Abstract
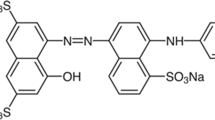
The textile, leather, and dyeing industries, as well as domestic wastewater, are among the most important sources of environmental pollution. In this study, modified multi-walled carbon nanotubes (m‑MWCNTs) powder was applied as an adsorbent in the adsorption process of Acid Orange 7 (AO7) pollutant in aqueous solution. The m-MWCNTs have been characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Brunauer–Emmett–Teller (BET) surface area analysis. The creation of functional groups on carbon nanotubes (CNTs) in acidic solution used. The presence of hydroxyl (–OH) and carboxylic (–COOH) functional groups on CNTs was proved using Fourier transform infrared spectroscopy (FT-IR). The influence of operating factors included pH, adsorbent amount and contact time in the process were studied and optimized. The Langmuir and Freundlich isotherms and pseudo first-second orders adsorption kinetics of dye were studied. Also the results showed that the dye adsorption onto m-MWCNTs followed Langmuir isotherm and pseudo-second-order kinetic.












Similar content being viewed by others
REFERENCES
D. Botkin and E. Keller, Environmental Science (Wiley, New York, 1995).
H. Zollinger, Color Chemistry Synthesis, Properties, and Applications of Organic Dyes and Pigments (Wiley-VCH, Weinheim, 2003).
C. L. Chen, J. Hu, D. Xu, X. L. Tan, Y. D. Meng, and X. K. Wang, J. Colloid Interface Sci. 323, 33 (2008).
N. M. Mahmoodi, Water Air Soil. Pollut. 224, 1419 (2003).
R. Gong, M. Li, C. Yang, Y. Sun, and J. Chen, J. Hazard. Mater. 121, 247 (2005).
S. S. Madaeni and Y. Mansourpanah, Desalination 161, 13 (2004).
S. Azizian, S. H. Jafari, and B. Jaleh, J. Industr. Eng. Chem. 18, 2124 (2012).
E. Guibal and J. Roussy, React. Funct. Polym. 67, 33 (2007).
R. Moradi and A. Ganjali, Russ. J. Phys. Chem. A 93, 2789 (2019).
R. Moradi, Iran J. Chem. Chem. Eng. 40, 1083 (2021).
M. E. Mosayebian, R. Moradi, and K. Mahanpoor, J. Nanoanal. 8, 209 (2021).
R. Moradi, Russ. J. Phys. Chem. A 92, 2781 (2018).
H. Hasar, J. Hazard. Mater. 97, 49 (2003).
M. Torkaman, R. Moradi, and B. Keyvani, Rev. Roum. Chim. 61, 763 (2016).
R. Moradi and K. Mahanpoor, Arch. Hyg. Sci. 7, 71 (2018).
M. Atieh, O. Bakather, B. Al-Tawbini, A. Bukhari, A. Abuilaiwi, and M. Fettouhi, Bioinorg. Chem. Appl. 21, 9 (2018).
F. Abuilaiwi, T. Laoui, M. Al-Harthi, and A. Mautaz, Arab. J. Sci. Eng. 35, 37 (2010).
Y. Ying, R. K. Saini, F. Liang, A. K. Sadana, and W. E. Billups, Org. Lett. 5, 1471 (2003).
M. Zahedniya and Z. Ghazi Tabatabaei, J. Water Wastewater 29, 1 (2018).
S. Lagergren, Vetenskapsakad Handling 24, 1 (1898).
Y. S. Ho and G. M. Mckay, Process. Biochem. 34, 451 (1999).
L. Jia, W. Liu, J. Cao, Z. Wu, and Ch. Yang, J. Environ. Manage. 262, 110260 (2020).
I. Langmuir, J. Am. Chem. Soc. 38, 2221 (1916).
H. M. F. Freundlich, Z. Phys. Chem. 57, 385 (1906).
F. Ghorbani, H. Younesi, S. M. Ghasempouri, A. A. Zinatizadeh, M. Amini, and A. Daneshi, Chem. Eng. J. 145, 267 (2008).
D. Pathania, Sh. Sharma, and P. Singh, Arab. J. Chem. 10, S1445 (2017).
F. S. Li, A. Yuasa, K. Ebie, Y. Azuma, T. Hagishita, and Y. Matsui, Water Res. 36, 4592 (2002).
ACKNOWLEDGMENTS
The author would like to gratefully acknowledge members of the Research Laboratory of Islamic Azad University, Tuyserkan Branch, Tuyserkan, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Moradi, R., Mehdizade, S. Adsorption of Acid Orange 7 Dye Pollutant from Aqueous Solutions Using Modified Multi-Walled Carbon Nanotubes. Russ. J. Phys. Chem. 97, 1550–1557 (2023). https://doi.org/10.1134/S0036024423070257
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423070257