Abstract
Introduction
We compared in vitro drug delivery characteristics of two budesonide/formoterol dry powder inhalers, the Bufomix Easyhaler® and the budesonide/formoterol Turbuhaler®, at different patient air flow rates, and to test dose delivery from the Easyhaler in stressed conditions exposed to moisture, dropping, vibration and freezing/thawing.
Methods
A total of 36 inhalers from two batches of both products (160/4.5 µg strength) were used when comparing the effect of flow rate; six inhalers for each of the three flow rates for uniformity of delivered dose (DD), fine particle dose (FPD), mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). Consistency of DD, FPD, MMAD and GSD were determined for each inhaler at three different flow rates: 10th, 50th and 90th percentile air flows. Dosing properties as a function of inhaler life were tested. The effects of moisture, dropping, vibration and freezing/thawing on DD and FPD were tested with two to four inhalers per test using all three strengths of Easyhaler: 80/4.5, 160/4.5 and 320/9 µg.
Results
The Easyhaler, 160/4.5 µg, showed statistically significantly better dose consistency (expressed as percentage of labelled dose) at all three inhalation flows compared with the Turbuhaler, 160/4.5 µg, (p < 0.001 for three flow rates). Exposure to moisture, dropping, vibration and freezing/thawing did not affect DD or FDP. These results were similar to all three tested Easyhaler strengths.
Conclusion
In vitro performance of the Easyhaler indicates reliable dosing. Dose consistency was superior compared with the Turbuhaler at all tested flow rates. Environmental moisture, dropping, vibration and freezing/thawing did not affect the performance.
Funding
Orion Corporation Orion Pharma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhaler devices containing an inhaled corticosteroid (ICS) and a long-acting β2-agonist (LABA) are widely used for the treatment of specified patient groups with asthma and chronic obstructive pulmonary disease (COPD) [1]. The first combination inhaler on the market containing the ICS budesonide and the LABA formoterol fumarate dihydrate (hereafter formoterol) was the Symbicort® Turbuhaler® (AstraZeneca, UK). Recently, secondary entry products containing the same active substances have been developed, among them the budesonide/formoterol Easyhaler® (Bufomix Easyhaler®, Orion Corporation Orion Pharma, Finland). Regulatory approval of the budesonide/formoterol Easyhaler was based on the demonstration of therapeutic equivalence (lung dose and systemic exposure) with the budesonide/formoterol Turbuhaler [2,3,4].
A prerequisite for any inhaler device used for the treatment of airway diseases is that it should perform consistently, delivering a predictable and reproducible drug dose during repeated use, i.e. from the first to the last labelled dose. The inhaled particles should also have a size making them respirable, i.e. particles of aerodynamic size ≤5 µm [5]. Depending on the particle size distribution, the lung deposition may be central or more peripheral. Particles smaller than 1 µm are exhaled to a large degree [5]. Due to differences in construction and powder formulation, great variations in aerodynamic particle sizes have been reported for dry powder inhalers (DPI) [6]. Criteria for the predictability of inhaler performance are required for successful management of asthma and COPD.
The Easyhaler is a multi-dose reservoir-type DPI used to deliver a wide range of various medications, the latest addition being the budesonide/formoterol combination product. The budesonide/formoterol Easyhaler is available in three strengths: 80/4.5, 160/4.5 and 320/9 µg/inhalation.
The Easyhaler is inspiratory flow-driven and, therefore, its performance is dependent on the inspiratory effort produced by the patient, the built-in resistance of the inhaler, as well as the aerosolization geometry of the device [7]. It has been previously shown that with clinically relevant flow rates, delivered doses (DD) and fine particle doses (FPD) are rather independent of flow rates with the budesonide/formoterol Easyhaler and budesonide/formoterol Turbuhaler [3], although in vitro flow-dependent properties of, e.g., the Turbuhaler have been reported [8]. However, the inspiratory effort may vary considerably from one patient to another and, thereby, potentially affecting the clinical efficacy of the product.
A previous study determined the mean inspiratory flow rates through the budesonide/formoterol Easyhaler and Turbuhaler in patients with COPD and in children and adults with asthma [3]. For the Easyhaler, the flow rates were 55.8 (13.0) L/min [standard deviations (SD)] in patients with COPD, 61.0 (11.1) L/min (SD) in asthmatic children, and 63.7 (11.5) L/min (SD) in adults with asthma. For the Turbuhaler, the corresponding mean values (SD) were 72.1 (15.1), 76.5 (14.1), and 79.4 (14.4) L/min [3]. An air flow resistance of 0.032 kPa0.5 min/L was documented for the budesonide/formoterol Turbuhaler while the resistance for the budesonide/formoterol Easyhaler was 0.036 kPa0.5 min/L [3]. Thus, both the budesonide/formoterol Easyhaler and budesonide/formoterol Turbuhaler could be described as medium-resistance devices [5]. For comparison, a study by Delvadia et al. showed a resistance of the budesonide Turbuhaler of 0.035 kPa0.5 min/L and for the salbutamol Easyhaler of 0.0435 kPa0.5 min/L [9]. Thus, the difference between flow rates through inhalers depends on the difference in their intrinsic resistance [9].
In this study, we wanted to compare the uniformity of the in vitro DD of the budesonide/formoterol Easyhaler and Turbuhaler and to analyze some key in vitro characteristics of the products such as FPD, mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). Additionally, the dosing properties (DD and FPD) through the inhaler life were tested. We also exposed the Easyhaler to various stress tests such as moisture, dropping, vibration and freezing/thawing in order to assess the robustness of the inhaler in simulated real-life conditions.
Methods
Materials
Orion Corporation Orion Pharma provided the budesonide/formoterol Easyhaler inhalers. The Easyhaler is a multi-dose, breath-actuated DPI containing budesonide and formoterol fumarate with lactose as carrier.
For the comparative dose uniformity studies versus the Turbuhaler, the commercially available reference products, the budesonide/formoterol Turbuhaler inhalers containing 160/4.5 µg per dose of budesonide and formoterol (Symbicort®), were used. A total of 54 inhalers from two batches of each product were used when comparing the effect of flow rate.
For the test of dose uniformity over the lifetime of the Easyhaler inhaler, two batches and three inhalers per batch from all strengths, 80/4.5, 160/4.5 and 320/9 µg/inhalation, were used; i.e. 3 × 18 = 54 inhalers.
For the environmental exposure and robustness tests, three inhalers were used for determination of DD and three for FPD.
Methods
The Orally Inhaled Product (OIP) guideline [10] and Pharmaceutical Quality on the Inhalation and Nasal Products guideline [11] address the need to perform a product comparison using air flows that are characteristic for the targeted patient population. Flow rate dependency of The Easyhaler and Turbuhaler inhalers was assessed with flow rate ranges achieved by the study patient population of an earlier study [3]. The minimum (10th percentile), median (50th percentile) and maximum (90th percentile) flow rates were used for DD and FPD measurements and are shown for both products in Table 1. The figures represent the patient sub-group proportions within the whole indicated patient population, and then balancing of the achievable sub-group flow rates accordingly [3].
Delivered Dose
DD was determined by using the sampling apparatus and procedure described in the European Pharmacopoeia [12]. Four liters of air were drawn through the inhaler at a flow rate corresponding to a 4-kPa pressure drop across the device. The number of doses collected is specified in tests characterized below. Budesonide and formoterol fumarate collected in the sampling apparatus were dissolved with 50:50 (v/v) water:methanol and samples were analyzed by high-performance liquid chromatography (HPLC).
Fine Particle Dose
FPD (<5 µm) was determined using a next-generation impactor (NGI) equipped with a pre-separator according to the procedure described in the European Pharmacopoeia. For each FPD analysis, ten doses were discharged into the NGI at a flow rate corresponding a 4-kPa pressure drop across the inhaler. NGI stages were coated with DS-515 Dekati collection substrate spray in order to eliminate particle bounce and re-entrainment, which may distort impactor measurements. Then, the doses were collected in the NGI, and the powder deposited on the surfaces was quantitatively recovered with 50:50 (v/v) water:methanol and the samples were analyzed by HPLC. In every FPD analysis conducted, it was verified that the mass balance was within ±15% of the delivered dose result of the batch.
MMAD and GSD values were determined from the measurements at the flow rates shown in Table 1 and were calculated from the number of particles deposited at different stages of the NGI.
Delivered Dose and Fine Particle Dose Through the Easyhaler Lifetime
The aim was to determine DD and FPD as a function of dosing interval. DD analyses were done at the beginning, middle and end of the labelled number of doses. FPD analyses were done at the beginning and end of the labelled number of doses. The number of doses analyzed through container life is presented in Table 2.
Effect of Moisture
Delivered dose was analyzed from the first five doses. After the fifth dose, the inhalers without an aluminum laminate pouch were placed in a 30 °C/75% relative humidity (RH) storage condition for 48 h. After storage, the next five doses were analyzed. The FPD was analyzed from the first ten doses. After the tenth dose, the inhalers without an aluminum laminate pouch were placed in a 30 °C/RH75% storage condition for 48 h. After storage, the next ten doses were analyzed.
Effect of Dropping
The dropping test was carried out according to the principles described in the ISO/FDIS 20072:2009 standard by dropping inhalers on a wooden surface from one meter height [13]. For this test, two Easyhaler inhalers from one batch were used and the DD and FPD were analyzed. Measurements were done both in the beginning and at the end of the labelled number of doses. DD was determined from three doses before the inhaler was dropped. After dropping, the DD from three doses were analyzed again. In the beginning of the labelled number doses, FPD was analyzed before and after the drop from the same inhaler, while toward the end of doses, FPD was analyzed from a dropped inhaler and an inhaler which had not been dropped. For the inhalers 80/4.5 and 160/4.5 µg, the inhaler doses 111–120 were analyzed after dropping and without dropping. For the 320/9 µg inhaler, the doses 51–60 were analyzed in a similar way.
Effect of Vibration
The aim of the vibration test was to simulate inhaler transportation and especially the vibration caused by the transportation. The DD and FPD were determined after the products were exposed to vibration stress. The vibration test was carried out in the vertical axis according to the publication IEC 60068-2-64, test Fh (2008-04) [14]. The test duration was 60 min and the test level conditions were as follows: frequency range 5–500 Hz, acceleration spectral density (ASD) level 1 m2/s3 5–20 Hz, ASD level 3 decibels/octave 20–500 Hz, total spectral acceleration 0.9 g, and uncertainty of measurements 5%. The DD and FPD results after vibration were compared to DD and FPD results of inhalers from the same batch that had not been vibrated. Two batches and two inhalers per batch were studied.
Effect of Freezing/Thawing
For the freeze/thaw study, the budesonide/formoterol Easyhaler inhaler was subject to three freeze/thaw cycles over 2 weeks. The Easyhaler inhaler 160/4.5 µg/inhalation, 120 doses, was selected as it covers the product containing 60 doses. The product was kept in a freezer (temperature target: −20 °C; ± 5 °C) for 2 days (or 3 days on weekends) as such without the laminate package or in the laminate package. After the inhalers were removed, they were transferred into elevated temperature (25 ± 2 °C; 60% ± 5% RH) for 2 days (or 3 days on weekends). The samples were then removed and transferred into the freezer for 2 days and so on for a total of three freezing/thawing cycles. After the third freeze/thaw cycle, the FPD and DD were analyzed.
Reference samples inside a laminate pouch were kept in a 25 °C/60% RH condition for the same time as three freeze/thaw cycles (2 weeks). They were not subjected to freezing. After 2 weeks, FPD and DD were analyzed.
HPLC Quantitation of Budesonide and Formoterol Fumarate
Budesonide and formoterol fumarate was determined from all samples by using validated HPLC. The method used a Spherisorb ODS-1 column (5 µm, 4.0 × 250 mm) with a mobile phase consisting 0.015 M sodium dihydrogen phosphate monohydrate buffer (pH 2.1) and acetonitrile (50:50, v/v). Mobile phase was delivered at a flow rate of 1.5 mL/min and injection volume was 30 µL. UV detection at 214 nm and a run time of 10 min was used. Samples were dissolved in H2O:methanol (50:50, v/v). The quantitation limits of budesonide and formoterol fumarate were 0.5 and 0.02 µg/mL, respectively. The linearity of response of budesonide and formoterol fumarate was 0.5–100 and 0.02–2.5 µg/mL, respectively.
Statistical Analyses
Mean values and SD were calculated for the delivered doses from the budesonide/formoterol Easyhaler and Turbuhaler at the three different flow rates. The equality of variance was assessed for all different flow rates using Levene’s test because of the non-normality of the data with a significance level of 0.05. The null hypothesis of equal variances is rejected with p values less than the significance level and it is concluded that there is a statistically significant difference between the variances. All statistical analyses were performed with Minitab version 17 (Minitab Inc., State College, PA, USA).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was not applicable as there is no clinical data.
Results
Delivered Doses and Fine Particle Doses Over Patient Flow Rate Range
Figure 1 shows the DD of budesonide (Fig. 1a) and formoterol (Fig. 1b) at the three different flow rates expressed as a percent of the labelled doses delivered from the budesonide/formoterol Easyhaler and Turbuhaler. The variability in dose delivery from the Easyhaler was statistically significantly smaller than from the Turbuhaler for both budesonide and formoterol and this was true at all three flow rates.
a, b Dose delivery of budesonide (Fig. 1a) and formoterol (Fig. 1b) from two budesonide/formoterol multi-dose DPIs, the Bufomix Easyhaler® and the Symbicort® Turbuhaler® (160/4.5 µg) at three different flow rates. The delivered dose is expressed as a percent of the nominal labelled dose. Each data print represents a single dose actuation
For budesonide at a 10th percentile air flow, the mean delivered dose (SD) was 159 (14) µg/inhalation for the Easyhaler and 148 (24) µg/inhalation for the Turbuhaler. The p value for the difference was p = 0.004. For the median percentile air flow, the corresponding figures were 169 (14) µg/inhalation for the Easyhaler and 155 (39) for the Turbuhaler (p < 0.001). For the 90th percentile air flow, the figures were 175 (13) µg/inhalation for the Easyhaler and 153 (35) µg/inhalation for the Turbuhaler (p < 0.001).
For formoterol at a 10th percentile air flow, the mean delivered dose (SD) was 4.1 (0.4) µg/inhalation for the Easyhaler and 4.1 (0.7) µg/inhalation for the Turbuhaler. The p value for the difference was p = 0.002. For the median percentile air flow, the corresponding figures were 4.6 (0.4) µg/inhalation for the Easyhaler and 4.3 (1.0) for the Turbuhaler (p < 0.001). For the 90th percentile air flow, the figures were 4.7 (0.4) µg/inhalation for the Easyhaler and 4.2 (1.0) µg/inhalation for the Turbuhaler (p = 0.001).
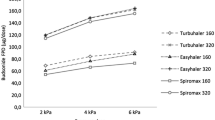
Figure 2 shows the DD (Fig. 2a) and the FPD (Fig. 2b) of the three strengths of the budesonide/formoterol Easyhaler at the three different flow rates [the minimum (10th percentile), median (50th percentile) and maximum (90th percentile) flow].
Delivered Doses and Fine Particle Doses Throughout the Budesonide/Formoterol Easyhaler Inhaler Lifetime
Figure 3a shows the DD of the three budesonide/formoterol Easyhalers taken in the beginning (doses 1–3), at the middle and at the end of the Easyhaler lifespan when the first doses are set to 100%. Overall, DD varied from 94% to 103% for the three presentations at the middle and at the end of the Easyhaler content. Fig. 3b shows FPD of doses at the beginning and at the end of the Easyhaler.
It is concluded that the influence of the number of actuations had only a slight effect on product properties. Additionally, DD and FPD show that patients can inhale the correct dose throughout the inhaler life.
Mass Median Aerodynamic Diameter and Geometric Standard Deviation
MMAD values (µm) and GSD for the budesonide/formoterol Easyhaler and budesonide/formoterol Turbuhaler at the different flow rates are presented in Table 3. Higher flows resulted in lower MMADs for both inhalers when going from the 10th percentile of flow rates to the 90th percentile. For budesonide, MMAD decreased for the Easyhaler from 2.5 to 2.2 µm and for the Turbuhaler, from 2.3 to 2.0 µm. The corresponding values for formoterol were from 3.0 to 2.7 µm for the Easyhaler, and from 2.7 to 2.5 µm for the Turbuhaler. GSDs of the particle size distributions were the same between the products through the different flow rates.
Delivered Doses and Fine Particle Doses after Dropping, Vibration or Freezing/Thawing
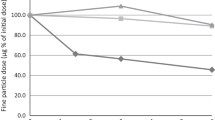
The effect of environmental moisture (30 °C/75% RH) on DD of the three strengths of the budesonide/formoterol in Easyhaler is shown in Fig. 4a. With the initial value set to 100%, the deviations from 100% after exposure were minor. The effect on FPD is shown in Fig. 4b.
Dropping the Easyhaler from one meter height did not result in changes in DD of budesonide or formoterol (Fig. 5). The DD of three doses in the beginning of the inhaler life before and after dropping is shown in Fig. 5a and at the end of the inhaler life, in Fig. 5b. Dropping did not influence DD of either budesonide or formoterol.
The results of the vibration tests are shown in Fig. 6a (DD) and Fig. 6b (FPD). When the DD without vibration was set to 100%, the deviations for the three doses of the budesonide/formoterol Easyhaler varied from 99% to 103% for budesonide and from 100% to 104% for formoterol. The corresponding values for FPD were 97–104% for budesonide and from 100% to 107% for formoterol. Thus, vibration did not affect DD or FDP of the budesonide/formoterol Easyhaler.
a–d Effect of vibration on delivered dose (Fig. 6a) and fine particle dose (Fig. 6b) of 80/4.5, 160/4.5 and 320/9 µg in the budesonide/formoterol Easyhaler. Effect of freezing/thawing on delivered dose (Fig. 6c) and fine particle dose (Fig. 6d) of 80/4.5, 160/4.5 and 320/9 µg in the budesonide/formoterol Easyhaler
The results of the freezing/thawing tests are shown in Fig. 6c (DD) and Fig. 6d (FPD). When the reference values are set to 100% (the 160/4.5 µg Easyhaler product), the DD values for budesonide after the freezing/thawing procedure were 98% for the inhaler as such and 99% for the laminated package, and 100% and 104% for formoterol. The FPD values were 95% and 96% for budesonide and 97% for formoterol (both presentations). Thus, freezing and thawing did not affect the DD and FPD of the budesonide/formoterol Easyhaler.
Discussion
Since the early introduction of multi-dose DPIs, investigators and reviewers have discussed the function of the devices, i.e. the built-in resistance, the required inspiratory flow/effort, and the consistency of dose delivery. Most recently, these questions were discussed in a review by Dal Negro [15]. According to him, a DPI should (1) be effective, i.e. able to consent the inhalation of a sufficient fraction of drug with a particle size ≤6 µ, independently of the patient’s inspiratory flow; (2) reproducible, i.e. able to always consent to the inhalation of the same drug amount, also in terms of its respirable fraction; (3) precise, i.e. able to consent to know at any moment (or the number of doses) of the drug remaining in the device, and whether or not the inhalation was correctly performed: thus, the need for providing DPIs of a “dose counter” and of a “double-dosing protection counter”, in order to avoid a further inhalation if the patient is unaware or not sure of having taken the previous one; (4) stable. i.e. able to protect the drug(s) contained from the effects of temperature and/or humidity changes; (5) comfortable, i.e., easy to use in different circumstances (particularly in clinical conditions) and possibly containing several doses of the drug(s) for a long-term use; (6) versatile, i.e. it should consent to the use of other drugs by inhalation; and (7) environmentally compatible, i.e. not containing chemical contaminants.
Commercially available DPIs do not fulfill all the above-mentioned criteria perfectly. All requirements may be achievable but at a complicated design and high cost. Therefore, for practical reasons, compromises have to be made, especially from a cost-effectiveness point of view. We can hope for the “ideal inhaler” but have to live with the “real-life inhaler” [16].
Previous reviews of in vitro performances of the Easyhaler inhaler concluded that the device, compared with other DPIs on the market, came closer to the “ideal inhaler” than many other inhalers [17, 18]. The comparison was mainly made versus the Turbuhaler inhaler—the first multi-dose reservoir DPI on the market.
The second entry of a budesonide/formoterol DPI, the Easyhaler, has been granted marketing authorization based on established therapeutic equivalence in comparison with the original product, the budesonide/formoterol Turbuhaler, according to the current European guidelines [2,3,4].
For comparison of orally inhaled products, it is essential to understand the performance of the products in the hands of different patient groups, i.e. asthmatic children and adults, and patients with COPD. The recent European Guideline on the requirements for clinical documentation for orally inhaled products, including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and COPD in adults and for use in the treatment of asthma in children and adolescents, requires in vitro flow dependency studies applying, e.g. 10th percentile, 50th (median) and 90th percentile, achievable air flow rates by the targeted patient population [10]. For the Easyhaler and Turbuhaler, the achievable air flow rates have been previously described [3]. In the current study, the final version of the budesonide/formoterol Easyhaler inhaler was compared with the commercially available budesonide/formoterol Turbuhaler inhaler.
The achievable air flow is determined by the air flow resistance of the device. The higher the resistance, the lower the achievable air flow. According to pharmacopoeias, in vitro studies should be carried out using a 4-kPa pressure drop through the inhaler [12]. Recently, a lot of progress has been made concerning more realistic in vitro measurements applying anatomical throats instead of pharmacopeia USP throat, e.g. Alberta idealised throat (AIT) [19], other more realistic physical airway models [20] as well as anatomically correct inlet throats [21]. In addition, different authors have published substantial work related to the use of more realistic patient air flow profiles, e.g. Koening et al. [22] and Delvadia et al. [23]. These advanced methods in combination with pharmacokinetic results would possibly establish more depth of understanding in vitro/in vivo (IVIV) correlation between different products and provide valuable tools for the development in the future. That will be the subject of further work in the future for us. The reported studies have been carried out based on pharmacopeial standard methods as that approach is in line with current regulatory requirements. A combination of this work in addition to Malmberg et al. [3] and Lähelmä et al. [4, 24] provides an overview for the performance of the budesonide/formoterol Easyhaler and Turbuhaler in vitro and in hands of the patients.
The air flow dependency of FPD of both products was demonstrated in the flow dependency study [3]. Further analysis of single delivered doses in that study showed that the Easyhaler dose delivery worked accurately and repeatedly across different flow rates. A similar finding was reported in an earlier study conducted with salbutamol Easyhaler and Turbuhaler devices [25]. Since then, the Turbuhaler device has been updated for some products, including budesonide/formoterol. The new budesonide/formoterol Turbuhaler device incorporates a printed dose counter and has a slightly reduced air flow resistance compared to the earlier Turbuhaler versions marketed with the single components, e.g. budesonide or formoterol [26, 27].
In this study, we further evaluated the flow rate dependency of all three budesonide/formoterol Easyhaler presentations, i.e. 80/4.5, 160/4.5 and 320/9 µg at the three different flow rate ranges achieved by the study patient population in an earlier study [3]. The minimum (10th percentile), median (50th percentile) and maximum (90th percentile) flow rates were used for DD and FPD measurements. The same presentations of the budesonide/formoterol Turbuhaler were used and consistency of dose delivery was compared. The study showed a statistically significantly better dose consistency for the Easyhaler compared with the Turbuhaler at all three levels of tested flow rates. Thus, the excellent dosing accuracy of the Easyhaler documented with other substances has been maintained with budesonide/formoterol. It can be speculated that the detected difference in dosing accuracy characteristics could potentially relate to differences in formulation and device technology between the Easyhaler and Turbuhaler. The mass and volume of a dose is fairly small in the Turbuhaler (160/4.5 µg/inhalations strength approx. 0.94 mg, whereas in the Easyhaler, it is approximately 4.0 mg) and, therefore, the Turbuhaler has a higher concentration of drug substance than the Easyhaler. The small volume and high concentration could lead to some lack of control in dosing volume and drug retention to the plastic surfaces of the inhalers. If drug retention occurs in an inhaler, it could potentially sometimes lead to lower and higher doses than normally, which could be an interesting subject of a further study. For lactose, besides the important formulation function as a carrier of the drug substance, a sufficient amount may additionally provide the patient with the feedback of received dose by taste.
From previous studies, it is well known that MMAD of the Turbuhaler is dependent on the inspiratory flow [28, 29]. In our study, we used the same 10th, median and 90th percentile patient flow rates as when studying DD uniformity. The MMADs and GSDs were very similar for the Easyhaler and Turbuhaler throughout the different patient flow rates. In another in vitro study using clinically relevant patient air flow rates, a slight flow-dependent increase in FPD was found for both budesonide and formoterol for both the devices [3]. In contrast, the Turbuhaler has earlier been reported being a highly air flow-dependent device [8, 30]. That conclusion may have partially been drawn from performance characteristics with low air flows, e.g. 30 L/min, which is far below the applicable 10th percentile air flow of 54 L/min reported by Malmberg et al. [3] and also reported by Brown et al. for the Turbuhaler [31].
Concerning the flow rate resistance, the DPIs with a high built-in air flow resistance give the best lung penetration because it reduces the velocity of the aerosol particles in the respiratory tract [32]. The preferences of patients and healthy volunteers for resistance have been studied with different outcomes. In one study, the highest preference was found for a low-resistance inhaler (0.015 kPa0.5 min/L) by asthmatics and COPD patients [33]. In contrast, in another study, 82% of the healthy volunteers gave preference for a moderate- or high-resistance inhaler (0.021–0.047 kPa0.5 min/L) [34]. The conflicting aspects of patient preferences (moderate to low resistance) and lung penetration (high resistance) may find an optimal solution with inhalers, such as the Easyhaler and Turbuhaler, having a medium resistance (e.g. 0.032–0.036 kPa0.5 min/L) [32]. Clinical experience shows that most patients can use a medium- to high-resistance DPI effectively, even during exacerbations [30, 31].
DPIs may be affected by environmental factors. A patient-reported survey indicates that two-thirds of the patients store their inhaler devices in suboptimal conditions, and only a minority had received instruction regarding inhaler handling [35]. These Easyhaler studies showed that the Easyhaler is tolerant to real-life environmental stress as DD and FPD are virtually unaffected by environmental factors.
What are the possible clinical implications of our findings? In the end, it is the clinical efficacy and safety that determines the usefulness of the inhalers. Also, inhaler handling easiness and patient preferences should be considered as important elements of patient adherence to the treatment. As stated in the recent European consensus statement (5), patients should be prescribed inhalers that they can and will use. In addition to pharmacokinetic studies [4], appropriate in vitro comparisons between products for flow rate dependency [3], particle size distributions and dosing accuracy with a target population of relevant flows are needed to support therapeutic equivalence. Based on our results and the previously published studies [3, 4, 24], there is no reason to believe that differences in clinical safety and efficacy would be demonstrable between the two tested inhalers.
The MMAD and GSD of the particles in the budesonide/formoterol Easyhaler are comparable with particles of the budesonide/formoterol Turbuhaler over the different patient flow ranges. The Easyhaler appears to be robust in dosing accuracy and is a viable device for treatment of patients with asthma and COPD.
Limitations of the current study: as discussed above, our paper reports studies that have been carried out based on pharmacopeial standard methods which are in line with current regulatory requirements. However, progress has been made in developing more advanced in vitro methods to mimic the patients’ inspiratory flow profiles to further improve their applicability to real-world situations where inhalers are used.
Conclusion
The results of studies reported here indicate that the budesonide/formoterol Easyhaler delivers consistently accurate doses throughout inhaler life. Dose consistency was superior compared with the budesonide/formoterol Turbuhaler at all tested flow rates. Consistency of overall dosing was maintained under exposure of the inhaler to stressed conditions as variations in temperature and humidity as well as after dropping, vibration and freeze/thaw tests.
References
Mapel DW, Roberts MH. Management of asthma and chronic obstructive pulmonary disease with combination inhaled corticosteroids and long-acting β-agonists: a review of comparative effectiveness research. Drugs. 2014;74:737–55.
https://docetp.mpa.se/LMF/Bufomix%20Easyhaler%20inhalation%20powder%20ENG%20PAR.pdf. Accessed 26 Jun 2015.
Malmberg LP, Everard ML, Haikarainen J, Lähelmä S. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol Easyhaler®. J Aerosol Med Pulm Drug Deliv. 2014;5:329–40.
Lähelmä S, Sairanen U, Haikarainen J, Korhonen J, Vahteristo M, Fuhr R. Equivalent lung dose and systemic exposure of budesonide/formoterol combination via Easyhaler and Turbuhaler. J Aerosol Med Pulm Drug Deliv. 2015;28:462–73.
Laube BL, Janssens HM, Jongh FHC, Devadason SG, Dhand R, Diot P, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–31.
Tamura G, Sakae H, Fujino S. In vitro evaluation of dry powder inhaler devices of corticosteroid preparations. Allergol Int. 2012;61:149–54.
Clark A, Hollingworth A. The relationship between powder inhaler resistance and inspiratory conditions in healthy volunteers: implications for in vitro testing. J Aerosol Med. 1993;6:99–110.
Tarsin W, Assi KH, Chrystyn H. In-Vitro intra- and inter-inhaler flow rate-dependent dosage emission from a combination of budesonide and formoterol in a dry powder inhaler. J Aerosol Med. 2004;17:25–32.
Delvadia R, Hindle M, Longest PW, Byron PR. In vitro tests for aerosol deposition II: IVIVCs for different dry powder inhalers in normal adults. Aerosol Med Pulm Drug Deliv. 2013;26:138–44.
CHMP (2009) Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. http://www.ema.europa.eu/docs/en_GB/document_library/Scientificguideline/2009/09/WC500003504.pdf. Accessed 05 Nov 2014.
CHMP (2006) Guideline on the pharmaceutical quality of inhalation and nasal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf). Accessed 31 Aug 2016.
European Pharmacopoeia monograph. Preparations for Inhalations. 5th Supplement. Strasbourg: Council of Europe, 2010.
ISO/FDIS 20072:2009 standard.
IEC 60068-2-64, Test Fh (2008-04).
Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10:13. doi:10.1186/s40248-015-0012-5.
Borgström L, Asking L, Thorsson L. Idealhalers or realhalers? A comparison of Diskus and Turbuhaler. Int J Clin Pract. 2005;59:1488–95.
Chrystyn H. Closer to an ‘ideal inhaler’ with the Easyhaler: an innovative dry powder inhaler. Clin Drug Investig. 2006;26:175–83.
Chrystyn H, Haahtela T. Real-life inhalation therapy—inhaler performance and patient education matter. Eur Respir Dis. 2012;8:11–8.
Mitchell J, Copley M, Sizer Y, Russell T, Solomon D. Adapting the abbreviated impactor measurement (AIM) concept to make appropriate inhaler aerosol measurements to compare with clinical data: a scoping study with the “Alberta” idealized throat (AIT) inlet. J Aerosol Med Pulm Drug Deliv. 2012;25:188–97.
Delvadia R, Hindle M, Worth Longest P, Byron PR. In vitro tests for aerosol deposition II: IVIVCs for different dry powder inhalers in normal adults. J Aerosol Med Pulm Drug Deliv. 2013;26:138–44.
Olsson B, Borgström L, Lundbäck H, Svensson M. Validation of a general in vitro approach for prediction of total lung deposition in healthy adults for pharmaceutical inhalation products. J Aerosol Med Pulm Drug Deliv. 2013;26:355–69.
De Koning JP. Dry powder inhalation: technical and physiological aspects, prescribing and use. Doctoral thesis. University of Croningen (2001).
Delvadia RR, Wei X, Longest PW, Venitz J, Byron PR. In vitro tests for aerosol deposition. IV: simulating variations in human breath profiles for realistic DPI testing. J Aerosol Med Pulm Drug Deliv. 2016;29:196–206.
Lähelmä S, Vahteristo M, Metev H, Taseva M, Stamatova N, Bartha A, Schlezák J, Sairanen U. Equivalent bronchodilation with budesonide/formoterol combination via Easyhaler and Turbuhaler in patients with asthma. Respir Med. 2016;120:31–5.
Palander A, Mattila T, Karhu M, Muttonen E. In vitro comparison of three salbutamol-containing multidose dry powder inhalers. Buventol Easyhaler®, Inspiryl Turbuhaler® and Ventoline Diskus®. Clin Drug Investig. 2000;20:25–33.
Koning JP, Mark TW, Coenegracht PM, Tromp TF, Frijlink HW. Effect of an external resistance to airflow on the inspiratory flow curve. Int J Pharm. 2002;234:257–66.
Lööf T, Elfman P, Ström P, Törngren A, Borgström L. Sustained mechanical and clinical functionality of the Flexhaler dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2008;21:381–8.
Ross DL, Schultz RK. Effect of inhalation flow rate on the dosing characteristics of dry powder inhaler (DPI) and metered dose inhaler (MDI) products. J Aerosol Med. 1996;9:215–26.
Hindle M, Byron PR. Dose emissions from marketed dry powder inhalers. Int J Pharm. 1995;116:169–77.
Malton A, Sumby BS, Smit IJ. A comparison of in vitro dose delivery from two multidose powder inhalation devices. Eur J Clin Res. 1995;7:177–93.
Brown PH, Ning ACWS, Greening AP, McLean A, Crompton GK. Peak inspiratory peak flow through Turbuhaler in acute asthma. Eur Respir J. 1995;8:1940–1.
Frijlink HW, Boer AH. Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv. 2004;1:67–86.
Andersen PB, Hanssen NCG. Which magnitude of inhaler resistance against airflow is preferred by patients using dry powder inhalers. Eur Respir J. 1993;6:148s.
Boer AH, Winter HMI, Lerk CF. Inhalation characteristics and their effect on in vitro drug delivery from dry powder inhalers, part 1, inhalation characteristics, work of breathing and volunteers’ preference in dependence of the inhaler resistance. Int J pharm. 1996;130:231–44.
Norderud Lærum B, Telg G, Stratelis G. Need of education for dry powder inhaler storage and retention—a patient-reported survey. Multidiscip Respir Med. 2016;11:21. doi:10.1186/s40248-016-0057-0.
Acknowledgements
The studies and publication charges were funded by Orion Corporation Orion Pharma, Espoo, Finland. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Jussi Haikarainen is an employee of Orion Corporation. Tero Löytänä is an employee of Orion Corporation. Anita Happonen is an employee of Orion Corporation. Paula Rytilä is an employee of Orion Corporation. Olof Selroos and Sirpa Metsärinne have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was not applicable as there is no clinical data.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article, go to http://www.medengine.com/Redeem/CB47F06016DADD14.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Haikarainen, J., Selroos, O., Löytänä, T. et al. Budesonide/Formoterol Easyhaler®: Performance Under Simulated Real-Life Conditions. Pulm Ther 3, 125–138 (2017). https://doi.org/10.1007/s41030-016-0025-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-016-0025-z