Abstract
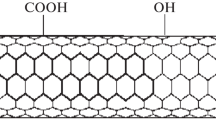
Multi-walled carbon nanotubes (MWCNTs) were oxidized for removal of indigo carmine (IC) from aqueous solution through batch experiment. The effects of contact time, temperature, pH and dosage of adsorbent on the removal of indigo carmine were investigated. The optimal pH for maximum dye adsorption was found to be 5.0. The adsorption kinetic of IC dye onto oxidized MWCNTs fitted the pseudo-second-order model and the equilibrium experimental results showed good correlation with Freundlich model. The maximum adsorption capacity for indigo carmine was obtained as 71.42 mg g−1.








Similar content being viewed by others
References
Abdel Salam M, Gabal MA, Obaid AY (2012) Preparation and characterization of magnetic multi-walled carbon nanotubes/ferrite nanocomposite and its application for the removal of aniline from aqueous solution. Synth Met 161:2651–2658
Abuilaiwi FA, Laoui T, Al-Harthi M, Atieh MA (2010) Modification and functionalization of multiwalled carbon nanotube (MWCNT) via fischer esterification. Arab J Sci Eng 35(1C):37–48
Ahn ChK, Park D, Woo SH, Park JM (2009) Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J Hazard Mater 164:1130–1136
Arasteh R, Masoumi M, Rashidi AM, Moradi L, Samimi V, Mostafavi ST (2010) Adsorption of 2-nitrophenol by multi-wall carbon nanotubes from aqueous solutions. Appl Surf Sci 256:4447–4455
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47–52
Barka N, Assabbane A, Nounah A, Ait Ichou Y (2008) Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. J Hazard Mater 152:1054–1059
Brito SMO, Andrade HMC, Soares LF, Azevedo RP (2010) Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J Hazard Mater 174:84–92
Erdem E, Colgecen G, Donat R (2005) The removal of textile dyes by diatomite earth. J Colloid Interface Sci 282:314–319
Fan L, Zhou Y, Cheng W, Yang F (2008) Electrochemical degredation of aqueous solution of Amaranth azo dye on ACF under potentiostatic model. Dyes Pigm 76:440–446
Freundlich HMF (1906) Über die adsorption in lösungen. Z Fur Phys Chem 57A:385–470
Freundlich H (1926) Colloid and capillary chemistry. Metheum, London, p 993
Gong J, Wang B, Zeng GM, Yang CP, Niu CG, Niu QY, Zhou WJ, Liang Y (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164:1517–1522
Goyanes S, Rubiolo GR, Jimento A, Corcuera MA, Mondragon I (2007) Carboxylation treatment of multiwalled carbon nanotubes monitored by infrared and ultraviolet spectroscopies and scanning probe microscopy. Diam Relat Mater 16:412–417
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Juang LC, Wang CC, Lee CK (2006) Adsorption of basic dyes onto MCM-41. Chemosphere 64:1920–1928
Kannan N, Rengasamy G (2005) Comparison of cadmium ion adsorption on various activated. Water Air Soil Pollut 163:185–201
Kaur S, Rani S, Mahajan RK (2013) Adsorption kinetics for the removal of hazardous dye congo red by biowaste materials as adsorbents. J Chem 2013:1–12
Lakshmi UR, Srivastava VC, Mall ID, Lataye DH (2009) Rice husk ash as an effective adsorbent: evaluation of adsorptive characteristics for Indigo Carmine dye. J Environ Manag 90:710–720
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. J Am Chem Soc 33:2221–2295
Li YH, Ding J, Luan Z, Di A, Zhu Y, Xu C, Wu D, Wei B (2003) Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 41:2787–2792
Lin YF, Chen HW, Chien PS, Chiou CS, Liu CC (2011) Application of bifunctional magnetic adsorbent to adsorb metal cations and anionic dyes in aqueous solution. J Hazard Mater 185(2-3):1124–1130
Long RQ, Yang RT (2001) Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc 123:2058–2059
Madrakiana T, Afkhami A, Ahmadi M, Bagheri H (2011) Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J Hazard Mater 196:109–114
Manu B (2007) Physico-chemical treatment of indigo dye wastewater. Color Technol 123(3):197–202
Merkoci A (2006) Carbon nanotubes in analytical sciences. Microchim Acta 152:157–174
Mittal A, Mittal J, Kurup L (2006) Batch and bulk removal of hazardous dye, indigo carmine from wastewater through adsorption. J Hazard Mater B137:591–602
Oguz E, Keskinler B (2007) Comparison among O3, PAC adsorption, O3 = HCO3, O3/H2O2 and O3/PAC processes for the removal of Bomaplex Red CR-L dye from aqueous solution. Dyes Pigments 74:329–334
Pang Y, Zeng G, Tang L, Zhang Y, Liu Y, Lei X, Li Z, Zhang J, Xie G (2011) PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 281:278–284
Qu S, Huang F, Yu S, Chen G, Kong J (2008) Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J Hazard Mater 160:643–647
Ramakrishna KR, Viraraghavan T (1997) Dye removal using low cost adsorbents. Water Sci Technol 36:189–196
Regina Furlan F, de Meloda Silva LG, Ferreira Morgado A, Augusto Ulson de Souza A, Guelli Ulson de Souza SAM (2010) Removal of reactive dyes from aqueous solutions using combined coagulation/flocculation and adsorption on activated carbon. Resour Conserv Recycl 54:283–290
Rosca ID, Watari F, Uo M, Akasaka T (2005) Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 43:3124–3131
Sheng GD, Shao DD, Ren XM, Wang XQ, Li JX, Chen YX, Wang XK (2010) Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes. J Hazard Mater 178:505–516
Sobhanardakani S, Zandipak R, Sahraei R (2013) Removal of Janus Green dye from aqueous solutions using oxidized multi-walled carbon nanotubes. Toxicol Environ Chem 95(6):909–918
Sobhanardakani S, Farmany A, Abbasi S (2014) A new modified multiwalled carbon nanotube paste electrode for quantification of tin in fruit juice and bottled water samples. J Ind Eng Chem 20(5):3214–3216
Sohrabnezhad S, Pourahmad A (2010) Comparison absorption of new methylene blue dye in zeolite and nanocrystal zeolite. Desalination 256:84–89
Tak-Hyun K, Park C, Yang J, Kim S (2004) Comparison of disperse and reactive dye removals by chemical coagulation and Fenton oxidation. J Hazard Mater B112:95–103
Wang S, Boyjoo Y, Choueib A, Zhu ZH (2005) Removal of dyes from aqueous solution using fly ash and red mud. Water Res 39:129–138
Wang X, Zhu N, Yin B (2008) Preparation of sludge-based activated carbon and its application in dye wastewater treatment. J Hazard Mater 153:22–27
Wu CH (2007) Adsorption of reactive dye onto carbon nanotubes: equilibrium, kinetics and thermodynamics. J Hazard Mater 144:93–100
Xu D, Tan X, Chen C, Wang X (2008) Removal of lead (II) from aqueous solution by oxidized multi walled carbon nanotubes. J Hazard Mater 154:407–416
Vazquez I, Rodriguez-Iglesias J, Marañon E, Castrillon L, Alvarez M (2007) Removal of residual phenols from coke wastewater by adsorption. J Hazard Mater 147:395–400
Yavari R, Huang YD, Ahmadi SJ (2011) Adsorption of cesium (I) from aqueous solution using oxidized multiwall carbon nanotubes. J Radioanal Nucl Chem 287:393–401
Acknowledgements
The authors are grateful to the Hamedan Branch, Islamic Azad University for providing facilities to conduct and complete this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobhanardakani, S., Ghoochian, M., Jameh-Bozorghi, S. et al. Assessing of Removal Efficiency of Indigo Carmine from Wastewater Using MWCNTs. Iran J Sci Technol Trans Sci 41, 1047–1053 (2017). https://doi.org/10.1007/s40995-017-0312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-017-0312-z