with cirrhosis and portal hypertension in their institution over a 12-year period, finding 77 of 495 patients (16%) had HH; 44 of these 77 patients (57%) subsequently died over the next 12 months [6]. Additionally, Hung et al. analyzed 3487 patients with cirrhosis and pleural effusion requiring drainage in a Taiwanese dataset; after adjusting for comorbidities, the authors conclude that the presence of pleural effusion was associated with a significantly increased mortality at 3 years [7]. However, it was not possible to differentiate between specific causes of pleural effusion in this study and, as such, these data need to be interpreted with some caution.
Pathophysiology of Hepatic Hydrothorax
Pleural effusions develop when the rate of fluid accumulation within the pleural cavity exceeds the natural absorptive capacity of the pleural membrane to remove this fluid. For HH, this occurs in the context of liver cirrhosis and portal hypertension, and is frequently associated with the presence of significant ascites within the peritoneal cavity [6, 7]. A number of mechanisms have been proposed to underpin the development of HH (Fig. 1), including hypoalbuminemia, trans-diaphragmatic lymphatic drainage of ascitic fluid, and azygos vein hypertension [8, 9]. However, the most widely accepted explanation relates to the direct passage of ascitic fluid via small defects present within the tendinous structure of the diaphragm [10, 11]. Such defects (typically less than 1 cm in diameter) can give rise to a direct pleuro-peritoneal communication, facilitating the flow of ascitic fluid into the pleural cavity [12] (Table 1).
Diagrammatic representation of the proposed mechanisms underpinning the development of hepatic hydrothorax (HH). The thoracic cavity is represented in blue, while the abdominal cavity is represented in orange. The black line between the two cavities denotes the diaphragm. The proposed mechanisms are mentioned in white boxes and numbered 1 to 4, with ‘Direct passage through defects in the diaphragm’ being the most widely accepted one. Each mechanism involves the movement of fluid along a pressure gradient, either from the peritoneal cavity or systemic vasculature, into the pleural space, thereby giving rise to a HH
In a previous study utilizing video thoracoscopic examination, Huang et al. [13] proposed four distinct morphological types of diaphragmatic defect associated with HH (see Table 2). The flow of fluid through these resultant defects is unidirectional (i.e., into the pleural space), largely driven by an inherent negative intrathoracic pressure gradient in conjunction with raised intra-abdominal pressure secondary to accumulation of ascitic fluid. Several image-based studies have confirmed this pathophysiological process, for example through the injection of intra-peritoneal dye or technecium-labeled colloids [14, 15], demonstrating one-way migration into the pleural space. Nonetheless, it is important to recognize that HH can occur even in the absence of demonstrable ascites [16, 17]; this likely arises when the rate of fluid uptake in the pleural space (via the natural pressure gradient) matches, or exceeds, accumulation within the peritoneal cavity.
The intrinsic mechanism of HH formation may help to explain the right-sided predominance of pleural effusions associated with this condition (outlined further in the following section). Specifically, it is postulated that during embryological development, the left hemi-diaphragm assumes a more muscular composition than the right hemi-diaphragm, which in turn contains more collagenous fibers [12]; as a consequence, the left hemi-diaphragm appears more resistant to bleb formation and rupture—key components in the pathogenesis of HH. Moreover, it is possible that the close apposition of the liver to the right hemi-diaphragm serves to augment the migration of fluid into the right hemithorax in a piston-like manner [18].
Clinical Features
As previously discussed, HH typically presents in the context of decompensated liver disease, characterized by portal hypertension and ascites. Depending on the size of effusion, patients with HH may remain asymptomatic (e.g., with small and/or incidental effusions), or present with frank respiratory failure (e.g., with large effusions causing complete lung collapse); most commonly, patients experience non-specific symptoms, including dyspnea, cough, nausea, and pleuritic chest pain [6].
In their retrospective case series, Badillo and Rockey identified right-sided pleural effusions as the most frequent radiological abnormality (present in 73% of patients); by contrast, only 17% had left-sided effusions, while 10% had bilateral effusions, respectively. The right-sided predominance in HH has been repeatedly borne out in practice, with reports of up to 85% of cases localized to the right hemithorax [18]. Notably, while the majority of patients analyzed had concomitant ascites, roughly 9% had no evidence of ascites—a finding that is supported by numerous case studies [14, 19] and is consistent with the underlying pathophysiology outlined in the previous section.
Diagnosis
The diagnosis of HH should be suspected in patients with, or without, known cirrhosis and ascites presenting with unilateral (especially right-sided) effusions in the absence of underlying cardiac, pulmonary, or renal pathology. Pleural aspiration is essential to confirm the diagnosis, with fluid analysis classically indicating a transudative effusion according to Light’s criteria [19]. Nonetheless, it is recognized that some transudates may be incorrectly classified as exudates by this method, especially patients receiving long-term diuretic therapy. Consequently, Bielsa et al. have recommended the use of either the serum-pleural albumin gradient (threshold > 1.2 g/dl) or the pleural-serum albumin ratio (threshold < 0.6 g/dl) as a more accurate measure of transudative effusions, with the latter being more appropriate for diagnosis of HH [20,21]. Notably, pleural effusions in the context of liver cirrhosis can also present as a chylothorax [22]; however, this is often associated with the presence of chylous ascites and is easily distinguished from HH by its milky appearance and high triglyceride content. In cases of diagnostic uncertainty (e.g., in the absence of known liver disease or ascites) imaging techniques such as scintigraphy, color Doppler, and magnetic resonance imaging may be used to confirm the migration of fluid through the diaphragm [14, 15].
An important differential and potential complication of HH is the development of spontaneous bacterial empyema (SBEM), which has been reported in 10–16% of patients with HH [23]. There may be a history of fever and there may be features of spontaneous bacterial peritonitis (SBP) in patients with ascites. The differential cell count of pleural fluid can accurately distinguish HH and SBEM: an absolute neutrophil count (ANC) < 250 cells/mm3 is characteristic of HH, whereas an ANC > 250 cells/mm3 with positive fluid culture, or an ANC > 500 cells/mm3 with negative fluid culture, is diagnostic of SBEM [24]. The condition is usually managed with intravenous antibiotics and intravenous human albumin solution, though the role of pleural drainage is uncertain; mortality may be as high as 20–38%, emphasizing the need for prompt recognition and treatment [25].
Management of Hepatic Hydrothorax
The primary goal of HH treatment is centered on addressing the underlying pathophysiological mechanisms leading to excess fluid accumulation. A number of therapeutic strategies may be employed, ranging from simple dietary modifications and diuretic therapy to more invasive interventions, such as diaphragmatic repair, transjugular intrahepatic portosystemic shunts (TIPSS), and liver transplantation. This section provides an overview of these current available treatment options. The specific role of pleural interventions in the management of HH is also discussed.
Medical Management Options
The principles of initial management of HH in a patient with or without ascites remains the same, i.e., centered on sodium restriction and the use of diuretics. HH is initially managed by treating the underlying ascites, if present, through restriction of dietary sodium intake, aiming to consume no more than 5–6.5 g salt/day. Patients should receive nutritional input and should be advised with regards to a no-added-salt diet and the avoidance of pre-cooked meals [26]. It is also vital that any driver of ongoing decompensation of liver disease is addressed—for example, the promotion of abstinence from alcohol, or treatment of viral hepatitis.
When diuretic therapy is required, aldosterone receptor antagonists (e.g., spironolactone) are typically the first line of treatment. Spironolactone acts by preventing aldosterone from binding to receptor proteins in distal tubular cells, thereby preventing sodium reabsorption [27]. Spirinolactone is usually commenced at a starting dose of 100 mg/day, with sequential increases of 100 mg every 72 h to a maximum dose of 400 mg/day (although this dose is rarely tolerated). Furosemide (a diuretic acting in the loop of Henle) can be added in patients who do not respond to spironolactone monotherapy; this is usually commenced at an initial dose of 40 mg/day, increasing incrementally in steps of 40 mg to a maximum dose of 160 mg/day [28]. The aim of diuretic therapy is to induce a negative fluid balance, leading to a reduction in body weight of 0.5 kg/day in patients without peripheral edema and of 1 kg/day in patients with peripheral edema [29]. It is important that patients have their renal function monitored closely after commencing diuretics, since diuretic-induced renal impairment may occur when the rate of ascites (and/or hydrothorax) reabsorption is exceeded by the rate of diuresis, leading to intravascular volume depletion. This is usually reversible upon withdrawal of diuretic therapy [27]. In addition to diuretic therapy, intermittent large volume paracentesis (LVP) may be required for control of ascites, which may also benefit the management of HH. Intermittent LVP can also be performed in isolation if there are diuretic-induced complications.
Splanchnic and peripheral vasoconstrictors—such as terlipressin, octreotide, and midodrine—may be beneficial by increasing renal sodium excretion. Midrodine, an alpha-1 agonist, in addition to standard medical therapy has been shown to be superior to standard medical therapy with regards to controlling ascites when administered for 3 months. Midodrine increases mean arterial pressure and systemic vascular resistance and decreases plasma renin activity, although the numbers included in this study were small [29]. This may also aid hydrothorax control, although the optimum duration of therapy required to treat HH is unknown [29]. The role of these therapies in the management of HH requires further evaluation.
The role of intermittent albumin infusion has been studied in cases of refractory ascites, with variable results. Importantly, the ATTIRE study demonstrated that, in patients who were hospitalized with decompensated cirrhosis, albumin infusions to increase the serum albumin > 30 g/l did not prevent infection or reduce renal dysfunction, and were associated with more severe or life-threatening adverse events in the albumin group [30]. The ANSWER trial randomized patients with ascites to either standard medical therapy (SMT) or SMT with weekly administration of albumin, concluding that long-term administration of human albumin (HA) provides some survival benefit after 18 months [31]. Patients in the SMT plus HA group had a 38% reduction in mortality compared with the SMT group. Currently, however, there are no data available regarding the role of albumin infusions in the management of HH. Table 3 provides a summary of the principal treatment modalities employed in the initial medical management of HH.
Definitive Interventional Procedures
Transjugular Intrahepatic Portosystemic Shunt
Despite optimal medical therapy and often successful treatment of ascites, many patients will continue to experience considerable symptoms due to persistent hydrothorax, known as refractory hepatic hydrothorax (RHH). Such patients should be considered for a transjugular intrahepatic portosystemic shunt (TIPSS), which has been shown to relieve symptoms of RHH in 70–80% of patients [32]. This can be either a definitive procedure or be used as a bridge to liver transplant [26]. TIPSS aims to reduce portal venous pressure by forming a shunt between the portal vein (higher pressure system) and the hepatic veins (lower pressure system) [33], thereby reducing the portosystemic gradient. A systematic review of 198 patients who underwent TIPSS for RHH found that 55.8% of patients had a complete response, with an incidence of post-TIPSS encephalopathy of 11.7% and a 45 days mortality of 17.7% [34]. Another study reported that 82% of patients had some improvement in their hydrothorax following TIPSS, with a 64% 1-year survival rate [35]. Predictors of poor outcome following TIPSS for RHH include older age, severe underlying liver disease (as assessed by MELD or Child–Pugh score) and renal dysfunction. TIPSS should not be performed for HH without discussion with a local liver transplant center. General complications of TIPSS include hepatic encephalopathy (in 30–40%), heart failure and deterioration in liver function. Careful patient selection is therefore vital when considering the role of TIPSS in the management of HH. The contraindications to TIPSS in the management of RHH can be assumed to be the same for those patients with refractory ascites, and include: significant pulmonary hypertension; heart failure or severe cardiac valvular insufficiency; rapidly progressive liver failure; severe or uncontrolled hepatic encephalopathy; uncontrolled systemic infection or sepsis; unrelieved biliary obstruction; polycystic liver disease; and extensive primary or metastatic hepatic malignancy [35]. These contraindications are summarized in Table 4 below.
Liver Transplantation
Once a patient develops a HH, their suitability for liver transplantation should be determined as a priority due to the associated mortality rate described above. Goals of treatment include prevention of respiratory complications and infection until liver transplantation can be performed, and provision of symptomatic relief in patients who are and are not deemed liver transplant candidates. HH is a recognized indication for liver transplantation, which remains the most definitive treatment for this decompensating event. Patients must have a United Kingdom model for end-stage liver disease (UKELD) score of greater than 49 to be listed for liver transplant for HH. UKELD is a widely adopted scoring system that predicts mortality while awaiting liver transplant, based on the patient’s sodium, international normalized ratio (INR), creatinine, and bilirubin levels. A UKELD score greater than 49 predicts a 1-year mortality of 9% [36]. In one study of 28 patients whose indication for transplant was HH, there was no difference in ventilation, post-operative mortality, or long-term survival compared with a control group of patients transplanted for other indications [37]. Setsté et al. studied 11 patients who were transplanted for HH—none of these patients required thoracentesis following liver transplantation [38]. However, these studies are limited by relatively small patient numbers. Notably, in the US, patients listed for liver transplant for HH and meeting defined criteria are afforded additional priority on the waiting list due to the increased mortality associated with this decompensating event; no such prioritization scheme exists in the UK [39].
Pleural Interventions for Hepatic Hydrothorax
Interventions are central to the investigation and management of pleural disease. Routine procedures include pleural aspirations (the removal of approximately 50–150 ml of fluid from the pleural space for diagnostic purposes); therapeutic aspirations or thoracocentesis (the removal of larger volumes of air or fluid—usually no more than 1.5 l at a time—for symptomatic benefit); and intercostal chest drain insertion, either via the Seldinger technique or by blunt dissection, allowing removal of large volumes of air and/or fluid from the pleural space. More advanced procedures, such as medical thoracoscopy (the insertion of a camera into the pleural cavity after induction of an artificial pneumothorax to allow biopsies under direct visualization) or insertion of an indwelling pleural catheter (IPC) for symptomatic management of pleural effusions, can also be performed.
As outlined above, pleural fluid analysis of a potential HH will typically show a transudative effusion. If initial medical management with diuretics and salt restriction is unsuccessful, and/or there are persisting symptoms such as dyspnea (the main symptom resulting from a pleural effusion) [40], removal of pleural fluid is usually required.
Thoracocentesis
Thoracocentesis is often the first-line pleural intervention for symptomatic HH. In general, it has a very low risk of complications even in the presence of coagulopathy and thrombocytopenia when performed by experienced operators [41, 42]. However, patients with HH often re-accumulate fluid very rapidly; expert opinion therefore suggests that the pleural space should be fully drained, as the risk of re-expansion pulmonary edema is low [43]. The concurrent administration of human albumin solution, as discussed previously, has neither been studied nor is it routinely recommended [44].
Recurrent thoracocenteses are often performed and have been advocated in the 2020 American Association for the Study of Liver Diseases guidance on HH [45]. Nonetheless, it should be noted that these procedures are not without risk, which inevitably increases with repeated intervention. Shojaee et al. performed a retrospective analysis of serial thoracenteses and found a cumulative risk of complications (namely, pneumothorax and hemothorax) approaching 12% in patients with HH compared to a non-HH population [46].
Chest Drain Insertion and Pleurodesis
Importantly, several studies have also commented on the poor outcomes observed in patients with HH requiring intercostal chest drain insertion. Specifically, Yoon et al. studied patients with refractory HH and found that the 12-month mortality of those undergoing thoracocentesis (11 patients) was 18.2%, but in those requiring pig tail drainage (16 patients), this increased to nearly 90% [47]. Further retrospective studies conducted by Orman and Lok (27 patients with HH) [48] and Liu et al. (24 patients with HH) [49] reported 3-month mortality rates of approximately 40 and 27%, respectively, for those patients undergoing chest drain insertion. Moreover, a Taiwanese propensity matched study comprising 1278 patients with HH undergoing chest tube drainage and 1278 undergoing therapeutic thoracenteses revealed 30-day mortalities of 23.5 and 18.6%, respectively [50]. Together, these studies highlight the fragile nature of managing patients with HH through pleural intervention, where rates of complication and death remain high. Pleurodesis through talc slurry in patients with HH and tube drainage often fails due to the rate and volume of fluid production, although case reports attest to variable success [48]. Negative pressure suction to those patients to promote pleural apposition has been tried but not formally studied [51].
Video-Assisted Thoracoscopy and Diaphragmatic Repair
As outlined above, the pathophysiology of HH principally relates to the direct migration of ascitic fluid into the pleural cavity through diaphragmatic defects, most commonly within the right hemidiaphragm. Medical thoracoscopy can be used to interrogate the pleura for the presence of adhesions and non-expandable lung, diaphragmatic defects, and to explore the possibility of alternative diagnoses [52]. However, its use in HH is not widespread, and has not been formally evaluated. Diaphragmatic repair (with or without talc pleurodesis) may also be achieved via video-assisted thoracoscopy (VATS). The largest review of VATS ± pleurodesis in HH analyzed 180 patients and found a pooled pleurodesis rate of 72%; however, complication rates (fever, renal failure, pneumothorax, pneumonia, liver failure, pleural infection) were very high at 82% [53]. Additionally, Huang et al. reviewed 63 patients with HH who underwent VATS with diaphragmatic defect repair, reporting rates of successful resolution approaching 94% with a complication rate of 32% [54]. However, these studies represent single-center, retrospective analyses and, as such, the results may not be widely generalizable.
Indwelling Pleural Catheters
Indwelling pleural catheters (IPCs) are increasingly utilized in the long-term management of pleural effusions (typically in the context of underlying malignancy) and offer a patient-centered approach to care that can be successfully delivered within the community setting [55]. Several randomized clinical trials (discussion of which is beyond the scope of this article) have been performed, generating a robust evidence base for the development of clinical guidelines that support their use in malignant pleural effusions [56]. However, there is a growing body of research investigating the role of IPCs in addressing patient-centered symptoms associated with non-malignant effusions. Retrospective case series have shown pleurodesis success rates of 11 and 51% following IPC insertion [57,58,59,60,61,62], and also attest to the use of IPC as a bridge to liver transplant in refractory HH [58,59,60]. Infection rates were between 5 and 35%, with associated mortality (where reported) between 0 and 3.2% [57,58,59,60,61,62]. Notably, Walker et al. performed the only randomized controlled trial to date of IPCs in refractory transudative effusions [63]. In this study, 220 patients were screened, leading to the randomization of 33 patients to undergo IPC insertion and 35 patients to undergo serial thoracocenteses. The study did not recruit to its pre-specified targets and the underlying etiology was HH in 16 cases. There was no demonstrable difference in breathlessness (the primary outcome measure) between the two study arms over a 12-week period. Importantly, however, while patients in the IPC arm underwent fewer pleural procedures, they experienced a higher rate of complications. Thus, the insertion of IPC needs to be carefully balanced against the risks.
Palliative Care for Hepatic Hydrothorax
Patients with end-stage decompensated liver disease are increasingly recognized to have complex symptom needs, on par with those experienced by patients with cancer [64]. Principal barriers to accessing specialist palliative care services include uncertainty surrounding prognostication, as well as a perception that transplantation and disease-modifying treatments preclude referral [65]. Up to 15% of patients listed for transplantation are removed from the list or die while on the waiting list each year [66]; thus, the importance of supporting symptom control even while awaiting transplantation is clear. The need to consider an approach that enables discussion about best- and worst-case scenarios is important for patients with conditions that have high prognostic uncertainty, including those with HH, as part of managing their end-stage liver disease [67, 68]. This involves the active management and use of disease-modifying approaches, while also supporting patients and their families to prepare for a potentially rapid and unexpected deterioration. The general principles and priorities for managing palliative and end-of-life care for patients with end-stage liver disease have been described elsewhere [64, 65]; here, we focus on the cardinal symptom of dyspnea associated with HH.
When considering symptom management for patients with dyspnea due to HH, the principal approach is to first adopt interventions for symptom relief (e.g., diuresis, or intermittent thoracocentesis), as outlined above. Currently, there is no evidence for any specific treatment approach for HH when it is not possible to provide relief through intervention; a pragmatic approach is therefore recommended. Crucially, it is important to recognize that this patient group reports high symptom scores which are inadequately addressed [69]. Caution in prescribing medication such as opioids to aid symptoms of dyspnea is appropriate, given the reduced metabolism of various drugs in end-stage liver disease. However, this should not prevent patients from receiving appropriate symptom control. The British Association for the Study of Liver disease End of Life (BASL EOL) Special Interest Group have published guidelines for symptom control in advanced liver disease (https://www.basl.org.uk/) [70], but do not make specific recommendations for the management of breathlessness. The following table (Table 5) summarizes general principles for the management of dyspnea in advanced (non-malignant) disease [71] and may be adopted in patients with HH. Outcomes specifically for patients with end-stage liver disease, however, are yet to be determined.
The provision of psycho-social support for patients, relatives and those close to patients is essential to consider as part of multi-disciplinary team discussions, especially as this patient group are likely to have a high informal-carer burden [72].
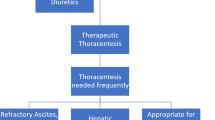
Figure 2 provides a summary of the various approaches to the management of HH, including primary medical treatments, the role of interventional procedures, and palliative care.
Conclusions
HH represents an important complication of end-stage liver disease and portal hypertension that presents challenges for management and is often associated with poor clinical outcomes. This review offers a summary of the pathophysiology, clinical manifestations, and treatment of HH, and highlights an important point that ascites does not necessarily need to be present to make the diagnosis. Management of HH should be based on patient needs and guided by a multi-disciplinary team approach. The mainstay of treatment includes dietary salt restriction and diuretic therapy, though pleural interventions are frequently required for additional symptomatic benefit. The presence of a refractory HH is an indication for liver transplantation (the most definitive treatment option) and is associated with significant morbidity and mortality; the use of TIPPS and IPC insertion may offer an effective ‘bridge’ to transplantation in select patients. Addressing the palliative care needs of affected individuals is central to the effective provision of patient-centered care.
References
Bodtger U, Hallifax RJ, et al. Epidemiology: why is pleural disease becoming more common? In: Maskell NA, Laursen CB, Lee YCG, et al., editors. Pleural disease (ERS monograph). Sheffield: European Respiratory Society; 2020. p. 1–1.
Bedawi EO, Guinde J, Rahman NM. Advances in pleural infection and malignancy. Eur Respir Rev. 2021;30(159): 200002.
Singh A, Bajwa A, Shujaat A. Evidence-based review of the management of hepatic hydrothorax. Respiration. 2013;86:155–73.
Thomas R, Jenkins S, Eastwood PR, et al. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med. 2015;21(4):338–45.
Mummadi SR, Stoller JK, Lopez R, et al. Epidemiology of adult pleural disease in the United States. Chest. 2021;160(4):1534–51.
Badillo R, Rockey DC. Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore). 2014;93:135–42.
Hung TH, Tseng CW, Tsai CC, et al. The long-term outcomes of cirrhotic patients with pleural effusion. Saudi J Gastroenterol. 2018;24(1):46–51.
Kiafar C, Gilani N. Hepatic hydrothorax: current concepts of pathophysiology and treatment options. Ann Hepatol. 2008;7(4):313–20.
Lazaridis KN, Frank JW, Krowka MJ, et al. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med. 1999;107(3):262–7.
Roussos A, Philippou N, Mantzaris GJ, et al. Hepatic hydrothorax: pathophysiology diagnosis and management. J Gastroenterol Hepatol. 2007;22(9):1388–93.
Zenda T, Miyamoto S, Murata S, et al. Detection of diaphragmatic defect as the cause of severe hepatic hydrothorax with magnetic resonance imaging. Am J Gastroenterol. 1998;93(11):2288–9.
Lv Y, Han G, Fan D. Hepatic hydrothorax. Ann Hepatol. 2018;17(1):33–46.
Huang PM, Chang YL, Yang CY, et al. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thorac Cardiovasc Surg. 2005;130(1):141–5.
Benet A, Vidal F, Toda R, et al. Diagnosis of hepatic hydrothorax in the absence of ascites by intraperitoneal injection of 99m-Tc-Fluor colloid. Postgrad Med J. 1992;68(796):153.
Ajmi S, Sfar R, Nouira M, et al. Role of the peritoneopleural pressure gradient in the genesis of hepatic hydrothorax. An isotopic study. Gastroenterol Clin Biol. 2008;32(8–9):729–33.
Kim JS, Kim CW, Nam HS, et al. Hepatic hydrothorax without ascites as the first sign of liver cirrhosis. Respirol Case Rep. 2015;4(1):16–8.
Rubinstein D, McInnes IE, Dudley FJ. Hepatic hydrothorax in the absence of clinical ascites: diagnosis and management. Gastroenterology. 1985;88(1 Pt 1):188–91.
Garbuzenko DV, Arefyev NO. Hepatic hydrothorax: an update and review of the literature. World J Hepatol. 2017;9(31):1197–204.
Sukcharoen K, Dixon S, Mangat K, et al. Hepatic hydrothorax in the absence of ascites. BMJ Case Rep. 2013;2013:bcr2013200568.
Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13.
Bielsa S, Porcel JM, Castellote J, et al. Solving the Light’s criteria misclassification rate of cardiac and hepatic transudates. Respirology. 2012;17(4):721–6.
Doerr CH, Allen MS, Nichols FC III, et al. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80(7):867–70.
Chen CH, Shih CM, Chou JW, et al. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011;31(3):417–24.
Xiol X, Castellote J, Baliellas C, et al. Spontaneous bacterial empyema in cirrhotic patients: analysis of eleven cases. Hepatology. 1990;11(3):365–70.
Xiol X, Castellví JM, Guardiola J, et al. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23:719–23.
Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9–29.
Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Int Ascites Club Hepatol. 1996;23(1):164–76.
European Association for the Study of the Liver. Clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60.
Singh V, Dhungana SP, Singh B. Midrodine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol. 2012;56(2):348–54.
China L, Freemantle N, Forrest E. A randomized trial of albumin infusions in hospitalised patients with cirrhosis. N Engl J Med. 2021;384:808–17.
Caraceni P, Riggio O, Angeli P, ANSWER Study Investigators, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417–29.
Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20(3):271–9.
Rossle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988–1000.
Ditah I, Al Bawardy BF, Saberi B, et al. Transjugular intrahepatic portosystemic stent shunt for medically refractory hepatic hydrothorax: a systemic review and cumulative meta-analysis. World J Hepatol. 2015;7(13):1797–806.
Tripathi D, Stanley AJ, Hayes PC, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69(7):1173–92.
Neuberger J, Gimson A, Davies M, Liver Advisory Group; UK Blood and Transplant, et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57(2):252–7.
Xiol X, Tremosa G, Castellote J, et al. Liver transplantation in patients with hepatic hydrothorax. Transpl Int. 2005;18:672–5.
Setsté T, Moreno C, Francoz C, et al. The impact of preoperative hepatic hydrothorax on the outcome of adult liver transplantation. Eur J Gastroenterol Hepatol. 2010;22(2):207–12.
Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74(2):1014–48.
Thomas R, Jenkins S, Eastwood PR, et al. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med. 2015;21(4):338–45.
McVay PA, Toy PTCY. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164–71.
Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10(4):336–41.
Feller-Kopman D, Berkowitz D, Boiselle P, et al. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656–61.
Gilbert CR, Shojaee S, Maldonado F, et al. Pleural interventions in the management of hepatic hydrothorax. Chest. 2022;161(1):276–83.
Banini BA, Alwatari Y, Stovall M, et al. Multidisciplinary management of hepatic hydrothorax in 2020: an evidence-based review and guidance. Hepatology. 2020;72(5):1851–63.
Shojaee S, Khalid M, Kallingal G, et al. Repeat thoracentesis in hepatic hydrothorax and non-hepatic hydrothorax effusions: a case-control study. Respiration. 2018;96(4):330–7.
Yoon JH, Kim HJ, Jun CH, et al. Various treatment modalities in hepatic hydrothorax: what is safe and effective? Yonsei Med J. 2019;60(10):944–51.
Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3(4):582–6.
Liu LU, Haddadin HA, Bodian CA, et al. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest. 2004;126:142–8.
Hung TH, Tseng CW, Tsai CC, et al. Mortality following catheter drainage versus thoracentesis in cirrhotic patients with pleural effusion. Dig Dis Sci. 2017;62(4):1080–5.
Shimbo A, Matsuda S, Tejima K, et al. Induced negative pressure proposed as a new method for diagnosing hepatic hydrothorax involving minor leaks. Clin Case Rep. 2014;2(6):296–302.
Jackson K, Johnston R, Mackay L, et al. A difficult pleural effusion in a cirrhotic patient. Breathe (Sheff). 2020;16(2): 200049.
Hou F, Qi X, Guo X. Effectiveness and safety of pleurodesis for hepatic hydrothorax: a systematic review and meta-analysis. Dig Dis Sci. 2016;61(11):3321–34.
Huang PM, Kuo SW, Chen JS, Lee JM. Thoracoscopic mesh repair of diaphragmatic defects in hepatic hydrothorax: a 10-year experience. Ann Thorac Surg. 2016;101(5):1921–7.
Davidson R, Carling M, Jackson K, Aujayeb A. Indwelling pleural catheters: evidence for and management. Postgrad Med J. 2022. https://doi.org/10.1136/postgradmedj-2021-141200.
Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839–49.
Bhatnagar R, Reid ED, Corcoran JP, et al. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax. 2014;69(10):959–61.
Chen A, Massoni J, Jung D, et al. Indwelling tunneled pleural catheters for the management of hepatic hydrothorax. A pilot study. Ann Am Thorac Soc. 2016;13(6):862–6.
Kniese C, Diab K, Ghabril M, et al. Indwelling pleural catheters in hepatic hydrothorax: a single-center series of outcomes and complications. Chest. 2019;155(2):307–14.
Shojaee S, Rahman N, Haas K, et al. Indwelling tunneled pleural catheters for refractory hepatic hydrothorax in patients with cirrhosis: a multicentre study. Chest. 2019;155(3):546–55.
Frost N, Ruwwe-Glo¨senkamp C, Raspe M, et al. Indwelling pleural catheters for non- malignant pleural effusions: report on a single centre’s 10 years of experience. BMJ Open Respir Res. 2020. https://doi.org/10.1136/bmjresp-2019-000501.
Li P, Hosseini S, Zhang T, et al. Clinical predictors of successful and earlier removal of indwelling pleural catheters in benign pleural effusions. Respir Int Rev Thorac Dis. 2019;98(3):239–45.
Walker SP, Bintcliffe O, Keenan E, et al. Randomised trial of indwelling pleural catheters for refractory transudative pleural effusions. Eur Respir J. 2022;59(2):2101362.
Roth K, Lynn J, Zhong Z, et al. Dying with end stage liver disease with cirrhosis: insights from support. Study to understand prognoses and preferences for outcomes and risks of treatment. J Am Geriatr Soc. 2000;48(1):122–30.
Esteban JPG, Rein L, Szabo A, et al. Attitudes of liver and palliative care clinicians toward specialist palliative care consultation for patients with end-stage liver disease. J Palliat Med. 2019;22:804–13.
Woodland H, Hudson B, Forbes K, et al. Palliative care in liver disease: what does good look like? Frontline Gastroenterol. 2020;11:218–27.
Kimbell B, Boyd K, Kendall M, et al. Managing uncertainty in advanced liver disease: a qualitative, multiperspective, serial interview study. BMJ Open. 2015;5: e009241.
Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates: “too well for transplant, too sick for life.” JAMA. 2006;295(18):2168–76.
Peng J-K, Hepgul N, Higginson IJ, et al. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med. 2019;33:24–36.
British association for the study of the liver. https://www.basl.org.uk/. Accessed 22 Apr 2022.
Chin C, Booth S. Managing breathlessness: a palliative care approach. Postgrad Med J. 2016;92:393–400.
Nguyen DL, Chao D, Ma G, et al. Quality of life and factors predictive of burden among primary caregivers of chronic liver disease patients. Annal Gastroenterol. 2015;28:124–9.
Acknowledgments
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
AA conceptualized the idea. All authors contributed to the drafting and revising of the manuscript, and agreed on the final submitted version.
Disclosures
Benjamin Pippard, Malvika Bhatnagar, Lisa McNeill, Mhairi Donnelly, Katie Frew, and Avinash Aujayeb have nothing to disclose.
Compliance with Ethics Guidelines
This review is based solely on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pippard, B., Bhatnagar, M., McNeill, L. et al. Hepatic Hydrothorax: A Narrative Review. Pulm Ther 8, 241–254 (2022). https://doi.org/10.1007/s41030-022-00195-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-022-00195-8