Abstract
Renewed interest in inorganic binders for sand molding has also intensified research on different forms of it. In this study, solid inorganic sodium silicate binder was tested with different additives to see how these affected the silica mold quality. The five additives used were: glucose, sucrose, boric acid, aluminum oxide and iron(III)oxide powders. The mold quality was assessed through tests like bending strength, tensile strength, hot distortion, wear resistance, gas evolution and collapsibility tests. In addition, SEM imaging was done on some select mold fracture samples. In the end, a casting trial was carried out followed by a surface roughness and defects analysis. A reduction in mold strength was noticed with glucose and boric acid, while collapsibility was improved by glucose, sucrose and boric acid additives. Casting trials have shown the best surface finish to be obtained with sucrose additive. All the casts in general showed some penetration; however, repeat casts have proven that altering some casting parameters could result in casts with excellent surface finish using solid silicates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sand casting is the most widely used type of casting method worldwide. A good sand mold is a compulsory prerequisite to obtaining a good quality casting. However, producing functional castings are no longer enough as manufacturing industries must ensure sustainability of the process as well. Environmental regulations are becoming stricter and as the world is moving toward more sustainable manufacturing; sand casting is following suit. More sustainable mold materials, i.e., sand, binder and additives are always being researched. One of the effects of this is more interest generated toward inorganic foundry binders that are more environmentally friendly and less hazardous to foundry workers than organic binders. Of the inorganic binders, sodium silicate(water glass) is considered to be the inorganic binder with most potential for achieving green foundry production.1 First used in mid-twentieth century, (Ch. 1)2 a gradual decline in their use took place as the popularity of organic no-bake binders increased. Organic binders gained popularity for their adequate strength, convenience, good knockout and reclamation properties.3,4 However, gas emissions and thermal breakdown at elevated temperature are a source of concern for worker health and safety, and also one of the most widely used organic binder, furan, is a suspected carcinogen.5 Although sodium silicate binders have advantageous characteristics when it comes to environmental friendliness, there are certain challenges to overcome. These include poor resistance to moisture, poor collapsibility after pouring and poor reclamation properties compared to organic binders. (p. 219).6, (p. 204)7, (p. 927).8 Research is going on to overcome these challenges with the use of additives and modifiers. Other advantages of using sodium silicate binder include its low-cost advantage, little to no odor, savings in air filtration equipment resulting from less investment being necessary due to reduced presence of toxic fumes and binder aerosols (p.219).6,9,10
The Case for Solid Inorganic Binder(Solid Silicates)
Solid hydrous silicates are dry counterparts of liquid sodium silicates. Water is added to dissolve the dry powder, after which it could be used similarly to the liquid silicate. The advantage of the use of the solid silicates is in the ease of storage and transport due to the reduced weight and volume of the material.11 Apart from that, solid silicates have the potential to simplify 3D printing of sand molds. 3D printing of sand mold and cores allows exceptional design freedom and reduction in lead time for small series products.12,13 As the world of additive manufacturing is evolving and increasing number of foundries are considering 3D printing of sand molds, there is an additional concern as most state-of-the-art 3D sand printers are using organic binders, and a higher binder amount is usually required with 3D printing of sand mold compared to traditional molding, and a higher binder content usually leads to more gas defects.14,15,16 Apart from that, jetting binders through a printhead causes issues like clogging of printhead nozzles. The use of solid silicates could potentially aid in these two issues. Firstly, it is inorganic with a reduced gas evolution rate and secondly, if only water is jetted through the printhead over a mixture of solid silicates and sand,17 the difficulty associated with the blocking and cleaning of nozzles could be overcome. The longevity of the printheads will increase and their maintenance will be easier and more economical. Ablation casting is another emerging sand casting technology that uses water soluble binder.18 It was shown in19 that thermally hardened hydrated sodium silicate binders perform very well in ablation casting. Therefore, molding with thermally hardened solid silicates could also be a good candidate for ablation casting.
The application of solid silicates could be in two different forms; one of them is to use separate solid silicates mixed with silica sand and another way is to use silicate coated sand. If foundries need only to add water to a premixed sand and solid silicates or to sodium silicate coated sand, it could simplify foundry operations to a great extent. However, before introducing solid silicates as main line foundry binders, or their widespread use in 3D printing, more data and trials are needed to overcome the challenges already known, or to identify any new challenges associated with their use. High surface roughness of castings was obtained with solid silicate molds in,20 which the authors attributed to the difficulty of metal pattern release from the mold, as the mold stuck strongly to the pattern.
Modus Operandi of Sodium Silicate Binder
The two most used ways of hardening sodium silicate are done physically through heat and chemically through carbon dioxide and esters. The requirement of heat is of course a hindrance to this type of binder as the additional energy demand results in more time and money being required. Heating large molds evenly could pose operational challenges for the foundries. Microwave hardening could be much faster compared to processes like furnace heating. It was shown in21 that the cost of hardening sodium silicate bonded cores could be much reduced with microwave and required strength could be obtained even with much smaller dosing amount of binder, as found out in.22 Chemical hardening through CO2 has remained quite popular for core making; however, it has some inherent challenges of its own. For example, the required amount of binder is very high which increases difficulties with collapsibility; also, the core must be made more permeable for CO2 diffusion, which means that the maximum attainable strength is never reached. However, there have been numerous recent studies that achieved quite good strength with CO2 hardening using 2–2.5% of binder, whereby the authors attempted different techniques like blowing compressed air after CO2 blowing, use of heated CO2 and different modifiers.23,24,25 Another semi-inorganic chemical hardening of sodium silicate is achieved through the use of esters. Although this approximates the ease of use similar to that of no-bake organic binders, hydrated sodium acetate formed in this process negatively affects the quality of reclaim from sodium silicate bonded sands.26,27
Additives
Many different additives have been used in foundry practices to improve different mold properties in all types of molding techniques. Additives could be used to improve surface finish or to reduce defects like penetration, veining, porosity in the castings produced. These could also be added to improve mold properties like strength, flowability and collapsibility. One major area of focus with sodium silicate was the use of different additives and modifiers to overcome the challenges originally faced with liquid sodium silicate binder. For example, modification using organic colloidal solutions containing metal oxides (ZnO, Al2O3 or MgO) was found to have improved the wettability28 as well as the strength and collapsibility29 of molds made with liquid sodium silicate. The addition of potassium hydroxide, sodium hexametaphosphate and white sugar was shown to improve the flowability, tensile strength and collapsibility.30 In another study, the addition of potassium hydroxide, lithium hydroxide and disodium phosphate was shown to have improved the tensile strength, although it resulted in a higher residual strength at 800 °C(decreased collapsibility).25 Addition of ultrafine metal oxides of Aluminum, Zinc, Titanium and Magnesium were studied where the author found that different expansion rate of the powders reduced residual strength of the sand and sodium silicate system.31 The modification of liquid sodium silicate to overcome the challenges faced had been a popular contemporary research topic, which is now carried forward to solid silicates in this study. Five different additives were shortlisted for this study to be used with solid hydrous silicates.
Glucose and Sucrose
Sugars and many sugar-based additives are used to improve the collapsibility of liquid sodium silicate bonded sand, (pp. 208–209).7 Sucrose is the only commonly used carbohydrate that dissolves physically in sodium silicate solution without a chemical reaction, (p. 208).7 It was projected that sugars would improve collapsibility of molds made with solid silicates as well. Two different sugars were tried in this study. One is a monosaccharide, glucose and the other a disaccharide, sucrose. Sucrose is a dimer of glucose and fructose.
Boric Acid
The presence of boron compound in the form of sodium tetraborate decahydrate(borax) was discussed in11 as an important additive for sodium silicate binder which allows storage of cores in moist atmosphere and also aids in collapsibility. However, owing to a better solubility of boric acid in water than borax, (pp. 4–53, 4–91)32, the author recommended the use of boric acid rather than borax as an additive for solid silicates.11 Boric acid was also studied as an additive for furan no-bake sand as it has flame retardant properties to reduce the burning of magnesium alloy casting.33 Boric acid powder was hence chosen as an additive in this study.
Metal Oxides: Aluminum and Iron Oxide.
Different metal oxides in the form of ultrafine powders and nanoparticles have been widely researched as an additive for sodium silicate binder. By using an additive mixture of Al2O3, SiC and BN, authors in1 were able to increase ester cured 1 hour tensile strength (from 28.3 to 40.4 N/cm2) and achieved a decrease in residual strength where Al2O3 was the principal constituent. Iron(III)oxide, Fe2O3, was found to reduce porosity overwhelmingly even in very small addition with Phenolic Urethan binder.34 It was shown that core protective coatings made with 100% Fe2O3 as well as mixture of waterglass and Fe2O3 were very successful in preventing porosity formation.35 Although Fe3O4 was not as effective in porosity prevention.36,37 Both Aluminum Oxide and Iron(III) oxide were selected to be tested in this study.
Purpose of This Study
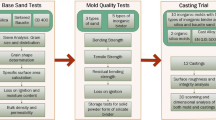
Although many contemporary studies focus on the use of liquid silicates and also its modification to overcome the challenges, there are very few on solid silicates. This study was aimed to learn more about the behavior of solid silicates in molding and casting and to learn how these behavior changes with different additives. Liquid silicate is also included in the study to provide a fiducial point for comparison. Figure 1 illustrates the experimental process followed in this study.
Material and Methods
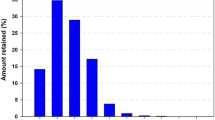
Base properties of the sand used in this study are given in Table 1. The solid binder used in this study was hydrated sodium disilicate powder with molar ratio 2.0, water content of 16–20% and mean particle size of 70 µm. Figure 2 shows the distribution of the sand particle size.
Sand Mixture Preparation
For each batch of sand and binder mixture, 2500 g of sand was used. Addition rate of solid silicate was 0.83% by mass of sand and water addition of 1.17% by mass of sand. Additive addition rate of 15% of the solid binder were used. Example, sand: 2500 g, solid silicate: 20.75 g, water: 29.25 g, additive: 3.11g. This moderately high rate of additives addition was chosen so that a more pronounced effect could be noticed in the mixture. When the sodium silicate mold was heat hardened in a drying furnace, they were heated at 160 °C for 1 hour. The liquid silicate used for comparison is a modified sodium silicate binder, which was used at an addition rate of 2.5% (manufacturer recommendation) and heated at 160 °C for 1 hour. Naming convention used in the study as well as the content of different samples is explained in Table 2.
Strength Test
A good mold must possess sufficient strength to withstand molten metal pressure, to support its own weight and the weight of the metal being cast. To evaluate this, bending strength tests were conducted in this study. Standard test samples of length 172 mm and square cross section of 22.4 mm sides were made for the bending strength tests. All the tests were done using Morek Multiserw Universal Strength Tester LRu-2e/w. Bending strength tests were conducted on the same day, after 4 days and after 7 days to see if the strength level remains similar or if there is much change. The same day test was done 30 min after taking out the sample from furnace. Residual bending strength was measured after heating the test bars at 900 °C for 30 mins and then cooling it to room temperature. In addition to bending strength tests, tensile strength tests were also done on dog bone specimen. The results presented for all the different tests are the mean of 3 samples tested. For information regarding strength level obtained with different organic and inorganic binders with different types of sands, the reader is referred to.20,38
Wear Resistance Test(Using Friability)
With this test, the resistance of the mold surface to abrasion was measured. The research was conducted with the usage of an apparatus designed in Poland by the HSW S.A. company. Standard Ø50 mm x 50 mm cylindrical samples were used. The mass of the sample was first measured; then, the sample was rotated along horizontal axis (1 rev/s) and 1750 g of steel shots of 1 mm dia fall onto the cylindrical specimen from a height of 307 mm.39 The steel shots cause abrasion and wears away some sand. After all the steel shots fell through, the cylindrical specimen was taken out and its mass was measured again. The % change in mass gives a value for the friability of the sample. The lower the friability, the more wear resistant that sample is. Three specimens were measured for each molding sand, and the arithmetic mean is presented. Figure 3 shows the schematic diagram of the friability test.
Gas Emission Test
One of the challenges with organic binders is the large amount of gas release upon pouring. Thermal decomposition at high temperature leads to the emission of VOCs which are harmful for human health.4 Apart from that, large amount of gas release could lead to casting defects as well if the gases cannot escape from the mold fast enough. Inorganic binders perform very well in this regard, as they produce very little fumes compared to the organic counterparts. However, some organic additives are used in this study, e.g., glucose and sucrose, and this gas emission test shows if any big emissions are coming from those additives. For this test only, organic phenolic binder was included as well to show the comparative result between organic and inorganic binder.
Gas emission test was conducted according to Polish standard BN-76/4024-05. After reaching a temperature of 1000 °C, a corundum boat with a weighed sample of 2 g is introduced into the quartz tube. The pipe is closed tightly. The other end of the pipe is connected to a peristaltic pump, which is turned on to create a negative pressure. The measurement is started, and the quartz tube is moved to the position where the sample is in the heating zone. Placing the sample in the heating zone causes the release of gases that are products of the reactions taking place. This increases the pressure in the system. The pump is automatically turned on to remove the generated gases. The recording of the volume of released gases continues until the pressure stabilizes at the initial value.40
Hot Distortion Test
The behavior of cores and molds in high temperature shows the real conditions of casting. During pouring, the mirror of liquid metal rises up, and cores and molds are intensively heated. Cores in initial stage are heated one-sided. During heating many different phenomena occur such as the thermal deformation (expanding and shrinking), the thermoplasticity and thermal and mechanical destruction. All the phenomena depend on the binder type and determine the final shape of the casting, its dimensional accuracy and finally, the quality of the manufactured castings. Hot distortion parameter allows the observation of the core behavior in conditions simulating the real conditions in the mold, when the core is heated by radiation.41
Hot distortion(thermal deformation) parameters were investigated using a DMA apparatus by Morek Multiserw. The 114 mm × 25.4 mm × 6.3 mm specimen was used in this test. One end of the sample is fixed in the jaws of the device, while a tilt sensor rests on the other end(free) of the sample. Additionally, a temperature sensor is provided for more accurate temperature reading. The sample is then heated in the middle from below, with two halogen lamps with a total power of 500 W. The heating temperature ranges from room temperature to 900 °C. The apparatus provides deformation readings as a function of both time and temperature. Maximum deformation reading was set at 6 mm. Schematic diagram of hot distortion test is shown in Figure 4.
Schematic diagram of the Hot Distortion Test, adapted from. 42
Collapsibility
Collapsibility refers to how easily the mold could be broken to take out the casting. Organic binders exhibit very good collapsibility as the binders burn out during the casting process. For example, the tensile strength of furan and silica mold nearly becomes zero in as low a temperature as 400 °C.33 However, such is not the case with sodium silicate as the binders do not burn out. Collapsibility of sodium silicate binders had been a challenge, and there are certain challenges in measuring collapsibility itself.
In this study, the collapsibility test was carried out according to Polish standard PN-85/H-11005. Standard Ø50 mm × 50 mm cylindrical specimen made from the tested sand are placed as cores in the mold cavity and cast with gray cast iron. The cast is then taken, and the core is rammed using the device designed for testing collapsibility according to the standard. The number of hits it takes to completely push out the core is taken as a measure of collapsibility. The smaller number of hits it takes, the more collapsible the sample is. Total work done to remove the core is measured as per equation 1.43
Where 1.63 is the work done by one hit of the weight, in Joules(J);
n is the number of hits of the weight until the core is pushed out of the casting.
Molding and Casting Trial
The pattern used in this study was a thermally resistant 3D printed plastic pattern, with a claimed thermal resistance up to 200 °C as shown in Figure 5. The molds were heated together with the pattern for 1 hour at 160 °C. Afterward, the pattern was removed as it cooled down outside. A mold made in such way is shown in Figure 6. Two such molds were combined to make a bigger phenolic mold as shown in Figure 7. The down sprue was in the middle, and the mold cavities were in the drag side of the mold. Target pouring temperature was varied in the range 1300–1400 °C, and casting alloy used was GJL-300(C:3.4%, Si: 1.6% and Mn:0.6%). High pouring temperatures were chosen in part to exhibit any potential issues that might occur from elevated temperature. The mold combinations used in the casting trial are shown in Table 3.
Fracture Mechanism and SEM Imaging
The mechanical strength of molding and core sands is one of the most important property of these materials and determines not only the quality of the mold and casting, but also the economic aspect of a used technology. The strength of the molding sand in the initial state, after hardening or at elevated temperature is mainly the result of the interaction of the binder and the grain matrix. Therefore, it is realized by the adhesive and cohesive properties of the binder. With mechanical destruction of the bond between the grains, the surface of destruction may run in different places, depending on the ratio of the cohesive forces to the adhesion forces. The place where the surface of destruction runs may cause good or poor collapsibility of molding sands. SEM imaging is a good way of studying fractured surface and identifying fracture mechanism. Figure 8A shows an instance of bonded sands under tension. The following fracture cases can be observed26,43,44:
-
1.
the cohesion forces, i.e., the force of attraction between the layers of the binding material, are greater than the adhesion forces, i.e., the force of attraction between the binding substance and the surface of the sand matrix grains; then, the destruction occurs on the surface of the sand matrix grains—good collapsibility; (Figure 8B)
-
2.
the forces of adhesion exceed the forces of cohesion; then, the destruction of the binder layer itself takes place—poor collapsibility; (Figure 8C)
-
3.
the forces of adhesion and cohesion are balanced; then, destruction can occur on the surface of the sand matrix grains and inside the layer of the binding material;
-
4.
both cohesion and adhesion exceed the strength of the sand matrix grains themselves; then, destruction of sand grains may occur; (Figure 8D)
In order to see the fractured surface, SEM imaging was done using Tescan MIRA4 GMU Scanning Electron Microscope, equipped with Electron source—field emitter with high brightness, and smooth adjustment of the beam current in the range from 2 to 400 nA. The microscope is equipped with two imaging detectors: secondary electron (SE) and backscatter (BSE) detector, and an additional GSD detector of secondary electrons (SE) designed to work in low vacuum mode, which allows the observation of non-conductive samples in their natural state. This means that the mold samples did not have to be coated with conductive material like gold or carbon. 4 chosen samples were studied, LS, SS, SSGC and SSSC. The study was conducted on fractured surfaces, to reveal if there was any difference in the way they fractured.
3D Scanning and Optical Surface Roughness Analysis
3D scans were taken of the molds with a structured light 3D scanner(manufacturer claimed accuracy up to 0.05 mm and resolution of 0.13 mm). The 3D scans of the molds were used to confirm mold surface geometry and also as backup data that could be investigated as part of the defects analysis(for example to confirm if certain defect came from the molding or casting phase).
As-cast surface roughness is assessed commonly through use of Gar Microfinish comparator C-9 or the SCRATA plate(ASTM A802 standard).46,47 An operator visually compares the surface of the casting and the reference plate, which results in only discrete assessment levels. Results might vary between operators, and it is very difficult to decide between surfaces with very close surface roughness. For this study, a 3D optical profilometer was used for evaluating surface quality. The profilometer software (vision64) gives an average Ra reading for a sampled area, 2 mm-by-2 mm in the case of this study. The built in function of the software allows for converting slight curvatures in the surface to planar ones, which gives a more accurate value of the surface roughness.
Results
Mold Quality Tests
The observation showed that the produced molds had very good surfaces. The use of thermal resistant plastic patterns instead of metal ones allowed for easier pattern removal which resulted in excellent surface quality in the as-molded state, with little to no visible damage. It could also be confirmed from 3D scans of the molds. One such example, SSSC mold is shown in Figure 9. As a comparison, a mold made with solid silicate and metal pattern(different geometry but similar) is shown in Figure 10.
Strength Tests
Results of strength tests are shown in Figure 11. Good bending strength, in excess of 260 N/cm2, was obtained with the solid silicates without any additives. With boric acid, it decreased to around 200 N/cm2 and with glucose; it decreased even further to around 170 N/cm2. Addition of sucrose, aluminum oxide and iron oxide increased the strength to over 300 N/cm2. The highest strength was obtained with liquid silicate in excess of 380 N/cm2(Same day maxima: 414.3 N/cm2; same day minima: 365.3 N/cm2). A similar trend was noticed with the tensile strength as well where the lowest strength of solid silicates was obtained with SSGC. The highest strength was again obtained with liquid silicate, LS(Same day maxima: 212.4 N/cm2; same day minima: 174.3 N/cm2). Not much difference in residual bending strength of the samples was noticed. It is to be emphasized that the same day specimens were tested 30 mins after taking out from furnace heat, and it is possible that the strength continued to increase for some time after that.
Wear Resistance Test(Friability method)
The lower the friability, the more wear resistance the sample has. Results of Friability test are shown in Figure 12. The most wear resistant sample was the liquid silicate, with friability less than 0.7%. Solid silicates without additives had the second lowest friability at about 1.4%. Addition of boric acid, aluminum oxide and iron oxide increased the friability of solid silicates slightly but still quite good at less than 1.8%. The highest friability was obtained with glucose which means SSGC had the least wear resistance. The results of the tests well complement the sand strength tests.
Gas Emission Test
Figure 13 shows the result of gas emission tests. The lowest emission was seen with LS, SS and SSBA at 16.5 cm3/g. Organic addition of sucrose increased the emission to 17 cm3/g, while the glucose increased it further to 19 cm3/g. These values are still lower than that of reference organic binder phenol, which produced 23.5 cm3 of gas per gram of molding sand.
Hot Distortion
The results of Hot Distortion tests are presented in Figures 14 and 15.
The thermal deformation of all tested molding sands has a typical pattern for molding sands with sodium silicate42 with a very slight (less than approx. 0.3 mm) deformation in the opposite direction to the heat source.
Tested molding sands were characterized with good heat stability. All tested mixtures exhibited almost no thermal deformation in the temperature range of 0—approx. 200 °C. After crossing this temperature, samples are subjected to mild deformation until they are damaged. The samples made from molding sand with sodium silicate begins to degenerate after about 50 sec. The best thermal stability was achieved for molding sand with solid silicate and glucose additive (SSGC) and molding sand with solid silicate without additives (SS). Additionally, in case of SSGC molding sand, longer time needed for the sample destruction was observed.
Collapsibility Tests
Results for the collapsibility tests are shown in Figure 16. The best result was obtained with liquid silicate(LS), which was expected as LS was modified for good collapsibility. Solid silicate(SS) had lower collapsibility value(11.41J of work required to push core out) than LS. However, the addition of glucose, sucrose and boric acid had improved the collapsibility of the solid silicates very close or equal to that of LS, while addition of aluminum oxide and iron oxide has decreased the collapsibility compared to solid silicates.
SEM Imaging of Fractured Surface
Some example photos of fracture surface are shown in Figures 17, 18 and 19. Bonding bridges are marked in red highlighter, while remains of a broken bonding bridge is marked with yellow highlighting. Arrows are used to show sheared sands. Presence of a high number of fractured sand, rather than the destruction of bonding bridge was seen with SS, as shown in Figure 17 A. Remnants of bonding bridge were distinguished from sand by elemental analysis with EDS(Energy-Dispersive Spectroscopy) and mainly through tracking presence of sodium. Examples of such analysis are shown in Figure 17 B, C.
With LS, fractures were seen both on the surface of the sand and some destruction of sand as shown in Figure 18. Fracture of the bonding bridge rather than within the sand body itself was noticed with glucose as shown in Figure 19A. Addition of sucrose revealed a similar fracture to SS, as sand shearing was seen, and many intact bonds as shown in Figure 19B. This confirms the high strength values of the bonds with SSSC. The EDS also revealed little to no undissolved solid silicate on the surfaces of sand.
Casting Trial and Integrity Analysis
Figure 20 shows the casting obtained with SS. Due to penetration, some sand particles can be seen embedded to the cast in the top flange, and a bit more in the middle of the casting. The heaviest penetration was seen in the region between top flange and middle. No penetration was seen on the right and left flanges. Figure 20 also shows a stereo microscope image of the right flange, and surface analysis using optical profilometer is shown for the left flange. Similar condition was achieved with casts using the different additives; therefore, it was decided to take surface roughness readings on the left and right flange only. Table 4 shows the surface roughness values for casts.
As can be seen in Table 4, except for SSAM all the other additives resulted in better surface roughness readings compared to SS. The best was obtained with SSSC. Liquid silicate also resulted in higher surface roughness values compared to SS. It was decided to repeat some casts with solid silicate alone(no additives) as the understanding of the authors was that it was more of a fluidity of the molten metal and elevated temperature that caused the penetrations, and penetrations could be reduced with some alterations. Hence, 4 casts were repeated with SS: at higher pouring temperature of 1400 °C but graphite coating applied(SS1), same pouring temperature but higher amount of binder(SS2), lower pouring temperature of 1320 °C(SS3) and even lower pouring temperature of 1300 °C(SS4). Surface roughness values were measured and shown in Table 5.
Improved results were obtained with SS1, SS3 and SS4 with very reduced penetration. A higher amount of binder did not improve the surface quality(SS2). SS1 along with a surface micrograph using optical profilometer is shown in Figure 21. Average of the roughness values for all the measured specimens is shown in Figure 22.
Discussion
This study was aimed at studying the effect of different additives on the bonding properties of solid sodium silicate binder. However, the authors took the opportunity to learn more about solid silicate binder itself as well, as there is very little information regarding these in literature. In previous study, metal pattern sticking to solid silicates was reported.20 This challenge was overcome in this study using a 3D printed thermal resistant plastic pattern. The molds produced had excellent surface finish with little to no visible damage as demonstrated in Figures 6, 7 and 9.
From the strength results, it was generally seen that the addition of sucrose, aluminum oxide and iron oxide had increased the strength of solid silicates, while that of glucose and boric acid had decreased the strength level, although even the decreased level seen in this study would be widely acceptable for most casting applications. Glucose and boric acid also exhibited good collapsibility behavior, which was expected looking at the tensile and bending strength properties. Sucrose exhibited good collapsibility behavior too, although it increased the bending and tensile strength. One interesting aspect of this could be in the 3D printing of sand molds using the solid silicates. Instead of jetting water through the printhead, glucose or sucrose solution could be jetted for example if that simplifies the process instead of mixing it with sand itself. Further test is of course required to find a feasible solution that works better. Increase in strength by aluminum oxide and iron oxide could be exploited, for example, by decreasing the amount of solid silicate binder usage. This might help both with economics of the process and with the eventual collapsibility and reclamation of the sand. The friability tests have revealed weaker resistance of glucose added solid silicates(SSGC) compared to only solid silicates(SS); however, it did not seem to affect the surface quality of casts negatively in the trials conducted.
From the gas emission tests, it was evident that the addition of organic additives increased the emissions to a small extent; however, the emission was still considerably less than a reference organic phenolic binder. There was no evidence of gas porosity occurring in any of the castings with additives. A considerable portion of gases from sodium silicate is steam and some foundries use this phenomenon to avoid using expensive facing sands like zircon or chromite. Facing sands like zircon and chromite are used for their excellent heat conducting abilities, to produce a chilling effect on casts. When free water and water of crystallization are removed from sodium silicate binders, a substantial amount of latent heat is transferred out, resembling the chilling effect produced by the heat conducting facing sands, (p. 938).8 Another noteworthy point was that both liquid silicate(LS) and solid silicate(SS) produced the same amount of gases, as was expected. Only the volume of emitted gases was measured in this study, however. Further study related to this could be for example to study the composition of the emitted gases with each additive.
The hot distortion tests have revealed good results of solid silicates, with and without additives compared to liquid silicates. Low deflection could be an advantage in the case of pattern release in the case where the pattern has to be heated together with the mold (as was done in this study) and also for smaller dimensional variation. After the 200 °C mark, all the additives exhibited a similar behavior except the one with glucose. All other additives exhibited a similar trajectory which is observed with liquid silicate(LS). For glucose, the gradual deflection rather than a steep one could indicate more ductility than brittleness of the mold. However, the longer time needed for the sample destruction was observed with SSGC could be beneficial in terms of the time of contact of the molding/core sand with elevated temperature during and after the pouring process.
The best collapsibility was demonstrated by LS; this liquid silicate was chosen in this study as it was modified for collapsibility. Although SS had lower collapsibility than LS, addition of glucose, sucrose and boric acid improved the collapsibility very close to that of LS. However, it must still be emphasized that more research is required to improve the collapsibility even further to match that of the organic binders. Measuring true collapsibility is a challenge for sodium silicate binders, as the strength relation with temperature is not linear, rather there are two maxima’s that occur, one around 200 °C and the other around 800 °C. Depending on the temperature of the melt and also on the size and geometry of sand mold, some sand could end up in the maxima’s which would negatively hinder collapsibility of the whole mold. Any additives that stop the second maxima from occurring will improve collapsibility of sodium silicate bonded sands used for cast iron and cast steel castings, which could be the reason of improved collapsibility with glucose, sucrose and boric acid. Other methods used to assess collapsibility include the retained strength and there were also recommendations of using volumetric expansion of silica at high temperature as an estimation of collapsibility.48
The casting trial had revealed some important considerations. Penetrations were seen at numerous places as explained in section "Casting trial and integrity analysis." Penetration was seen in the top flange and in the middle and even more in the region between top flange and the middle. However, the right and left flanges generally had much better as-cast condition as no sand inclusions were present. A couple of things to note here. Firstly, gray cast iron is generally known to cause high penetrations, which was evident, and it was seen that the liquid silicate(LS) had greater penetration than solid silicates without additives(SS). Secondly, except for aluminum oxide, rest of the additives which were tested resulted in better as-cast condition. It was assumed then that casting conditions could be modified a bit and tested with solid silicates alone. If improvements could be achieved with solid silicates alone, it could be generally accepted that conditions will improve with the additives as well. The recasting trial with SS1, SS2 and SS4 had resulted in much better surface than was originally obtained with SS. This proved the fact that the penetrations could be reduced with better temperature control, application of coating, etc. So, trials are needed to find a compromise for each alloy type, as the fluidity of the melt is also a function of both alloy type and pouring temperature. Reduced temperature led to much better surface quality in SS3 and SS4; however, some evidence of cold shut was starting to be seen. Thirdly, organic binders suffer at elevated temperature as well and that’s why different coatings are applied in molds for cast iron and steel castings. In the case of gray cast iron, hydrocarbon rich (e.g., organic binder or green sand with coal dust additives) mold performs better at preventing penetrations as hydrocarbons pyrolyze a film of solid carbon on the advancing liquid melt, which then meets a pyrolyzed layer of carbon on the sand surface, producing a carbon-on-carbon interaction and a shiny metal surface. This could also be a potential reason why the best surface finish was obtained with organic sucrose additive and also why the application of graphite-based coating improved the casting surface finish. Fourthly, any other measures that generally reduce penetration could be tried to improve the casts. Examples include the use of sand with smaller particle size which helps in reducing the pore size, thereby hindering penetration. Also, this reduces the permeability of mold, hence the trapped gases could potentially exert an opposing pressure to the advancing molten liquid, which would help reduce penetration. Fifthly, it is a common practice to subject most casts to some kind of post treatment to improve the surface finish. Such treatment could include bead blasting, sand blasting, etc. Hence, having slightly less than optimal as-cast condition does not render the cast useless as there are several techniques available to improve the surface finish of the castings. Therefore, considering the casting alloy in question, sand particle size, use of additives and coatings and suitable pouring temperature, excellent casting results should be possible to obtain with solid silicates and gray cast iron. Casting other less penetrating cast irons should then be of no greater challenge with solid silicates.
This study focused on the effect of individual additives to inorganic solid silicate binder. The additives were used in a moderately high amount to find their individual effect on mold and cast qualities. Some of the additives were found to have desirable qualities. However, it remains to be seen if a blend of the additives could be found that elevate all the desirable qualities in the mold and casts and reduce the undesirable characteristics. Orthogonal tests could be done to find out optimum mixture amount. However, it could be said with some degree of certainty that solid silicates offer a promise of sustainable molding that has the potential to be used for 3D printing of sand molds and also to be a main line foundry binder for foundries. However, to be ascertained of true sustainability of solid sodium silicates, the difficulty of reclamation of sodium silicate-based binder needs to be addressed, also more efficient heating is required. Use of microwave hardening is a potential solution for more efficient heating. Mechanical reclamation is not very efficient for sodium silicate sands. Wet reclamation of sodium silicate-based sands, on the other hand, works very well as it is water soluble. Although wet reclamation is more expensive and there is a risk of secondary pollution from the wastewater, if a process could be developed to reclaim and reuse this dissolved sodium silicate from wastewater, it opens up the door for a fully circular ecosystem for foundries where both the sand and binder could be recycled.
Future Work
-
Orthogonal test to find out a blend of additives that could further improve the desired qualities and reduce the undesirable ones.
-
Exploring other ways of hardening solid silicates: ester, CO2, microwave hardening, etc.
-
Investigating reclamation properties of solid silicates, both with and without additives.
Conclusion
The pursuit of manufacturing sustainability is an ongoing phenomenon across the whole world. A sodium silicate-based binder is deemed to be a forerunner in increasing the sustainability of the sand casting process. Solid silicate binder has the potential to play a significant role in this as it provides additional advantage compared to liquid silicates. The authors have revealed several important findings in this study regarding solid silicates and how it is affected by five different additives.
Addition of glucose has generally reduced the strength of the mold and wear resistance; however, it improved the collapsibility and reduced distortion at elevated temperature. Addition of sucrose increased both the strength and collapsibility of the solid silicates, while boric acid reduced the strength and improved collapsibility. Both aluminum oxide and iron oxide had increased mold strength, which could play an important role in reducing binder addition rate.
Only aluminum oxide produced casts that had worse surface roughness compared to solid silicates without additives, while addition of sucrose produced the best as-cast surface condition. The recasting trial had shown evidence that selecting proper pouring temperature for a particular alloy in use and also using other improvement techniques like use of coatings, very good quality castings can be produced with solid silicates that has the potential of becoming the norm of sustainable foundries in future.
References
H. Miao, X. Du, Y. Sun, M. Zhang, G. Song, Effect of powder breakdown additives on properties of ester-hardened sodium silicate bonded ceramic sand. Int. J. Metalcast.Metalcast. 15(2), 710–718 (2021). https://doi.org/10.1007/s40962-020-00517-z
H. Polzin, Inorganic binders: for mould and core production in the foundry, 1st ed. Fachverlag Schiele and Schön GmbH, 2014
J.T. Fox, F.S. Cannon, N.R. Brown, H. Huang, J.C. Furness, Comparison of a new, green foundry binder with conventional foundry binders. Int. J. Adhes. Adhes.Adhes. Adhes. (2012). https://doi.org/10.1016/j.ijadhadh.2011.11.011
A. Kmita, C. Fischer, K. Hodor, M. Holtzer, A. Roczniak, Thermal decomposition of foundry resins: a determination of organic products by thermogravimetry–gas chromatography–mass spectrometry (TG–GC–MS). Arab. J. Chem. 11(3), 380–387 (2018). https://doi.org/10.1016/j.arabjc.2016.11.003
N. Bakhiya, K.E. Appel, Toxicity and carcinogenicity of furan in human diet. Arch. Toxicol.Toxicol. (2010). https://doi.org/10.1007/s00204-010-0531-y
M. Holtzer, A. Kmita, Mold and core sands in metalcasting: chemistry and ecology. Sustain. Dev. (2020). https://doi.org/10.1007/978-3-030-53210-9_4
J. R. Brown, Ed., Foseco Ferrous Foundryman’s Handbook, 11th ed. Elsevier, 2000
J. Campbell, Complete Casting Handbook: Metal Casting Processes, Tech Des. 2011
L. Zaretskiy, Modified silicate binders new developments and applications. Int. J. Metalcast.Metalcast. (2016). https://doi.org/10.1007/s40962-015-0005-3
A. Fortini, M. Merlin, G. Raminella, A comparative analysis on organic and inorganic core binders for a gravity diecasting Al alloy component. Int. J. Metalcast.Metalcast. 16(2), 674–688 (2022)
L. Zaretskiy, Hydrous solid silicates in new foundry binders. Int. J. Metalcast.Metalcast. 12(2), 275–291 (2018). https://doi.org/10.1007/s40962-017-0155-6
S.R. Sama, T. Badamo, G. Manogharan, Case studies on integrating 3D sand-printing technology into the production portfolio of a sand-casting foundry. Int. J. Metalcast.Metalcast. 14(1), 12–24 (2020). https://doi.org/10.1007/s40962-019-00340-1
E.S. Almaghariz et al., Quantifying the role of part design complexity in using 3d sand printing for molds and cores. Int. J. Metalcast.Metalcast. 10(3), 240–252 (2016). https://doi.org/10.1007/s40962-016-0027-5
D.A. Snelling, C.B. Williams, A.P. Druschitz, Mechanical and material properties of castings produced via 3D printed molds. Addit. Manuf.. Manuf. 27, 199–207 (2019). https://doi.org/10.1016/j.addma.2019.03.004
P.M. Hackney, R. Wooldridge, 3D sand printing for automotive mass production applications. Int J Rapid Manuf 6(2–3), 134–154 (2017). https://doi.org/10.1504/IJRAPIDM.2017.082156
D. Snelling, R. Kay, A. Druschitz, and C. Williams, Mitigating gas defects in castings produced from 3D printed molds, 2013
R. Ramakrishnan, B. Griebel, W. Volk, D. Günther, J. Günther, 3D printing of inorganic sand moulds for casting applications. Adv. Mater. Res. Mater Res (2014). https://doi.org/10.4028/www.scientific.net/AMR.1018.441
Q. Han, Ablation casting: solidification characteristics, microstructure formation, and mechanical properties. Int. J. Metalcast.Metalcast. 15(4), 1213–1222 (2021). https://doi.org/10.1007/s40962-020-00544-w
K. Major-Gabryś, S. Puzio, A. Bryłka, J. Kamińska, The influence of various matrixes on the strength properties of moulding sands with thermally hardened hydrated sodium silicate for the ablation casting process. J Cast Mater Eng 5(2), 31–35 (2021). https://doi.org/10.7494/jcme.2021.5.2.31
N. Anwar, K. Jalava, J. Orkas, Experimental study of inorganic foundry sand binders for mold and cast quality. Int. J. Metalcast.Metalcast. 17(3), 1697–1714 (2022). https://doi.org/10.1007/s40962-022-00897-4
A. Malachowska, M. Stachowicz, K. Granat, Innovative microwave hardening of water-glass containing sandmixes in technical-economic approach. Arch. Foundry Eng. 12(1), 75–80 (2012). https://doi.org/10.2478/v10266-012-0015-z
K. Granat, D. Nowak, M. Pigiel, M. Stachowicz, R. Wikiera, The influence of hardening method on basic properties of water glass molding sands. Visnik Chmel’nickogo Nacional’nogo Universitetu 1(4), 98–104 (2007)
L. Song, K. Zhao, W. Liu, F. Xin, B. Liu, Y. Li, Effects of water and compressed air pressure on the adhesion of Na2SiO3 binders on silica sand surface: comparison of experimental data and molecular dynamics simulation. Int. J. Metalcast.Metalcast. 17(2), 1350–1360 (2023). https://doi.org/10.1007/s40962-022-00862-1
Q. Li et al., Effect of carbon dioxide temperature on adhesion of Na2SiO3 binder to silica sand surface: comparison of experimental data with molecular dynamics simulations. Int. J. Metalcast.Metalcast. (2023). https://doi.org/10.1007/s40962-023-01181-9
L. Song, X. Du, G. Song, Y. Sun, Effect of complex modifier on properties of heat-hardened sodium silicate-bonded sand for castings production. Int. J. Metalcast.Metalcast. (2023). https://doi.org/10.1007/s40962-023-01008-7
P. Jelinek, Binding systems of foundry moulding sands(in Czech). Ostrava, 2004
S.M. Dobosz, K. Major-Gabryś, M. Hosadyna, New look at the process of reclamation of moulding sands. Arch. Foundry Eng. 12(3), 19–24 (2012). https://doi.org/10.2478/v10266-012-0075-0
A. Kmita, A. Roczniak, Nanocomposites based on water glass matrix as a foundry binder: chosen physicochemical properties. Arch. Foundry Eng. 17(1), 93–98 (2017). https://doi.org/10.1515/afe-2017-0017
A. Kmita, Modification of water glass, the moulding sands binder, by nanoparticles of metal oxides in organic solvents., AGH, 2014
F. Xin, W. Liu, L. Song, Y. Li, Modification of inorganic binder used for sand core-making in foundry practice. China Foundry 17(5), 341–346 (2020). https://doi.org/10.1007/s41230-020-0018-2
Y. Cheng, Effect of ultra-fine powder modifying sodium silicate on foundry sand (in Chinese), Hot. Work Technol., pp 82–84, 2014, https://doi.org/10.14158/j.cnki.1001-3814.2014.19.020.
W. M. Haynes, Ed., CRC Handbook of Chemistry and Physics, 95th ed. Boca Raton: CRC Press, 2014. https://doi.org/10.1201/b17118.
F. Liu et al., Performance of resin bonded sand for magnesium alloy casting. J. Manuf. Process. 30, 313–319 (2017). https://doi.org/10.1016/j.jmapro.2017.10.002
R.L. Naro, Porosity in iron castings from mold-metal interface reactions. Modern Cast 90(4), 41–44 (2000)
C. Umezurike, W.O. Onche, Experimental analysis of porosity in gray iron castings. Global J Res Eng 10(7), 65–70 (2010)
R.W. Monroe, The use of iron oxides in no-bake bonded sand molds. Steel Founders’ Res. J. 5, 9–20 (1984)
AFS cured sand committee, The effects of iron oxide additions on core properties and casting quality, in Modern Cast., 1982, p 29
N. Anwar, T. Sappinen, K. Jalava, J. Orkas, Comparative experimental study of sand and binder for flowability and casting mold quality. Adv. Powder Technol. 32(6), 1902–1910 (2021). https://doi.org/10.1016/j.apt.2021.03.040
A. Bobrowski, K. Kaczmarska, D. Drożyński, F. Woźniak, M. Dereń, B. Grabowska, M. Szucki, 3D printed (binder jetting) furan molding and core sands—thermal deformation. Mech Technol Proper. Mater 16(9), 3339 (2023). https://doi.org/10.3390/ma16093339
A. Kmita, D. Drożyński, J. Mocek, A. Roczniak, J. Zych, M. Holtzer, Gas evolution rates of graphite protective coatings in dependence on the applied solvent and kind of atmosphere. Arch. Metall. Mater.Metall Mater 61(4), 2129–2134 (2016). https://doi.org/10.1515/amm-2016-0284
J. Jakubski, St. M. Dobosz, K. Major-Gabryś, and A. Grabarczyk, Hot distortion—important parameter of estimation quality of moulding and core sands, in The 2015 WFO International Forum on Moulding Materials and Casting Technologies, Changsha, China: WFO Moulding Materials Commission, Foundry Institution of Chinese Mechanical Engineering Society, Productivity Promotion Center of Foundry Industry of China, Oct. 2015, pp 86–89
K. Major-Gabryś, A. Grabarczyk, S.M. Dobosz, J. Jakubski, J. Morek, J. Beňo, Measurement of molding sand elasticity. J Cast Mater Eng 2(2), 38–44 (2018). https://doi.org/10.7494/jcme.2018.2.2.38
J.L. Lewandowski, Materials for foundry moulds (AKAPIT, Krakow, 1997)
S. M. Dobosz, Water in Molding sands(in Polish: Woda w masach formierskich ). Krakow: AKAPIT, 2006
S. Dobosz, Aktywacja cieplna piasków kwarcowych (Akad, Górniczo-Hutnicza, 1990)
R. Tuttle, S. Ramrattan, L. Wells, As-cast surface characterization for steel using disk-shaped chemically bonded sand specimens. Int. J. Metalcast.Metalcast. 15(2), 382–390 (2021). https://doi.org/10.1007/s40962-020-00520-4
D.W. Schimpf, F.E. Peters, Variogram roughness method for casting surface characterization. Int. J. Metalcast.Metalcast. 15(1), 17–28 (2021). https://doi.org/10.1007/s40962-020-00451-0
K. Major-Gabryś, S.M. Dobosz, P. Jelínek, J. Jakubski, J. Beňo, The measurement of high-temperature expansion as the standard of estimation the knock-out properties of moulding sands with hydrated sodium silicate. Arch. Metall. Mater. 59(2), 739–742 (2014). https://doi.org/10.2478/amm-2014-0123
Acknowledgement
Friability tests, collapsibility tests, hot distortion tests, gas emission tests and SEM imaging were conducted with equipment at Faculty of Foundry Engineering, AGH University of Krakow (Krakow, Poland). The authors extend their gratitude to the University and personnel, especially Prof. Aldona Garbacz-Klempka, for their sincere assistance. Authors also thank Soilikki Kotanen for her expertise regarding binders and chemicals, and also Kiilto Oy for supplying some required material for the experiments.
Funding
Open Access funding provided by Aalto University. This study was funded by Aalto University and Business Finland. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwar, N., Major-Gabryś, K., Jalava, K. et al. Effect of Additives on Heat Hardened Inorganic Solid Foundry Binder. Inter Metalcast (2024). https://doi.org/10.1007/s40962-024-01277-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40962-024-01277-w