Abstract
This work assesses the effect of cooling rate (\(\dot{T}\)) on the solidification kinetics and microstructural evolution of a 319 aluminum alloy with and without the addition of Al–5 wt%Ti–1wt%B grain refiner. Directionally solidified samples were produced under various cooling rates (from 0.05 to 40 °C/s). Microstructure coarsening was then quantitatively analyzed by measuring grain size (GS) and primary (λ1), secondary (λ2) and average (λL) dendrite arm spacing. The results show that polarized optical images can be used to identify dendrite fragments between neighboring primary dendrites, allowing a more accurate microstructural analysis after the addition of grain refiner. The results also show that, for a given \(\dot{T}\), although GS is reduced after the addition of grain refiner, the respective values of λ1, λ2 and λL remain practically the same. In other words, the grain refiner only affects nucleation and not grain growth. Experimental growth equations are proposed to represent this behavior. Furthermore, λL is shown to be a good parameter for evaluating microstructural refinement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of their exceptional combinations of properties, such as high strength-to-weight ratio associated with outstanding castability, excellent wear performance and high recyclability, Al-based alloys find widespread use in both the aerospace and automotive industries.1 Among these alloys, 319 Al alloy (Al–Si–Cu based) deserves special attention. The composition of this alloy is of particular interest in the manufacture of engine blocks because of its low density, good mechanical strength at relatively high temperatures and suitable wear resistance.2,3 Without reliable control of the microstructure, however, the optimized properties of products made from 319 alloy cannot be totally guaranteed.4
In processes such as conventional sand casting, die casting, welding and additive manufacturing, Al–Si–Cu alloys used to manufacture components with different shapes and sizes usually undergo solidification. Control of the cooling rate (\(\dot{T}\)) during these processes is of practical importance since the rate at which the temperature decreases plays a key role in the evolution of the microstructure. This is also true for rheocasting (directly) and for thixoforming (prior to forming, during the feedstock preparation step) as \(\dot{T}\) directly affects the microstructure of the semisolid slurry, which should consist of as fine and globular a solid phase as possible within the liquid for adequate thixotropy.5,6,7
One of the most important microstructural features observed in many castings is the dendritic microstructure. Over the past years, significant efforts have been made to achieve a better understanding of dendrite growth during directional solidification of several Al-based alloys.8,9,10,11,12,13 The key parameters used in dendrite growth analysis are primary (λ1), secondary (λ2) and tertiary (λ3) dendrite spacing, which are related to thermal solidification parameters such as \(\dot{T}\).9,10,11,12,13,14,15,16,17,18 Dendrite spacing is widely known to be a determining factor for many mechanical properties.8,13 For example, λ2 is known to affect the permeability of semisolid structures.16 However, very few of these studies focused on the effect of the addition of grain refiners.
The use of grain refiners of different types, such as Al–Ti–B or Al–Ti–C, with Al-based alloys is a fundamental practice in the foundry industry and should therefore be the subject of study.9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 An excellent strategy for systematic investigation into the effects of these grain refiners is the use of transient directional solidification techniques as with only one experimental run a wide range of \(\dot{T}\) can be achieved along the length of the casting and, in turn, a wide range of a microstructural scales. This experimental approach is adopted here to compare the evolution of the solidification microstructure of 319 alloy with and without Al–5 wt% Ti−1 wt% B grain refiner, referred to here as 319R and 319, respectively. One of the main contributions of this work is the application of the dendrite growth theory to predict and control casting, die casting, rheocasting and feedstock preparation parameters for thixoforming operations.
Experimental Procedure
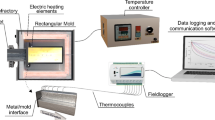
The chemical composition of the 319 alloy studied here was determined with a BILL OES optical spectrophotometer and is shown in Table 1. First, 750 g of the 319 alloy was put in a SiC crucible, which was then placed in a muffle furnace set to 750 °C. For the experiments with grain refiner, when the alloy had melted completely, the crucible was taken out of the furnace and a stoichiometric amount of an Al–5wt%Ti–1wt%B master alloy was added to the liquid to achieve a Ti solute content of 0.2 wt%. Next, the molten alloy was mechanically homogenized with a stainless-steel bar coated with alumina and poured into a mold inside a directional solidification apparatus15,16,17.Figure 1a presents a schematic overview of the solidification device. After the pouring procedure, argon was injected into the melt for at least 3 minutes to remove trapped gases.
Unsteady-state solidification experiments with the 319 and 319R alloy were carried out in the directional solidification apparatus, in which an electrical resistance around the mold allows the temperature of the melt to be controlled and ensures that heat is only extracted through a water-cooled bottom made of AISI 1020 steel following the same procedures described in previous articles.15,16,17,26,27 Prior to mold assembly, the inner surface of the mold’s bottom part (which was intended to come into contact with the molten alloy) was metallographically ground to achieve a standardized finishing using 1200-grit SiC abrasive paper. A stainless-steel mold with an internal diameter of 55 mm, a height of 110 mm and a wall thickness of 5 mm was used. The mold was constructed using two AISI 310 stainless-steel half-cylindrical shells, which were joined together using M6 screws and nuts. Along the height of the mold, eight through holes were designed for positioning type K thermocouples with an outer diameter of 1.6 mm. These thermocouples allowed for the temperature monitoring during solidification. Figure 1b provides a schematic illustration showcasing the precise dimensions of the mold, the locations of the thermocouples and details of the water-cooled bottom part. To avoid radial temperature gradients and facilitate casting removal, a ceramic coating was applied to the inner vertical surface of the mold. When the melt temperature was 10% higher than the liquidus temperature, the electric heaters were disconnected and the water-flow system turned on so that solidification could start.
Real-time temperature during solidification was monitored with a set of fine K-type thermocouples (1.6 mm external diameter) placed along the length of the castings at 15mm deep and connected by coaxial cables to a Lynx ADS1000 data logger. Temperature-time data were recorded at a frequency of 5 Hz and used with well-known techniques28 to determine the cooling rate (\(\dot{T}\)) when the liquidus isotherm passed each thermocouple. Basically, \(\dot{T}\) values were calculated using the derivative with respect to time of fitted regression profiles that represented the trend lines of the cooling curves in a comprehensive region around the liquidus temperature (± 15 °C) with a coefficient of determination (R2) greater than 0.9.
For macrographic examination, the directionally solidified (DS) 319 alloy casting was sectioned along its vertical axis, and one of the semicylindrical parts was ground with 100- to 1200-grit soft SiC papers and then etched with aqua regia solution (3:1 mixture of HCl and HNO3, respectively). For microstructural characterization, selected cross sections at different positions in relation to the metal/mold interface were extracted from the DS casting. The microstructure of the alloy was evaluated after polishing down to 0.1 µm with a metallographic vibratory polisher. Conventional metallography in black and white (B&W) involved solely polishing, while for color metallography the samples were subjected to electrolytic etching with a 1.8% HBF4 solution under mechanical stirring at 0.6 A and 30 V for 180 s. Polarizing filters were used to obtain color images allowing dendrites with varying crystal orientations to exhibit distinct colors. In both cases, a Leica DM ILM microscope was employed for observation.
Dendrite arm spacing was measured in longitudinal and transverse cross sections of samples extracted along the length of the DS castings. The triangle method29 was used with ImageJ to measure primary dendrite arm spacing (λ1) in transverse cross sections, while the intercept method was used to measure average grain size (GS) and average arm spacing (λL) in transverse cross sections, although the secondary arm spacing (λ2) was measured in the longitudinal sections (approximately 50 and 200 measurements were taken for each selected longitudinal and transverse position, respectively).30 Note that the average arm spacing (λL) is measured using the Heyns intercept method and represents the average distance between two consecutives branches of the dendritic structure, disregarding if represents the primary or secondary arm spacing.
Results and Discussion
In Figure 2a, the white vertical line indicates the composition of the 319 alloy in the partial phase diagram determined by Thermo-Calc® (V 5.01.61) computational thermodynamics software with the TTAL5 database. Starting from the liquid, an Al-rich primary phase (AlFCC for low silicon content and Alα for high silicon) is formed in the area highlighted in dark green), followed by a Si-rich main eutectic. Further tertiary eutectic phases consisting mainly of AlFeSi-β and small amounts of Al3Ni2 are formed at lower temperatures. The simulation also provides the liquidus (610.8 °C) and solidus (502.8 °C) temperatures of the alloy. The elements Al, Si, Cu, Fe, Mg, Zn, Mn, Sn, Ni and Cr were considered in the simulation. At room temperature, Thermo-Calc® predicts the existence of Alα and the Al/Si eutectic phase, as well as the AlFeSi-β and small amounts Al2Cu, Al5Cu2Mg and Al7Cu4Ni in addition to TiAl3, especially for the chemical refined 319R structure. This evaluation aims to provide the reader an idea of the possible metallic phases that can be found in the material under analysis and represents a calculation of the equilibrium phases.
Figure 2b shows the characteristic as-solidified microstructure of the 319 alloy. The microstructure is essentially formed of dendrite-like Al-rich primary phase (A) and intergranular Si-rich main eutectic (B) as predicted by Thermo-Calc®. Tertiary eutectic compounds are found in the interdendritic space, including acicular AlFeSi-β phase (C), platelets of θ–Al2Cu(Si) phase preferentially nucleated around β-phase (D) and clusters of Cu-rich Al–Al2Cu–Si phase (E). In addition, the complex Al15(FeMnCr)3Si2 compound (known as α-Fe phase) is present in the form of both Chinese (F) script and polyhedral morphologies (G) which differs from the microstructure predicted by Thermo-calc®; in fact, kinetic factors can sometimes result in the formation of non-equilibrium phases.30,31
Figure 3 shows the solidification cooling rate (\(\dot{T}\)) profile along the length of the DS casting alloys with and without Al–5Ti–1B [wt%] grain refiner (319 and 319R). To determine the points in the graph, the liquidus (610.8 °C) and solidus (502.8 °C) were considered to be the limits of the solidification range. As can be seen, it was possible in only one experimental run to achieve a wide range of \(\dot{T}\) from approximately 0.5 to 45 °C/s, values commonly found in the literature on commercial sand casting and die casting.1,10,18 Figure 3 also shows that the addition of grain refiner did not have a significant effect on \(\dot{T}\); in fact, a single experimental power equation is able to represent the dataset for both experiments:
where P is the position in mm.
Columnar grains prevailed along the entire length of the DS 319 alloy casting analyzed (Figure 4a). However, after the addition of grain refiner, the macrostructural morphology changed to equiaxed structure with finer grain size (Figure 4b). In the case of the 319R alloy, the absence of aligned grains prevented any analysis of vectorial thermal parameters such as the growth rate (v) and thermal gradient (G = \(\dot{T}\)/v). For this reason and for practical purposes, \(\dot{T}\) is the only thermal parameter considered for analysis here.
Figures 5 and 6 show representative micrographs of the 319 and 319R alloys, respectively. These images, obtained from cross sections of the castings, correspond to several \(\dot{T}\) values. As expected, an increase in \(\dot{T}\) led to a more refined structure. However, this effect differs considerably between the samples with and without grain refiner. While the magnification used in the color images is the same for all the conditions as this allows the grains to be more easily observed, the magnification in the B&W images was increased as \(\dot{T}\) increased so that the microstructure could be more readily observed and the effect of the thermal parameters on dendrite spacing could be compared. Note that the increase in \(\dot{T}\) promotes refinement of the dendrite arms (left) and grains (right) that constitute the microstructure of the alloys.
Microstructural evolution of 319 alloy with increasing cooling rate. Conventional B&W micrographs and color micrographs taken under polarized light for cooling rates of: (a) and (b) \(\dot{T}\) = 0.07 °C/s; (c) and (d) \(\dot{T}\) = 0.15 °C/s; (e) and (f) \(\dot{T}\) = 1.5 °C/s; (g) and (h) \(\dot{T}\) = 6.5 °C/s; (i) and (j) \(\dot{T}\) = 13 °C/s; (k) and (l) \(\dot{T}\) = 23 °C/s; (m) and (n) \(\dot{T}\) = 37.5 °C/s; and (o) and (p) \(\dot{T}\) = 84.5 °C/s.
Microstructural evolution of 319R alloy with increasing cooling rate. Conventional B&W micrographs and color micrographs taken under polarized light for cooling rates of: (a) and (b) \(\dot{T}\) = 0.06 °C/s; (c) and (d) \(\dot{T}\) = 0.1 °C/s; (e) and (f) \(\dot{T}\) = 0.6 °C/s; (g) and (h) \(\dot{T}\) = 2.5 °C/s; (i) and (j) \(\dot{T}\) = 6.5 °C/s; (k) and (l) \(\dot{T}\) = 13 °C/s; (m) and (n) \(\dot{T}\) = 23 °C/s; and (o) and (p) \(\dot{T}\) = 84.5 °C/s.
The grain refining effect is clearly more pronounced in the refined alloy (Figure 6) than in the unrefined alloy (Figure 5). In fact, the observed grain size in refined structures is half that found in unrefined structures. This effect is also observed in the B&W images, where the dendrite morphology varies from quite coarse to fine. For the microstructural analysis, micrographs like those shown in Figures 5 and 6 were used to measure the microstructural parameters studied here, namely, primary, secondary and average dendrite arm spacing and grain size (λ1, λ2, λL, GS). Unlike the other measurements, which were taken in transverse cross sections, the secondary dendrite spacing of the 319 and 319R alloys was measured using images obtained from longitudinal cross sections of the ingots, as shown later in this work.
In the color micrographs, where the grains can be observed in both the alloys, the microstructure starts out extremely coarse and decreases in size as one moves toward regions subjected to higher \(\dot{T}\). Although this is true for both alloys, in the case of the 319R alloy the effect of the grain refiner can already be observed even for low cooling rates. For higher rates, such as in die casting operations, the refined microstructure of 319R has the lowest observed GS, with grains in the 100 µm range.
Figure 7 shows the relationship between grain size and cooling rate (average ± standard deviation, trend line and experimental equation) of the microstructural parameters (GS, λ1, λL and λ2) in relation to \(\dot{T}\) during solidification of the 319 and 319R alloys. Addition of grain refiner clearly has a significant impact on GS. For a given value of \(\dot{T}\), the GS of the 319R alloy is about 54% smaller than that of the 319 alloy. For unrefined samples, there is a very large dispersion of the data, which is reflected in the standard deviation. Grain refining combined with a higher cooling rate resulted in a more homogeneous microstructure.
The addition of grain refiner, however, had little or no effect on λ1, λL and λ2, and the cooling rate was the main parameter that could be used to control the structure. For GS, we therefore have the following equations (Figure 7a):
In fact, the grains size of the refined microstructure is 1/2 of the non-refined microstructure.
The heterogeneity of the unrefined structure should be emphasized once again. However, for parameters λ1, λL and λ2, which are significantly affected by \(\dot{T}\), the following equations can be generated:
As they can predict the final microstructure of the part produced, these equations can be used to support the simulation of sand casting and die casting processes.
For a given value of \(\dot{T}\), λ1 is greater than λ2 for both alloys, which agrees with the theory of dendrite growth during solidification.8,15,16,17 This difference is explained by the fact that primary dendrites grow with a large degree of freedom because of the ease with which the solute is distributed in the liquid at the growth interface. As primary dendrites form, secondary dendrites grow in the narrow primary interdendritic space in which the solute is trapped and segregated, resulting in slower growth kinetics for the secondary arms.26 This effect is amplified for tertiary dendrites and so on.
The experimental growth laws are represented by the power function a*\(\dot{T}\)−b with the coefficients a(λ1) = 268 and a(λ2) = 36. The microstructural parameters λ1 and λ2 follow different, progressively slower growth kinetics as \(\dot{T}\) increases. This is expressed by the exponent b, where b(λ1) = Al–0.36 and b(λ2) = Al–0.30. The values of the coefficient a and exponent b found here for the alloys studied are compatible with those reported in the literature for similar systems, e.g., a(λ1) = 250 and b(λ1) = − 0.55 for Al–5%Cu, Al–8 wt%Cu and Al–15 wt%Cu alloys15; a(λ1) = 220 and b(λ1) = − 0.55 for Al–3 wt%Si, Al–5 wt%Si, Al–7 wt%Si and Al–9 wt%Si alloys15; and a(λ1) = 153 and b(λ1) = − 0.55 for Al–6% Cu–1 wt%Si and Al–6 wt%Cu–4 wt%Si alloys.17 Note that all the microstructural parameter refers to a non-refined structures.15,16
The data presented here indicate that the growth kinetics of λ1 (b =−0.36) were not significantly affected by the addition of refiner. Similarly, in the case of the 319R alloy, the value of the exponent λ2 is identical to that for the 319 alloy (b = − 0.30).
As observed in32 for multicomponent Al alloys, the addition of chemical refiner does not affect the magnitude of secondary dendrite arms. The kinetic growth of λ2 is exactly the same for both conditions studied here (a = 36, b = − 0.30) and is in agreement with the values found in the literature, i.e., a = 20.43, b = − 0.33 for Al–6 wt%Cu–1 wt%Si; a = 14.59, b = − 0.33 for Al–6 wt%Cu–4 wt%Si alloys14; a = 41, b = − 0.33 for Al–5.5 wt%Si–3 wt%Cu alloys; and a = 28, b = − 0.33 for Al−9 wt%Si–3 wt%Cu alloys.16
Figure 7c shows the correlation between λL and \(\dot{T}\) for 319 and 319R alloys. Again, the similarity between the individual models allowed the data to be fitted jointly, as shown in the graph, indicating that grain refining did not affect the change in λL with \(\dot{T}\).
Figure 8 shows primary, average and secondary dendrite arm spacing against GS (Figure 8a λ1, Figure 8b λL and Figure 8c λ2). Note that the dispersion of the data for 319 alloy indicates the absence of a correlation. For 319R alloy, however, there is an excellent correlation, indicating that the larger GS, the larger λ1, λL and λ2 (as expected). This alloy, to which grain refiner was added, has a more homogeneous microstructure, and all possible direct relationships resulted in a value of R2 greater than 0.8. Note that for conventional dendritic structures, the grain size differs greatly from the primary dendritic spacing, but for refined structures, the relationship is direct, in fact λ1 = 0.9997 GS, or simple λ1 = GS, with R2 = 0.8527. It is clear that for unrefined structures, there is no limitation against the growth of the Alα and the structure tends to present a three-dimensional skeleton forming a very complex structure, but for refined structures, due to the high number of nuclei formed, growth is limited, generating a situation in which a dendritic cell actually corresponds to a grain. In fact, this was observed by Kurz34: "the primary spacing in an equiaxed structure is not well-defined and usually corresponds to the mean grain diameter."
Figure 9 shows the correlation between λL and λ1 and between λL and λ2. As expected, the more homogeneous the structure, the greater the correlation between the thermal parameters. λL, which is much simpler to measure, is thus a good parameter for evaluating the microstructure. In the case of 319 alloy, λ1 and λ2 can be estimated with the following equations:
It should be noted that the values of λ1 for the 319 alloy are lower than for the 319R alloy for \(\dot{T}\) > 0.5 °C/s. At first sight, this may seem to be inconsistent with the fundamental role of grain refinement and the observed microstructure for these alloys. A possible explanation for this is the presence of dendrite fragments between neighboring Maltese crosses in the 319R alloy, as shown in Figure 10a. These dendrite fragments result in a higher average λ1 measured by the triangle method because of the triangle’s larger sides. This is shown by the dashed line in Figure 9a. There is a massive presence of dendrite fragments in the 319R alloy because of the radial growth caused by extensive nucleation. It should therefore be kept in mind that measurement of λ1 by the triangle method for chemically refined alloys may not always be very accurate. To overcome this drawback, colored images can be used to enable the identification of fragments (as in Figure 10a) so that these regions are avoided during the quantitative analysis. The morphological changes from Maltese-cross to rosette-like dendritic ramifications caused by the addition of chemical refiner are shown in Figure 10b and c, respectively.
(a) Dendrite fragments between three neighboring dendrites; (b) Maltese-cross dendrite in 319 alloy;25 rosette-like dendrite in 319R alloy.
Conclusions
Addition of Al–5Ti–1B [wt%] to 319 alloy as grain refiner had a significant effect on the observed GS. The resulting power law to describe the effect of cooling rate on grain formation had a proportionality constant a(319) = 660 (µms°C−1) for the unrefined structure and a(319R) = 311 (µms°C−1) for the refined alloy. However, the growth kinetics, represented by the exponent b, were similar: b(319) = − 0.22 and b(319R) = − 0.24. The grain refiner therefore only affects nucleation and not grain growth. In addition, the grain refiner did not significantly affect growth of the dendritic structure in terms of the primary and secondary dendrite spacing. The proportionality constant was a(λ1) = 268 (µms °C−1) and a(λ2) = 36, while the growth exponent was b(λ1) = − 0.36 and b(λ2) = − 0.30. Thus, only the cooling rate significantly affects the formation of the primary and secondary morphology, i.e., λ1 and λ2. A simpler analysis parameter, average dendrite arm spacing, λL, was introduced. This parameter, which is calculated using the Heyn intercept method, proved to have an excellent correlation with λ1 and λ2 and can be used to estimate the final microstructure. The proportionality constant and growth exponent were a(λL) = 62 (µms °C−1) and b(λL) = − 0.28.
REFERENCES
J.R. Davies, ASM specialty handbook—aluminum and aluminum alloys (Novelty, Enschede, 1993)
B. Donadoni, G.L. de Gouveia, A. Garcia, J.E. Spinelli, J. Manuf. Process 54, 14–18 (2000). https://doi.org/10.1016/j.jmapro.2020.02.047
B. Wang, J. Wang, X. Liu, Q. Li, X. Liu, Mater. Sci. Eng. A 858, 144090 (2022). https://doi.org/10.1016/j.msea.2022.144090
A.M. Samuel, H.W. Doty, S. Valtierra, F.H. Samuel, Beta Al5FeSi phase platelets-porosity formation relationship in A319.2 type alloys. Int. J. Metalcasting 12(1), 55–70 (2018). https://doi.org/10.1007/s40962-017-0136-9
M.C. Flemings, Metall. Trans. B 22(3), 269–293 (1991). https://doi.org/10.1007/BF02651227
Z. Fan, Inter. Mater. Rev. 47(2), 49–86 (2002). https://doi.org/10.1179/095066001225001076
M.S. Salleh, M.Z. Omar, J. Syarif, M.N. Mohammed, Inter. Sch. Res. Not. (2013). https://doi.org/10.1155/2013/679820
J.D. Hunt, S.Z. Lu, Metall. Trans. A 27(3), 611–623 (1996). https://doi.org/10.1007/BF02648950
G.K. Sigworth, T.A. Kuhn, Int. J. Metalcasting 1(1), 31–40 (2007). https://doi.org/10.1007/BF03355416
E. Vandersluis, N. Prabaharan, C. Ravindran, Inter. Metalcasting 14, 37–46 (2020). https://doi.org/10.1007/s40962-019-00329-w
E. Aghaie, J. Stroh, D. Sediako, A. Rashidi, A.S. Milani, Mater. Sci. Eng. A 793, 139899 (2020). https://doi.org/10.1016/j.msea.2020.139899
G.K. Sigworth, Int. J. Metalcasting 2(2), 19–40 (2008). https://doi.org/10.1007/BF03355425
D. Bouchard, J.S. Kirkaldy, Metall. Mater. Trans. B 28, 651–663 (1997). https://doi.org/10.1007/s11663-997-0039-x
M. Rappaz, W.J. Boettinger, Acta Mater. 47(11), 3205–3219 (1999). https://doi.org/10.1016/S1359-6454(99)00188-3
O.L. Rocha, C.A. Siqueira, A. Metall, Mater. Trans. A 34, 995–1006 (2003). https://doi.org/10.1007/s11661-003-0229-3
M.D. Peres, C.A. Siqueira, A. Garcia, J. Alloys Comp. 381(1–2), 168–181 (2004). https://doi.org/10.1016/j.jallcom.2004.03.107
D.J. Moutinho, L.G. Gomes, O.L. Rocha, I.L. Ferreira, A. Garcia, Mater. Sci. Forum 730–732, 883–888 (2012). https://doi.org/10.4028/www.scientific.net/msf.730-732.883
M. Tiryakioğlu, Metall. Mater. Trans. A 50, 3030–3032 (2019). https://doi.org/10.1007/s11661-019-05257-2
J.A. Spittle, S.G.R. Brown, Mater. Sci. Technol. 21(9), 1071–1077 (2005). https://doi.org/10.1179/174328405X51839
E. Quested, A.L. Greer, Acta Mater. 52(13), 3859–3868 (2004). https://doi.org/10.1016/j.actamat.2004.04.035
L. Bolzoni, N. Hari Babu, Metall. Mater. Trans. A 50(2), 746–756 (2019). https://doi.org/10.1007/s11661-018-5017-1
G. Salloum-Abou-Jaoude, P. Jarry, P. Celle, E. Sarrazin, Miner. Metals Mater. Ser. (2020). https://doi.org/10.1007/978-3-030-36408-3_134
A.M. Samuel, F.H. Samuel, H.W. Doty, S. Valtierra, Int. J. of Metalcasting 11(2), 305–320 (2017). https://doi.org/10.1007/s40962-016-0075-x
A.M. Samuel, F.H. Samuel, H.W. Doty, S. Valtierra, Int. J. Metalcasting 11(3), 475–493 (2017). https://doi.org/10.1007/s40962-016-0089-4
American Society for Testing and Materials, B179–18: standard specification for aluminum alloys in ingot and molten forms for castings from all casting processes (ASTM International, West Conshohocken, 2018). https://doi.org/10.1520/B0179-18
T. Soares, C. Cruz, A. Barros, A. Garcia, N. Cheung, Adv. Eng. Mater. 22(6), 1901592 (2020). https://doi.org/10.1002/adem.201901592
R. Kakitani, R. Oliveira, R.V. Reyes, A.V. Rodrigues, F. Bertelli, A. Garcia, J.E. Spinelli, N. Cheung, Case Stud. Therm. Eng. 26, 101144 (2021). https://doi.org/10.1016/j.csite.2021.101144
R. Oliveira, T.A. Costa, M. Dias, C. Konno, N. Cheung, A. Garcia, Mater. Today Comm. 25, 101490 (2020). https://doi.org/10.1016/j.mtcomm.2020.101490
M. Gündüz, E. Çadırlı, Mater. Sci. Eng. A 327(2), 167–185 (2002). https://doi.org/10.1016/S0921-5093(01)01649-5
G.L. Brollo, C.T.W. Proni, E.J. Zoqui, Metals 8(5), 332 (2018). https://doi.org/10.3390/met8050332
D. Kim, J. Kim, S. Wenner, E. Thronsen, C.D. Marioara, R. Holmestad, E. Kobayashi, Mater. Charact. 173, 110863 (2021). https://doi.org/10.1016/j.matchar.2020.110863
M. Rappaz, P. Thévoz, Acta Metall. 35(7), 1487–1497 (1987). https://doi.org/10.1016/0001-6160(87)90292-6
M. Easton, D. St John, Metall. Mater. Trans. A 41(6), 1528–1538 (2010). https://doi.org/10.1007/s11661-010-0183-9
W. Kurz, D.J. Fisher, Fundamentals of solidification, 4th edn. (Trans Tech Publications, Wollerau, 1998), p.305. (ISBN: 0878498044)
Acknowledgements
The authors would like to thank the Brazilian research funding agencies FAPESP (São Paulo Research Foundation—projects 2009/08478-1, 2013/09961-3, 2015/22143-3, 2021/11439-0 and 2022/05050-5), CNPq (National Council for Scientific and Technological Development—projects 470572/2011-6 and 140918/2018-3) and CAPES (Federal Agency for the Support and Improvement of Higher Education—notice 23/2016, ref. no. 88881.131045/2016-01) for providing financial support for this study. The authors are also indebted to the Faculty of Mechanical Engineering at the University of Campinas for the practical support very kindly provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Penha Gomes, I., Barros, A., Brollo, G.L. et al. The Effects of Cooling Rate on Microstructure Formation during Solidification of 319 Alloy. Inter Metalcast 18, 2079–2091 (2024). https://doi.org/10.1007/s40962-023-01145-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-023-01145-z