Abstract
Introduction
The aim of this economic evaluation was to estimate the cost-effectiveness of fluticasone propionate/formoterol (FP/FORM; Flutiform®) and compare it to those of fluticasone/salmeterol (FS) and budesonide/formoterol (BF) when used in the treatment of adult patients with moderate-to-severe asthma.
Methods
A Markov model was developed with five asthma health states: successful control, suboptimal control, outpatient-managed exacerbation, inpatient-managed exacerbation, and death. The time horizon was set at 12 months. Transition probabilities and indirect resource utilization were derived from previous international and Spanish publications. Univariate and probabilistic sensitivity analyses (SAs) were applied.
Results
FP/FORM was less expensive to acquire than FS or BF (20% lower than FS and 30% lower than BF), while the quality-adjusted life years (QALYs) of the three options compared were very similar. Cost per patient in the FP/FORM cohort was 9326€/year, making it the cheapest option, 1.5% cheaper than FS and 2.6% cheaper than BF. The suboptimal control health state dominated the costs (80% of the total cost) in each of the analyzed options and scenarios. The results of the SAs verified the data obtained from the base case scenario.
Conclusions
From a Spanish societal perspective, in 2014, FP/FORM produced a similar gain in QALYs but at a lower cost when compared to FS and BF in a highly meaningful number of replications and scenarios. FP/FORM can therefore be considered a cost-effective option in the treatment of moderate-to-severe asthma in Spain. The cost savings were mainly due to the significantly lower acquisition cost of FP/FORM than the other two options.
Funding
Mundipharma Pharmaceuticals, S.L., Madrid, Spain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is a heterogeneous disease that is usually characterized by chronic airway inflammation. Its pathophysiology involves cells and inflammation mediators, and a genetic predisposition influenced by environmental interaction mediators and cells. Asthmatics have a history of respiratory symptoms such as wheezing, shortness of breath, chest tightness, and coughing that vary over time and in intensity, together with variable expiratory airflow limitation [1, 2].
Of the various chronic respiratory diseases, asthma is among those that has the greatest impact on public health [3]. Its average prevalence in Spain is 5.7% [3], but depending on the geographical area it can reach more than 10% [4]. With a mortality rate of 2.22 per 100,000 inhabitants in 2005, it leads to high consumption of health and non-health resources. Its estimated annual cost to Spain is 1480 million euros, with the associated pharmacological treatment representing around 33% of that figure [5].
The main goals of asthma treatment are to control symptoms such as daytime symptoms, sleeping difficulties, and activity limitations, and to reduce the future risk of adverse outcomes such as fixed airflow limitation, medication side effects, and exacerbations that are independent of symptom control. Low-to-high inhaled doses of a combination of inhaled corticosteroids (ICS) and a long-acting β2-agonist (LABA) represent the first-choice maintenance treatment recommended by Spanish and international guidelines for patients with moderate-to-severe persistent asthma [1].
Adult patients with moderate asthma are characterized by daily symptoms, everyday reliever medication needs, night-time waking more than once a week, moderate activity limitation, an FEV1 (forced expiratory volume in one second) of between 60% and 80%, and two or more exacerbations a year. On the other hand, persistent severe asthma is defined as continuous daytime and frequent night-time symptoms with reliever medication needed on more than one occasion, high activity limitation, an FEV1 of <60%, and two or more exacerbations a year [2]. It is important to note, however, that asthma severity is not static and must therefore be assessed in order to determine the need for possible changes in treatment, such as increasing the dose of the ICS/LABA combination or the inclusion of add-on therapies. ICS/LABA has demonstrated a higher exacerbation control than maintenance treatment with monotherapy consisting of corticosteroids plus a SABA (short-acting β2-agonist) on an as-needed basis [1].
Although these treatments have proven efficacy, the European National and Wellness Survey shows that a high proportion (around 50%) of asthmatic patients have uncontrolled asthma, leading to significantly reductions in their quality of life and increased consumption of healthcare resources [6–8]. In Spain, 70% of the treatment cost can be attributed to the lack of disease control. Partly controlled patients have more than one exacerbation a year, whereas totally uncontrolled patients have more than one a week [2]. Asthma control is also associated with daytime and night-time symptoms, reliever medication use, activity limitations, and FEV1.
During the last year, different combinations of ICS/LABA in a single inhalation device have been developed, and these have been found to have a positive impact on patient acceptance, dosage convenience, and adherence, all of which may conceivably increase control and reduce the costs associated with this illness. These combinations are fluticasone/salmeterol (FS), budesonide/formoterol (BF), and, most recently, fluticasone propionate/formoterol (FP/FORM; Flutiform®).
Due to the high annual costs of asthma and the recent incorporation of FP/FORM, we decided to carry out an economic evaluation from a Spanish societal perspective to estimate the cost-effectiveness of fluticasone propionate/formoterol (Flutiform®) (FP/FORM) and compare it to those of fluticasone/salmeterol (FS) and budesonide/formoterol (BF) when used in the treatment of adult patients with moderate-to-severe asthma.
Methods
Study Design
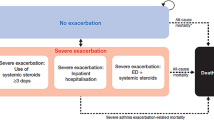
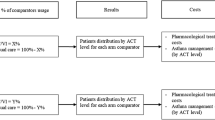
Based on the work of Price et al. [9], we developed a Markov model that was adapted to the new GEMA (Spanish Guidelines for Asthma Management 2015) and GINA (Global Initiative for Asthma: Management and Prevention Strategy) [1, 2] guidelines, which considered five asthma health states: optimal control, suboptimal control, outpatient-managed exacerbation, inpatient-managed exacerbation, and death (Fig 1).
Asthma control classification was based on daily symptoms, night-time waking due to asthma, need for reliever medication, and activity limitation. According to the GINA 2015 criteria (Table 1), well-controlled patients represent the state of successful control (SC), and partly controlled and uncontrolled patients correspond to the suboptimal control state (SOC).
Depending on its/their severity, worsening asthma and exacerbations can be self-managed, treated in the primary care center, or will require emergency department care with or without hospital admission. In our model, self-management corresponded to SC and SOC, the need for primary care and emergency department care corresponded to outpatient managed exacerbation (OME), and the need for hospitalization corresponded to inpatient managed exacerbation (IME) [1].
The death state includes deaths from all causes: asthma-related and non-asthma-related.
The time horizon was set at 12 months, and we used weekly probability transitions.
Treatment Comparisons, Efficacy, and Utilities Estimation
The treatment comparators in the analyses are the maintenance ICS/LABA combinations recommended in the national and international guidelines for patients with moderate-to-severe asthma, available in a single aerosol inhaler.
Two different clinical trials have proven that FP/FORM is as effective as FS and BF. The first open-label randomized multi-country phase 3 study was designed to demonstrate the noninferiority of FP/FORM compared with FS in controlling mild-to-moderate/severe persistent asthma in adult patients based on mean pre-dose forced expiratory volume in the first second (FEV1) at week 12. The results showed that FP/FORM is comparable to FS in the primary and various other secondary endpoints, such as other parameters of the lung function test, patient-reported outcomes, rescue medication use, asthma exacerbations, and asthma quality-of-life questionnaire scores. Noninferiority was tested using a covariance analysis with a 95% CI ≥ −0.2 L for the lower limit. The main results are presented in Table 2 [10].
A randomized double-blind multi-country study of asthmatic patients using FP/FORM or BF was performed in which the primary endpoint was the change in FEV1 from pre-dose at baseline to pre-dose at week 12 and the secondary endpoints were the mean change in FEV1 from pre-dose at baseline to 2 h post-dose at week 12 and the number of discontinuations due to lack of treatment efficacy. This study demonstrated that FP/FORM and BF present comparable efficacies in terms of primary and secondary endpoints. The predefined noninferiority baseline limit for the primary endpoint was established at −0.2 L (95% CI −0.130, 0.043 L; p < 0.01), and the results obtained are shown in Table 3 [11].
We adopted the same transition probabilities for the three options and incorporated FS values from the Gerzeli 2012 study, in which calculations were performed using the raw data from the ICAT SY trial (Inhaled Combination Asthma Treatment versus SYmbicort) [12]. The initial proportions of the patients in the SC and SOC states was taken from Demoli 2010: 53% and 47%, respectively. Weekly health utility weights were also derived from the mean utility values obtained in the Gerzeli 2012 study [8, 13] (Table 4).
Cost Estimation
The main economic analysis was conducted from a societal perspective (the direct healthcare cost, direct non-healthcare cost, and indirect cost were included). An expert panel composed of two allergists and two pneumologists from different hospitals and regions of Spain were recruited to validate the list of resources used (derived from a literature review) and complete a survey aimed at determining the units consumed in each Markov model state.
The direct healthcare cost was divided into pharma (maintenance, rescue, and other non-rescue-related) and non-pharma (primary care specialist visits, ancillary tests, emergency attendance, and hospital diagnostic-related group for bronchitis and asthma in patients over 17 years of age with or without complications) costs. Drug unitary costs were derived from Bot Plus (Spanish Official Pharmacist Association) [14], and direct non-pharma healthcare unit costs from autonomous communities that published prices weighted by population. All costs are expressed in euros, and refer to monetary values in 2014.
The current guidelines recommend medium-to-high doses of ICS/LABA for patients with moderate-to-severe asthma. The ICS doses in the GINA 2015 guidelines are 400–800 mcg of budesonide (or equivalent) as medium dosing and >800 mcg for high dosing; the corresponding values are 250–500 mcg and >500 mcg for propionate fluticasone. Table 5 shows the average (range), the unitary cost, and the average weekly cost (range) of maintenance treatment for each drug combination and state considered in the model.
The cost of home rescue medication (OME), associated with SC and SOC, includes adrenergic treatment and systemic corticosteroids (SCS) or SMART (Symbicort® maintenance and rescue treatment) with BF. The expert-panel-estimated number of exacerbations per cycle was 1.3 (0–2) for SC and 5.33 (3–7) for the SOC state. Total cost of rescue per R03 and SCS was 2.41€ for SC and 14.32€ for SOC, or 2.06€ and 11.97€, respectively, when SMART therapy was used (Table 6).
Other pharma costs not related to home rescue medication include those of the adrenergic inhaler, other COPD drugs, anticholinergics, and systemic corticosteroids. The data used to estimate this cost were derived from the EPAR (doses), from Idoctus [15] (unitary cost), and from Collados et al. [16] (the percentage of patients treated with them); see Table 7.
Direct non-pharma healthcare costs were assessed by calculating the average weighted populations of the different Spanish regions, available published prices, and the expert panel’s consumption data. Table 8 shows the average weekly costs of the different resources referred to by the experts surveyed.
Direct non-healthcare costs or costs derived from informal care were calculated based on the recommendations of Oliva et al. [17], in which the unitary cost per hour (in 2014) expressed in euros was 7.21€ (4.71–9.71€). The percentage of the week that patients devoted to receiving such care was, according to the expert panel, 0–2.5% for SC, 20–50% SOC, 30–60% OME, and 40–80% for IME. The resulting weekly cost was 7.21€ (4.71–9.71€) for SC, 93.73€ (37.68–194.20€) for SOC, 122.57€ (56.52–233.04€) for OME, and 165.83€ (75.36–310.72€) for IME [17].
The indirect cost of loss of productivity was estimated using the lost workday equivalent (LWDE), which is the number of workdays lost plus the number of days worked while suffering from the symptoms of asthma [18]. According to the expert panel, the LWDE was 0.27 (0.13–0.42) for SC, 3.71 (1.78–4.22) for SOC, 5.31 (3.9–6.1) for OME, and 6.63 (6–7) for IME. With a 87.96€ labor cost per day, the indirect cost was 23.75€ (11.43–36.94€) for SC, 273.56€ (156.57–371.19€) for SOC, 467.07€ (343.04–536.56€) for OME, and 583.17 (527.76-615.72€) for IME.
The total cost of each health state considered in our Markov model is summarized in Table 9.
Base Case Analyses
This analysis assumed that 53% of the patients were initially defined as SC and 47% were initially defined as SOC [8], that there were 1.23 and 5.33 weekly home management exacerbations, respectively, for SC and SOC patients, that there was a ratio of women to men of 1:1, and that the patients had a mean age of 55 years, according to the expert panel.
Effectiveness was expressed as quality-adjusted life years (QALYs) gained, and the results were assessed based on the incremental cost-effectiveness ratio (ICER).
Deterministic and Probabilistic Sensitivity Analyses
Uncertainty from the social and payer perspective was tested using univariate (OWSA) and probabilistic (PSA) sensitivity analyses to ensure the strength of the model.
The OWSA was developed by increasing and decreasing the deterministic value by 10% or by using the IC limits when they were available.
The results of the PSA were expressed graphically by plotting a “cloud” of iterations on a cost-effectiveness plane.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Base Case Analysis
In the base case analysis, FP/FORM proved to be less expensive than BF or FS (by 2.8% and 1.1%, respectively; see Table 10). This advantage was due to a cost reduction associated with the successful control of patients, as the costs relating to emergency and impatient exacerbations were quite similar for all drug combinations. The reason for this was that the cost of acquiring FP/FORM was 24% lower than that of FS and 32% lower than that of BF.
The suboptimal control health state dominated (was 80% of) the overall cost in all of the options and scenarios analyzed.
The QALYs of the three options were very similar, as there were minimal differences in efficacy between the strategies.
Univariate Sensitivity Analysis
The results of the univariate sensitivity analysis did not show any change from the base case results. Only costs relating to SC and SOC showed any changes, but FP/FORM was always found to be the most favorable option.
Probabilistic Results
When the PSA was run, results confirmed the data obtained from the base case scenario, which indicated that FP/FORM was the most economically attractive option (Table 11).
Total costs and total QALYs were expressed graphically to highlight differences in the ICER among iterations. Looking at the two probabilistic cost-effectiveness planes (Fig. 2a, b), it is apparent that the two “clouds” of points (where a cloud represents iterations for a particular drug combination) almost fully overlap with each other in each plot, reflecting the numerical results shown in Table 11.
Discussion
Cost-effectiveness evaluation is a tool used for health technology assessment as a means to support universal coverage. In Spain, the National Health Service, which is almost totally funded by taxes, has to comply with the Royal Decree Law 16/2012 in which different measures are established that are intended to guarantee service sustainability. These measures include a cost-effectiveness analysis, which must be carried out before decisions are made about prices and reimbursement (RD 12/2016) [19].
This study provides data that may help physicians, budget holders, and decision makers to decide on the treatment of moderate-to-severe asthma with ICS/LABA in a single inhaler.
FP/FORM is less expensive to acquire than the alternative drug combinations: FP/FORM is 24% cheaper than FS and 32% cheaper than BF. These cost differences are maintained in all of the clinical states defined in the model. When SC and SOC are compared and only direct costs are taken into account, the use of FP/FORM led to 17% and 14% lower costs than FS and BF in the successful control state and 10% lower costs in the suboptimal control state.
Noninferiority trials prove that FP/FORM and FS have comparable efficacies and safety profiles, as do FP/FORM and BF [10, 11], but FP/FORM provides better cost-effectiveness performance from National Health Service and social perspectives.
Nonetheless, it is important to bear in mind that the differences between the overall costs of the various strategies are small (1.5–2.6%), so the only advantage of FP/FORM is its lower acquisition cost. Indeed, a shift to less-expensive generic drugs could reduce the advantage of FP/FORM even more.
Due to a lack of data on the comparative efficacies of different types of devices for administering the drugs considered here, we have not considered the potential benefits and disadvantages of those different devices, but this issue should be explored in future trials assessing the efficacy and benefits of these drug combinations for patients.
The main limitation of the study was the use of the same transition probabilities and utilities for the different treatments because patient-level data were not available and the clinical trials were not designed to evaluate them.
Although FP/FORM has a more rapid onset of action, and this could not be modeled properly, FP/FORM seems to be the appropriate option for patients with moderate-to-severe asthma, as it is the least expensive option but is as effective as the other two options. Formoterol is a rapid and long-acting β2-agonist that has demonstrated a faster onset of action than salmeterol in patients with moderate-to-severe asthma in clinical trials [20–22]. This may increase the patient’s quality of life, with treatment adherence being reflected in better disease control. However, in this work, we used data from clinical trials where the rapid onset of FP/FORM—which reflects the faster bronchodilatory effects of formoterol compared with salmeterol—was not considered as an effectiveness outcome that could represent another advantage of FP/FORM aside from its lower acquisition cost.
The SOC state leads to direct health costs that are 50% higher than those associated with the SC state. This is one of the reasons why the international asthma management guidelines GINA and GEMA [1, 2] consider patient education about asthma to be a very important element of treatment, as it improves disease control and treatment adherence, which in turn reduce the risk of an exacerbation and lead to higher quality of life and adequate self-care.
Patients with asthma should know the symptoms of their disease and understand that asthma medication ought to be taken daily even if they do not experience any symptoms, as asthma is a chronic disease. Patients should also learn how to identify their symptoms and when their control over the asthma is decreasing, learn to distinguish maintenance and rescue medication and to recognize and avoid triggers, know how to implement their self-treatment plan, and they should get proper training in the inhalation technique.
Conclusions
From a Spanish societal perspective, in 2014, FP/FORM produced a similar gain in QALYs but at a lower cost when compared with FS and BF in a highly meaningful number of replications and scenarios. FP/FORM can be considered a cost-effective option for the treatment of moderate-to-severe asthma in Spain. The cost savings achievable with FP/FORM are mainly due to the significantly lower acquisition price of FP/FORM compared to the other two options.
References
GINA. Global strategy for asthma management and prevention. Vancouver: Global Initiative for Asthma; 2015.
FENAER, GRAP, SEAIC, et al. GEMA (Guía Española del Manejo del Asma) 4.0. Madrid; Luzán 5, S. A. de Ediciones; 2015. http://www.gemasma.com.
Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma. Allergy. 2004;59(5):469–78.
Urrutia I, Aguirre U, Sunyer J, Plana E, Muniozguren N, Martínez-Moratalla J, et al. Cambios en la prevalencia de asma en la población española del Estudio de Salud Respiratoria de la Comunidad Europea (ECRHS-II). Arch Bronconeumol. 2007;43(08):425–30. doi:10.1157/13108781.
Martínez-Moragón E, Serra-Batllés J, De Diego A, et al. Coste económico del paciente asmático en España (estudio AsmaCost). Arch Bronconeumol. 2009;45(10):481–6. doi:10.1016/j.arbres.2009.04.006.
Vollmer WM, Markson LE, O’Connor E, Sanocki LL, Fitterman L, Berger M, et al. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1647–52. doi:10.1164/ajrccm.160.5.9902098.
Guilbert TW, Garris C, Jhingran P, Bonafede M, Tomaszewski KJ, Bonus T, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. 2011;48(2):126–32. doi:10.3109/02770903.2010.535879.
Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev. 2010;19(116):150–7. doi:10.1183/09059180.00002110.
Price MJ, Briggs AH. Development of an economic model to assess the cost effectiveness of asthma management strategies. PharmacoEconomics. 2002;20(3):183–94.
Bodzenta-Lukaszyk A, Dymek A, McAulay K, Mansikka H. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulm Med. 2011;11:28. doi:10.1186/1471-2466-11-28.
Bodzenta-Lukaszyk A, Buhl R, Balint B, Lomax M, Spooner K, Dissanayake S. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. J Asthma. 2012;49(10):1060–70. doi:10.3109/02770903.2012.719253.
Papi A, Paggiaro PL, Nicolini G, Vignola AM, Fabbri LM. Beclomethasone/formoterol versus budesonide/formoterol combination therapy in asthma. Eur Respir J. 2007;29(4):682–9. doi:10.1183/09031936.00095906.
Gerzeli S, Rognoni C, Quaglini S, Cavallo MC, Cremonesi G, Papi A. Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig. 2012;32(4):253–65. doi:10.2165/11598940-000000000-00000.
Botplusweb. Website. Madrid: Consejo General de colegios oficiales de farmacéuticos. https://botplusweb.portalfarma.com/.
Idoctus. Website. http://es.idoctus.com/consulta/medicamentos.
Collados C, Martín V, González-Torralba F, Rejas J. Análisis coste-efectividad de beclometasona/formoterol frente a fluticasona/salmeterol en el tratamiento de pacientes con asma moderada a grave en España. PharmaEcon Span Res. 2014;12(2):53–62.
Moreno JO, Guerrero RO. Los costes de los cuidados informales en España. Madrid: Secretaría General de Presupuestos y Gastos; 2009.
Ojeda P, Sanz de Burgoa V. Costs associated with workdays lost and utilization of health care resources because of asthma in daily clinical practice in Spain. J Investig Allergol Clin Immunol. 2013;23(4):234–41.
Ministerio de la Presidencia. Real Decreto-ley 16/2012, de 20 de abril, de medidas urgentes para garantizar la sostenibilidad del Sistema Nacional de Salud y mejorar la calidad y seguridad de sus prestaciones. Boletín Oficial del Estado. 2016;5403. https://www.boe.es/boe/dias/2012/04/24/pdfs/BOE-A-2012-5403.pdf.
Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178.
Aalbers R, Brusselle G, McIver T, Grothe B, Bodzenta-Lukaszyk A. Onset of bronchodilation with fluticasone/formoterol combination versus fluticasone/salmeterol in an open-label, randomized study. Adv Ther. 2012;29(11):958–69. doi:10.1007/s12325-012-0058-0.
van Noord JA, Smeets JJ, Raaijmakers JA, Bommer AM, Maesen FP. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. Eur Respir J. 1996;9(8):1684–8.
Acknowledgments
Sponsorship for this study was provided by Mundipharma Pharmaceuticals, S.L., Madrid, Spain.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
J. Manuel Collar and C. Martín-Saborido contributed to the conception and design of the study. C. Antón Rodriguez and C. Martín-Saborido carried out all of the costing and data collection, data analysis, and model programming. J. Delgado, P. Ojeda, and L. Pérez del Llano contributed to the data collection and assisted in the interpretation of the data analysis. C. Antón Rodriguez, J. Manuel Collar, and C. Martín-Saborido drafted the manuscript. E. Martínez Moragón revised the manuscript.
Disclosures
J. Manuel Collar is an employee at Mundipharma Pharmaceuticals; S.L.E. Martínez Moragón, J. Delgado, P. Ojeda, L. Pérez del Llano, C. Antón Rodriguez, and C. Martín Saborido have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/A9E6F06074280500.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Moragón, E.M., Delgado, J., Ojeda, P. et al. Economic Evaluation of Fluticasone Propionate/Formoterol (Flutiform®) vs. Fluticasone/Salmeterol and Budesonide/Formoterol in Spain. Pulm Ther 2, 199–213 (2016). https://doi.org/10.1007/s41030-016-0021-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-016-0021-3