Abstract
Zn-based alloys have found increasing interest as orthopedic biodegradable implantable materials, hence it was the aim of this work to investigate the microstructure and corrosion behavior of Zn–0.5Al–xMg cast alloys with different Mg additions in simulated body fluid (SBF). The cast samples were prepared using a simple stir casting method and the molten alloys were poured in a cast iron mold. The SEM results showed that adding Mg greatly influenced the microstructure of the Zn-based alloys where the degree of fineness of the microstructure increased with a rise in the Mg content. Moreover, polarization measurements revealed that the Zn–0.5Al–0.6Mg alloy attained the lowest degradation rate of 0.33 mm/year as compared to the other investigated alloys which complies the requirements of ideal corrosion rates for biodegradable bone implants. This corrosion rate helps the implantable metal alloy to last in the body until healing of the bone tissue proceeds. The fine structure and uniform distribution of Aluminum oxide and MgZn2 intermetallic phases along the grain boundaries were most likely the main factors in the superior corrosion stability of the Zn–0.5Al–0.6Mg alloy in SBF. However, higher concentrations of Mg (1 wt%) lowered the corrosion resistance of the Zn–Al–Mg alloy which was attributed to the accelerated galvanic corrosion between Zn and Mg2 Zn11 phases and the inhomogeneous distribution of corrosion products on the alloy surface due to the increased grain size and the coarse structure of the Zn alloy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) and its alloys have attracted increased attention due to the diverse applications of Zn in many engineering and medical applications. Zn is commonly used as a protective coating1,2 to inhibit steel corrosion, since it will function as an anode and be dissolved rather than the underlying steel. Zn is also exploited as Zinc Oxide (ZnO) in pharmaceutical, textiles, cosmetics, rubber, biosensors and agricultural industries.3,4 Other applications that include zinc are Zn-air battery and fuel cells.5 Over the last few decades, research has been conducted on the corrosion manner of zinc and zinc-based alloys (as bulk or coated films) in chloride-containing environments for engineering applications;6,7 recently there has been a growing interest on zinc-based biodegradable implant materials.
Corrodible or biodegradable metals gradually corrodes in vivo by generating an appropriate host response and then dissolves completely upon tissue healing.8 A progressive transfer of the load to the healing tissue and the prevention of secondary surgery are the two main reasons why this category of metals are favorable alternatives to existing corrosion resistant metal implants used for temporary applications.9 Regarding the corrosion process of a biodegradable implant in vivo, it is essential to know which corrosion rate is acceptable for a particular application. Too fast an in-vivo degradation of an implant is undesirable because such an implant would degrade before the completion of the healing process.
Magnesium (Mg), Iron (Fe) and Zinc (Zn) have found growing acceptance as biodegradable implantable materials.10,11,12,13 Zn is the most recently introduced out of three competitive materials. The major drawback of Mg-based materials is that they degrade too fast and Fe-based materials degrade too slowly, leading to deficiencies in clinical applications.14 In vivo tests showed that pure iron corrosion products remained in the body for 9 months after implantation.15 On the other hand, zinc does not accumulate in the body and is excreted via urine and feces,16 which is another advantage over pure iron. Moreover, biocorrosion products of Zn are compatible with the human tissues.17 Zn has a standard corrosion potential of (−0.76V vs. Standard Hydrogen Electrode (SHE)), which is intermediate between Fe (−0.44V vs SHE) and Mg (−2.37V vs SHE), therefore, it exhibits intermediate rates of biodegradation as compared to the utmost cases of Mg and Fe. Hence Zn proved to be a more promising alternative to Fe and Mg and thus considerable interest increased in this metal.
Recent studies were conducted on Zn–based alloys as biodegradable bone implant material, owing to their good mechanical strength, corrosion rate, and biocompatibility.18,19 Zinc is also vital for biochemical reactions in the human body because it is involved in different forms of cellular metabolism. Zn is relevant for the suitable function of various enzymes and helps the immune system, protein and DNA synthesis, as well as wound healing. It also promotes natural growth as well as a healthy sense of taste and smell.20
However, pure zinc metal and most commercial Zn alloys struggle to satisfy the requirements of mechanical characteristics and corrosion resistance for metallic implants21 so the valuable tool to modify the corrosion resistance and applicability of zinc alloys as implantable biomaterials is controlling their alloying addition. It was shown in previous works that the concentration and the type of alloying elements affected the degradation and corrosion rates of Zn alloys. Moreover, alloying can alter the corrosion properties of a metal due a change in the microstructure (e.g., from single to multi-phase system, change in grain size) of the alloy.22,23
Studies on the corrosion behavior of Zn with other alloying elements such as Fe, Al, Mg, Mn, Sr, Ca, Cu and Ag were investigated,24,25,26,27,28,29,30,31 implying the performance of Zn alloys could be improved via grain refinement and strengthening of various second phases. Králová et al.32 found that Zn–1Fe and Zn–2Fe samples met the criteria for potential biodegradable implants. Xue et al.33 studied a few Zn–Fe–Mg alloys in simulated body fluid and concluded that the Zn–1Fe–1Mg shows a good corrosion rate and superior mechanical properties.
Recently, Zn–Mg alloying systems have been developed where zinc is often the major constituent, zinc as a more noble metal than magnesium, is well known to positively affect corrosion resistance and magnesium is assumed to enhance the strength, corrosion resistance, and biocompatibility of zinc. Since magnesium is insoluble in zinc, the magnesium inside the alloy nearly exists in the form of Zn-Mg secondary phases, mainly as \({\mathrm{Mg}}_{2}{\mathrm{Zn}}_{11}\) and \({\mathrm{MgZn}}_{2}\). Hence, the role of these intermetallic compounds in the corrosion performance of Zn-Mg alloys has been the area of interest of many research. The addition of low compositions of Mg (0-3 weight%) to Zn was reported to enhance the strength and resistance of Zn based alloys to corrosion due to solid solution hardening, and by the introduction of a harder secondary phase.28,34 Krieg et al.35 studied the microstructure and corrosion properties of Zn-Mg alloys with altering Mg contents. The authors found that the magnesium content had a great influence on the microstructure of the alloy where the degree of fineness of the microstructure increased with increasing magnesium contents. They also observed that an increase of the fineness of the microstructure lead to an improved corrosion performance.
Additionally, Zn–Al-Mg alloys have received a lot of interest because of their excellent mechanical and corrosion resistant properties. Zn–Al–Mg alloys can also be utilized in light metal structures due to their benefits such as high strength per weight ratio, low density, and high specific hardness.36,37 Aluminum as an alloying element improves the castability and mechanical strength of zinc. Additions of Al to zinc alloys was reported to enhance the tensile strength, hardness and corrosion resistance of pure zinc38
Bakhsheshi-Rad et al.39 studied the mechanical and electrochemical behavior of binary Zn–0.5Al and ternary Zn–0.5Al–xMg alloys with different Mg contents as biodegradable materials for implant applications. The authors concluded that Zn–0.5Al–0.5Mg alloy with appropriate mechanical properties, low corrosion rate, good biocompatibility and antibacterial activities was believed to be a good candidate as a biodegradable implant material. Panaghie et al.40 compared the biodegradation of Zn, Zn–Mg and Zn–Mg–Y alloys and they revealed that the three alloys showed a moderate degradation rate with uniform corrosion in simulated body fluid, and they can be considered as promising biodegradable materials for orthopedic applications.
Several other works on the effect of Mg on Zn–Mg-based ternary alloys were presented, it was also shown that increasing Mg content reduced the corrosion rates of cast Zn–0.1Mn–xMg (x = 1, 1.5)41 and of cast Zn–0.5Al–xMg (x = 0.1, 0.3, 0.5)42; but enhanced the degradation rate of extruded Zn–3Cu–xMg (x = 0.1, 0.5, 1).43
A recent work by Moussa et al.44 studied the influence of Mg additions on Zn–0.5Al alloys and investigated their corrosion behavior in NaCl solutions for cathodic protection applications. The authors concluded that the introduction of 0.7 wt% Mg to Zn–0.5Al alloy reduced the corrosion rates in NaCl solutions from (~0.00099 mm/year) without Mg to (~0.0005 mm/ year) due to microstructure refinement and uniform distribution of Al. However, the addition of higher amounts of Mg reduced the corrosion resistance of the alloy.
However, in the body environment, it is important for a biodegradable bone implant to satisfy suitable corrosion rates to service for enough time to allow the curing of the bone tissues. Moreover, the corrosion reactions and hence the corrosion products on the surface of the implant in body environment is different from NaCl solutions due to the presence of chloride, calcium and phosphorus ions present in body fluid. Hence it is relevant to choose a medium that resembles body fluid to explore the degradation processes of bone implants.
The main objective of this work is to develop a Zn biodegradable alloy having suitable degradation rates in body environment. Therefore, the corrosion behavior of Zn–Al–Mg alloys received from the previous work44 was explored in simulated body fluid to determine the best composition of the Zn ternary alloy having acceptable corrosion rates for bone implant applications. The effect of different concentrations of Mg on the microstructure and electrochemical properties of Zn–Al–Mg ternary alloys were explored. The influence of different microstructures on the corrosion performance of the as-prepared Zn–Al–Mg alloys was also discussed.
Materials and Methods
History of As-Received Samples
Zn–0.5wt%Al–Mg alloys with Mg additives of (0.0, 0.2, 0.4, 0.6, 0.8 and 1.0 wt%) were received from the previous report.44 These samples were prepared by melting pure Zn (99.99 %), Al (99.99 %) and commercial pure Mg (99.96 %) ingots. An electric resistance furnace was employed, and the temperature reached 690 °C ± 5 °C. The mixture was then stirred lightly with a graphite rod to ensure good mixing. After skimming the slag, the molten alloys were then poured into a preheated cylindrical cast iron mold (150 °C) of dimensions of 100 mm outer diameter, 40 mm inner diameter and 250 mm height. The specimens were prepared as discs of 6 mm in diameter and 2 mm in thickness. Each disc was held by stainless steel rod sheathed with teflon for complete isolation when immersed in the electrolyte solution. The discs were fine polished using SiC papers up to 2400 grit and then cleaned ultrasonically in deionized water for 5 minutes. Afterwards, the specimens were etched in a 3 wt% Nital solution for 5 minutes at room temperature then rinsed with water and ethanol followed by washing with anhydrous ethyl alcohol and drying. The chemical analysis of the investigated alloys was determined with X-ray fluorescence analyzer (XRF) (model Axios advanced-PANA- LYTCAL, Netherlands) and presented in Table 1.
Microstructure (Surface) Characterization
A scanning electron microscopy (JEOL- JSM-5410, Japan) equipped with an energy dispersive spectrometer unit (EDS-Oxford) was used to study the surface morphologies of alloys and chemical composition, respectively. X-ray diffractometer (Bruker AXS-D8, Advance, Germany, operated at 35 KV and 45 mA with CuKα radiation λ = 0.1540 nm) was performed to find out the structure of different phases. The specimens were cut at a position of height 80 cm from below having a depth of 10 mm. The position of specimens for microstructure and corrosion investigations were shown in Figure 1.
Position of specimens for microstructure investigation and corrosion tests.44
Electrochemical Test
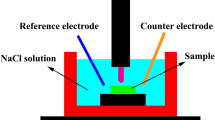
Electrochemical measurements using (potentiostat/galvanostat, Autolab PGSTAT 302N, Netherlands equipped with the NOVA software package) were performed at 37 ºC in SBF solution (pH 7.4) containing (g/l (NaCl :8, CaCl2 : 0.14, KCl :0.4, NaHCO3 : 0.35, C6 H12O6 : 1, NaH2PO4 : 0.1, MgCl2 .6H2O :0.1, Na2HPO4 .2H2O : 0.06 and MgSO4 .7H2O: 0.06.at pH 7.4.11,45 A conventional three-electrode system in which Ag/AgCl was utilized as the reference electrode, while a platinum as a counter electrode and tested alloys were used as the working electrodes. The circular area that was exposed to the electrolyte solution was 0.785 cm2. Stabilization time of 30 min at the open circuit potentials (OCP) was recorded before starting the potentiodynamic measurements. Electrochemical impedance spectroscopy (EIS) was completed in a frequency range of 10 mHz 100 kHz with a perturbation amplitude of 5 mV. Potentiodynamic polarization curves were performed from -1.4 mV to -0.2 mV at a scan rate of 1 mV/s. For reproducibly purposes, two tests were applied for each sample.
Results and Discussion
Microstructure
Figure 2 showed the optical microstructures of the investigated Zn-0.5Al-Mg alloys with and without Mg additions. It was obvious that without Mg additions, the microstructure of the Zn-0.5Al based alloy consisted of Zn dendrites with an average size of 164 μm and a Zn-Al eutectic phase. However, the microstructure of the alloys containing Mg, as shown in Figure 2 consisted of primary Zn dendrites surrounded by binary (Zn + \({\mathrm{Mg}}_{2}{\mathrm{Zn}}_{11}\)) or (Zn + \({\mathrm{MgZn}}_{2}\)) and ternary (Zn + \({\mathrm{Mg}}_{2}{\mathrm{Zn}}_{11}\)+ Al) eutectic phases.
Optical micrographs of the as-cast Zn–0.5Al–Mg alloys 44 (a) 0 (b) 0.2 (c) 0.4 (d) 0.6 (e) 0.8 (f) 1 wt% Mg concentrations.
With increased concentrations of Mg, the degree of fineness of Zn dendrites increased and their size decreased from 165 to 110 μm with 0.2 wt% Mg and further down to 40 μm with 0.8 wt% Mg and their morphologies changed gradually to nearly polygonal shapes with 0.8 wt% Mg addition (Figure 2e).
This grain refinement effect can be attributed to the growth restriction factor of Mg which restricted the growth of Zn dendrites.46 With further Mg additions up to (1 wt%), the microstructure of Zn became coarse dendrites (Figure 2f) and their size increased again. This result is in agreement with Krieg et al.35 who concluded in their work that the magnesium content had a great influence on the microstructure of the alloy where the degree of fineness of the microstructure increased with increasing magnesium contents.
Phase Structure
The XRD patterns of ternary Zn–Al–Mg alloys were shown in Figure 3 and are compared with those of binary Zn–Al alloy. The results are consistent with the microstructure shown in Figure 2. In the Zn–Al alloy, intermetallic compounds containing Zn–Al were not identified, it was made up of main Zn phases due to the high solubility of Al in Zn matrix. In the XRD graph of as-cast Zn–Al–Mg alloy, there were no Zn–Al–Mg ternary intermetallic compounds recognized. Two phases were mainly indexed: the Zn matrix and second intermetallic phases. In the Zn–Al–0.2 Mg, A peak appeared indicating traces of Orthorhombic \({\mathrm{Mg}}_{7}{\mathrm{Zn}}_{3}\) intermetallic compounds. However, on the addition of 0.6 Mg, peaks of Al and highly crystalline hexagonal \({\mathrm{MgZn}}_{2}\) phases were identified while highly crystalline cubic lattice \({\mathrm{Mg}}_{2}{\mathrm{Zn}}_{11}\) phases were detected in the Zn–Al–1.0 Mg.
Electrochemical Behavior
To study the influence of Mg content on the corrosion process of Zn-Al alloys, potentiodynamic polarization was utilized on the as Zn alloys in SBF solution at pH 7.4 and temperature 37 ºC. The Tafel extrapolation method was used to extract the corrosion current density \(i_{{{\text{Corr}}}}\) and the corrosion potential \(E_{{{\text{corr}}}}\) data from linear sections of polarization curves. Through this procedure, the linear part of the anodic and cathodic branches is extended, and their collision points indicate the \(i_{{{\text{Corr}}}}\) and \(E_{{{\text{corr}}}}\) coordinates and the slope of each branch shows the Tafel slopes. Higher values of \(E_{corr}\) suggest that the samples have better corrosion resistance, while elevated values of \(i_{{{\text{Corr}}}}\) indicates that the samples are suffering from fast corrosion or degradation rates. Polarization curves of the as-prepared Zn–0.5Al–Mg alloys in simulated body fluid were performed and compared to Zn–Al alloy in Figure 4.
It can be noticed from the polarization curves in Figure 4, that the corrosion potential \(E_{{{\text{corr}}}}\) of all alloys shifted to the more positive direction as compared to the Zn-Al alloy associated with higher corrosion current densities \(i_{{{\text{corr}}}}\) except Zn–Al–0.6Mg which had a more promising value of \(i_{corr}\) of 22 \(\mu A/cm^{2}\) and a slight negative shift in the corrosion potential \(E_{{{\text{corr}}}}\) reaching a value of −0.946 V. The negative shift of the corrosion potential of the Zn–Al–0.6Mg alloy can be attributed to a rise in the anodic reactions due to the formation of oxide protective films on the surface of the alloy at the initial stages of corrosion.
The corrosion behavior of Zn–Al–Mg alloys in simulated body fluid is proposed in the following reactions which mainly consist of the following anodic and cathodic reactions:
While the product formation reaction involves:
However, the main corrosion product precipitated on the surface is solid \({\text{Zn}}\left( {{\text{OH}}} \right)_{2}\) which protects the Zn alloy from further corrosion. Such protection effect is relatively weak since the \({\text{Zn}}\left( {{\text{OH}}} \right)_{2}\) layer is not compact. Simulated Body fluid (SBF) which is a corrosive medium containing aggressive chloride ions with small radius, easily penetrates the loose \({\text{Zn}}\left( {{\text{OH}}} \right)_{2}\) layer, forming soluble zinc chloride according to Eqn. (4).47 Therefore, the corrosion protection of Zn surfaces by \({\text{Zn}}\left( {{\text{OH}}} \right)_{2}\) is not efficient in presence of Cl − ions.
The released Zn ions can further react with phosphate ions to form insoluble Zn phosphate (or hepatite):
This insoluble compound tends to nucleate and form on the surface of the specimens decreasing the corrosion reactions. The deposition of biocompatible zinc phosphate on zinc substrate simulates new bone formation in human body and thereby accelerates the healing of bone tissue.47 Moreover, the production of magnesium hydroxyl carbonates was mentioned for Zn–Mg alloys:48
It was proposed that Mg can enhance the corrosion resistance of Zn-based alloys by the formation of electrochemically inert compounds such as Mg2(OH)2CO3 on the surface of investigated samples. Also, Mg-containing a form of simonkolleite has been reported to act as a corrosion inhibitor.49
The polarization resistance and the corrosion current densities are identified through Tafel slopes by Stern–Geary Eqn:50,51
Where (βa and βc) are the anodic and cathodic Tafel slopes. The corrosion current density (\(i_{{{\text{Corr}}}}\)) is related to the corrosion rate (\(R_{i}\)) by the following Eqn. (8)
Where: \(R_{i} \) is given in mm/year, \(i_{{{\text{Corr}}}}\) in µA/cm2 , \({\text{K = 3}}{.27} \times {10}^{ - 3} \;mm\;g/\mu A\;cm\;year\), \({\text{EW}}\) is the equivalent weight and d is the density of the base zinc metal which is 7.13 \({\text{g}}/{\text{cm}}^{3}\).52
The corrosion parameters derived from the polarization curves such as, corrosion potential (\(E_{{{\text{Corr}}}}\)), corrosion current density (\(I_{{{\text{Corr}}}}\)), polarization resistance (Rp) and corrosion rate (Ri) were recorded in Table 2.
The results in Table 2 revealed that the Zn–0.5Al and Zn–0.5Al-0.6Mg alloy exhibited good corrosion resistance as they were close to the suitable corrosion rates for orthopedic biodegradable materials. On the addition of 0.2-0.4 wt% Mg, the corrosion inhibition of the ternary alloys decreased suggesting surface reactivity and unstable corrosion products leading to increased corrosion attacks. However, the addition of 0.6 wt% Mg to the alloy exhibited a favorable corrosion resistance which can be ascribed to the formation of fine MgZn2 intermetallic phases distributed in the eutectic Zn matrix with the inclusion of higher amounts of Mg as confirmed by the XRD in Figure 3.53 These secondary phases are homogenously dispersed in the matrix, leading to the generation of a uniform surface film of Mg-containing corrosion products which act as a corrosion boundary that can hinder the occurrence of localized corrosion and can effectively protect the Zn-based alloy. Also, the high corrosion resistance of the alloy could be related to the uniform distribution of Al phases in the microstructure which can hinder the chance of corrosion attack on the passive film.
However, higher Mg concentrations up to 1% wt led to the increase in the corrosion rates of the Zn–Al–Mg alloy which can be as a result of formation of galvanic micro-cells between Zn and Mg2Zn11, therefore increasing the degradation rate of the base alloy.54 This result is in agreement with the XRD (Figure 3) where cubic lattice Mg2Zn11 phases were detected in Zn–Al–1.0 Mg. This suggestion was also proposed by Mostaed et al.55, who mentioned that galvanic corrosion due to Mg2Zn11 enhanced the corrosion rates in Zn-xMg alloys. Moreover, the decrease in corrosion resistance with higher Mg content up to 1.0 Mg are in good agreement with a previous study.56
It was clear from Table 2 that the Zn-0.5Al-0.6Mg ternary alloy had the lowest degradation rate of 0.33 mm/year as compared to the other investigated alloys which satisfies the requirements for ideal corrosion rates in orthopedic implants of less than 0.5 mm/year.57,58 This corrosion rate helps the implantable metal alloy to survive in the body during the healing process of the bone tissue. This finding is close to another work by Bakhsheshi-Rad et al.42 who studied the corrosion behavior and biocompatibility of Zn–0.5Al and ternary Zn–0.5Al–xMg biodegradable alloys by altering Mg contents. They concluded that the Zn–0.5Al–0.5Mg alloy exhibited the lowest corrosion degradation rates and attained suitable biocompatibility and mechanical properties as a biodegradable implantable material.
Figure 5 shows the Nyquist plots of the Zn–0.5Al–Mg alloys with different Mg contents using electrochemical impedance spectroscopy (EIS) technique in SBF solution at pH 7.4 and temperature 37 ºC. The impedance study confirms the previously obtained results by polarization. The obtained Nyquist plots consisted of one capacitive semicircle loop with different diameters; an increase in the size of the semicircle indicates an increase of corrosion resistance. It was observed that that the best corrosion performance was for the Zn–Al–0.6Mg since it attained the largest loop diameter.
In order to enable an accurate analysis of the impedance diagrams, the equivalent circuit model was shown in Figure 6. The most fitted electric model to explain the corrosion process is the simple model one-time constant. The equivalent circuit consisted of a constant phase element (CPE) which is parallel to a polarization resistance (charge transfer resistance) of the coating surface Rp, both are in series with solution resistance Rs. Constant phase element (CPE) included in this circuit compensates the action of capacitor owing to surface heterogeneity and is defined by impedance value which can be characterized by the following equation:
Where \(\alpha \) is an exponent account for surface heterogeneity, 0 ≤ \(\alpha\) ≤ 1, \(j \) is the imaginary number \((j = \left( { - 1} \right)^{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-0pt} \!\lower0.7ex\hbox{$2$}}}} , \omega = 2\pi f \) is the angular frequency in \({\text{rad}}/s\), \(f\) is the frequency in \(Hz = S^{ - 1}\). The results of EIS analysis are given in Table 3
It was seen from Table 3 that the Rp value of the Zn–Al–0.6Mg alloy (0.35 \(\mathrm{k\Omega }{\mathrm{cm}}^{2}\)) was higher as compared to the other alloys reflecting its high resistance to charge transfer. However, it is important to note that the Rp values are related to the rate of charge transfer reactions that give rise to the formation of a corrosion layer on the surface of the samples but say nothing about the protective characteristics of these films. Hence, it can be concluded from the previous results of this study and from the electrochemical behavior of the investigated Zn samples that the best corrosion performance was attained by Zn–Al–0.6Mg alloy which is proposed for use in biodegradable orthopedic implant applications.
Microstructure of the Corroded Specimens
SEM images (low-and high-resolution images) and EDS spectra analysis of the most corroded regions of the surfaces of the investigated Zn ternary alloys after electrochemical tests in SBF were shown in Figures 7 and 8. It was apparent that the surface of the Zn–0.5 Al alloy (Figure 6i) was covered with pores and a few number of large corroded pits due to the breakage of oxide films by attacking of chloride ions and phosphate ions in SBF solution. However, the corroded areas were increased with the addition of 0.2 wt% Mg (Figure. 7ii) On the addition of 0.6 wt% Mg, it was clear from Figure (7iii) that the corroded areas apparently decreased since many areas of the base alloy were not attacked by corrosion. Furthermore, the Zn-0.5Al-1Mg alloy (Figure. 7iv) showed increased areas of anodic attacks on the surface.
The EDS results of different regions on the surfaces of the Zn alloys were listed in Table 4 for purpose of comparison. The results revealed that besides the basic components of the alloys, one can observe that the corrosion products included Phosphorus, Chloride and Calcium originating from the simulated body fluid. Hence, it can be proposed that the surfaces of the corroded zinc alloys were dominated by aluminum oxide/hydroxide, insoluble calcium–Zinc phosphates, zinc hydroxide, zinc carbonates, insoluble calcium–magnesium phosphates or magnesium hydroxyl carbonates as shown from the previous corrosion reactions. These corrosion products were reported to be protective passive layers that protects the Zn-based alloys from further attack from the simulated body fluid (SBF).
It was also noticed from Table 4 that the main differences between corroded areas (A, C, E and G) and flat areas (B, D, F and H) are in the concentrations (wt%) of oxygen and aluminum where these concentrations greatly increased in the corroded regions which can be attributed to the formation of Aluminum oxides. However, there were not many differences between the concentrations of P, Cl or Ca. The corrosion products also included Mg phases so magnesium corrosion products like magnesium hydroxyl carbonates or phosphates might have been produced.
Hence, it was concluded in this work that the inclusion of 0.6 wt% Mg to the Zn based alloy helped in the increase of fineness of the microstructure of the alloy. According to the previous study,44 the authors concluded that Mg helped in the homogeneous distribution of Al phases in the Zn matrix which helped in the development of Aluminum oxide protective layers. It is also known that Al is less noble than zinc, therefore it is expected to be preferentially oxidized than Zn and hence protecting Zn from further corrosion. This was confirmed in this work since the EDS analysis of the corroded regions of the samples showed increased concentrations of Al and oxygen in the corroded areas revealing the formation of Aluminum oxide films. Volovitch et al.59 reported that aluminum formed basic aluminum-oxides in the initial stages of corrosion of a Zn-Al-Mg alloy
The fine microstructure of the Zn–0.5Al–0.6Mg alloy also helped to increase its corrosion resistance. This result is close to a work performed by Yao et al.49 who analyzed Zn-Mg alloys with different Mg concentrations and noticed that an alloy containing 3wt% Mg was more resistant to corrosion due to the nanostructure of the alloy. However, when 1 wt% Mg was added, it affected negatively on the corrosion rates due to the coarse structure of the alloy and the increase in grain size which contributes to the inhomogeneous distribution of corrosion products on the alloy surface and consequently less corrosion resistance.
Moreover, the formation of \({\text{MgZn}}_{2}\) and \({\text{Mg}}_{2} {\text{Zn}}_{11}\) intermetallic phases seem to have an important role in the corrosion resistance of the alloys. Owing to the higher standard electrode potential of Mg element than Zn, intermetallic phases (eutectic structures) are more vulnerable to be eroded than Zn matrix while the Zn cathode is protected. \({\text{MgZn}}_{2}\) intermetallic phases were formed in the Zn–0.5Al–0.6Mg alloy as shown from the XRD (Figure 3). The propagation of corrosion areas extending along the grain boundaries of eutectic structures was seen in Figure (7)iii) which might be attributed to the fine structure and homogeneous distribution of intermetallic phases in the Zn matrix and along the grain boundaries. This might have helped in the production of Mg-based oxide films along with other corrosion products preventing the Zn alloy from further exposition to the electrolyte.
Prosek et al.7 confirmed that the superior corrosion properties of Zn-Mg alloys are related to the presences of \({\mathrm{MgZn}}_{2}\) and \({\mathrm{Mg}}_{2}{\mathrm{Zn}}_{11}\) intermetallic (IMC) phases.
However, further additions of Mg above 0.6 wt % led to the increase in the percentage of \({\mathrm{Mg}}_{2}{\mathrm{Zn}}_{11}\) secondary intermetallic phases which caused accelerated galvanic corrosion between Zn and the precipitated secondary phases and consequently deteriorated the corrosion inhibition effect of Mg and reduced the corrosion resistance of the alloy.60
Conclusions
In this study, several Zn–0.5Al–Mg cast alloys were received to study the influence of Mg concentrations on the microstructure and corrosion behavior of the Zn-based alloys. It was the aim of this work to determine the best composition of the Zn ternary alloy to be employed in orthopedic biodegradable implant applications. The SEM analysis revealed that Magnesium had a great effect on the microstructure of the alloy where the degree of fineness of the microstructure increased with increasing magnesium contents up to 0.6 wt%. However further additions of Mg led to the formation of coarse microstructures. The X-ray diffraction confirmed the production of \({\text{MgZn}}_{2}\) and \({\text{Mg}}_{2} {\text{Zn}}_{11}\) secondary phases.
Moreover, polarization analyses revealed that the ZnAl0.6Mg ternary alloy had the lowest degradation rate of 0.33 mm/year as compared to the other investigated alloys which satisfies the requirements for ideal corrosion rates in orthopedic implants of less than 0.5 mm/year. This corrosion rate helps the implantable metal alloy to last in the body until healing of the bone tissue. The superior corrosion properties of Zn-Al-0.6Mg alloy was contributed to the fine structure, uniform distribution of Aluminum oxide passive layers and the uniform formation of MgZn2 phase precipitated in the Zn-rich matrix and along the grain boundaries which provided the best corrosion stability in SBF.
References
Z. Panossian, L. Mariaca, M. Morcillo, S. Flores, J. Rocha, J.J. Peña, F. Herrera, F. Corvo, M. Sanchez, O.T. Rincon, G. Pridybailo, J. Simancas, Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coatings Technol. 190, 244–248 (2005). https://doi.org/10.1016/j.surfcoat.2004.04.023
P.G. Salom, The metallurgy of steel. J. Franklin Inst. 120, 191–271 (1885). https://doi.org/10.1016/0016-0032(85)90312-6
A. Pola, M. Tocci, F.E. Goodwin, Review of microstructures and properties of zinc alloys. Metals (Basel). 10, 253 (2020). https://doi.org/10.3390/met10020253
P. Sharma, M.R. Hasan, N.K. Mehto, A.B. Deepak, J. Narang, 92 years of zinc oxide: has been studied by the scientific community since the 1930s- an overview. Sensors International 3, 100182 (2022). https://doi.org/10.1016/j.sintl.2022.100182
V. Caramia, B. Bozzini, Materials science aspects of zinc-air batteries: A review. Mater. Renew. Sustain. Energy. 3, 1–12 (2014). https://doi.org/10.1007/s40243-014-0028-3
N.P. Wasekar, A. Jyothirmayi, N. Hebalkar, G. Sundararajan, Influence of pulsed current on the aqueous corrosion resistance of electrodeposited zinc. Surf. Coatings Technol. 272, 373–379 (2015). https://doi.org/10.1016/j.surfcoat.2015.03.038
T. Prosek, A. Nazarov, U. Bexell, D. Thierry, J. Serak, Corrosion mechanism of model zinc-magnesium alloys in atmospheric conditions. Corros. Sci. 50, 2216–2231 (2008). https://doi.org/10.1016/j.corsci.2008.06.008
E. Aghion, Biodegradable metals. Metals 8(10), 804 (2018). https://doi.org/10.3390/met8100804
W.S. Pietrzak, B.L. Eppley, Resorbable polymer fixation for craniomaxillofacial surgery: development and engineering paradigms. J. Craniofac. Surg. 11, 575–585 (2000). https://doi.org/10.1097/00001665-200011060-00011
M.H. Emily Walker, Magnesium iron and zinc alloys, the trifecta of bioresorbable orthopaedic and vascular implantation - a review. J. Biotechnol. Biomater. 05, 1 (2015). https://doi.org/10.4172/2155-952x.1000178
S.A. Abdel-Gawad, M.A. Shoeib, Corrosion studies and microstructure of Mg−Zn−Ca alloys for biomedical applications. Surf. Interf. 14, 108–116 (2019). https://doi.org/10.1016/j.surfin.2018.11.011
H. Li, Y. Zheng, L. Qin, Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 24, 414–422 (2014). https://doi.org/10.1016/j.pnsc.2014.08.014
H. Hermawan, Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 7, 93–110 (2018). https://doi.org/10.1007/s40204-018-0091-4
X. Tong, W. Cai, J. Lin, K. Wang, L. Jin, Z. Shi, D. Zhang, J. Lin, Y. Li, M. Dargusch, C. Wen, Biodegradable Zn−3Mg−0.7Mg2Si composite fabricated by high-pressure solidification for bone implant applications. Acta Biomater. 123, 407–417 (2021). https://doi.org/10.1016/j.actbio.2020.12.059
M.F. Ulum, W. Caesarendra, R. Alavi, H. Hermawan, In-vivo corrosion characterization and assessment of absorbable metal implants. Coatings 9, 282 (2019). https://doi.org/10.3390/coatings9050282
Q. Yu, X. Sun, J. Zhao, L. Zhao, Y. Chen, L. Fan, Z. Li, Y. Sun, M. Wang, F. Wang, The effects of zinc deficiency on homeostasis of twelve minerals and trace elements in the serum, feces, urine and liver of rats. Nutr. Metab. 16, 1–8 (2019). https://doi.org/10.1186/s12986-019-0395-y
P.K. Bowen, R.J. Guillory, E.R. Shearier, J.M. Seitz, J. Drelich, M. Bocks, F. Zhao, J. Goldman, Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C. 56, 467–472 (2015). https://doi.org/10.1016/j.msec.2015.07.022
He. Huang, H. Liu, L.S. Wang, Y.H. Li, S.O. Agbedor, J. Bai, F. Xue, J.-H. Jiang, A high-strength and biodegradable Zn–Mg alloy with refined ternary eutectic structure processed by ECAP. Acta Metallurgica Sinica (English Letters) 33(9), 1191–1200 (2020). https://doi.org/10.1007/s40195-020-01027-x
P.K. Bowen, J. Drelich, J. Goldman, Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 25, 2577–2582 (2013). https://doi.org/10.1002/adma.201300226
S. Zhang, X. Zhang, C. Zhao, J. Li, Y. Song, C. Xie, H. Tao, Y. Zhang, Y. He, Y. Jiang, Y. Bian, Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 6, 626–640 (2010). https://doi.org/10.1016/j.actbio.2009.06.028
K. Pieła, M. Wróbel, K. Sztwiertnia, M. Jaskowski, J. Kawałko, M. Bieda, M. Kiper, A. Jarzębska, Zinc subjected to plastic deformation by complex loading and conventional extrusion: Comparison of the microstructure and mechanical properties. Mater. Des. 117, 111–120 (2017). https://doi.org/10.1016/j.matdes.2016.12.056
F.C. Walsh, Corrosion of metals physicochemical principles and current problems. J. Electroanal. Chem. 567(1), 151 (2004). https://doi.org/10.1016/j.jelechem.2004.02.028
M. Esmaily, J.E. Svensson, S. Fajardo, N. Birbilis, G.S. Frankel, S. Virtanen, R. Arrabal, S. Thomas, L.G. Johansson, Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 89, 92–193 (2017). https://doi.org/10.1016/j.pmatsci.2017.04.011
H. Huang, H. Liu, L. Wang, K. Yan, Y. Li, J. Jiang, A. Ma, F. Xue, J. Bai, Revealing the effect of minor Ca and Sr additions on microstructure evolution and mechanical properties of Zn-0.6 Mg alloy during multi-pass equal channel angular pressing. J Alloys Comp 844, 155923 (2020). https://doi.org/10.1016/j.jallcom.2020.155923
A. Jarzębska, M. Bieda, J. Kawałko, Ł Rogal, P. Koprowski, K. Sztwiertnia, W. Pachla, M. Kulczyk, A new approach to plastic deformation of biodegradable zinc alloy with magnesium and its effect on microstructure and mechanical properties. Mater. Lett. 211, 58–61 (2018). https://doi.org/10.1016/j.matlet.2017.09.090
S. Zhao, J.M. Seitz, R. Eifler, H.J. Maier, R.J. Guillory, E.J. Earley, A. Drelich, J. Goldman, J.W. Drelich, Zn-Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng. C. 76, 301–312 (2017). https://doi.org/10.1016/j.msec.2017.02.167
G. SathishKumar, P. Parameswaran, V. Vijayan, R. Yokeswaran, Effects of Ca, Cu concentration on degradation behavior of Zn alloys in Hank’s solution. Met. Powder Rep. 76, 40–42 (2021). https://doi.org/10.1016/j.mprp.2019.12.005
H.F. Li, X.H. Xie, Y.F. Zheng, Y. Cong, F.Y. Zhou, K.J. Qiu, X. Wang, S.H. Chen, L. Huang, L. Tian, L. Qin, Erratum: development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg. Ca and Sr. Sci. Rep. 5, 1–14 (2015). https://doi.org/10.1038/srep12190
P. Sotoudeh Bagha, S. Khaleghpanah, S. Sheibani, M. Khakbiz, A. Zakeri, Characterization of nanostructured biodegradable Zn-Mn alloy synthesized by mechanical alloying. J. Alloys Compd. 735, 1319–1327 (2018). https://doi.org/10.1016/j.jallcom.2017.11.155
W. Bednarczyk, M. Wątroba, J. Kawałko, P. Bała, Can zinc alloys be strengthened by grain refinement? a critical evaluation of the processing of low-alloyed binary zinc alloys using ECAP. Mater. Sci. Eng. A. 748, 357–366 (2019). https://doi.org/10.1016/j.msea.2019.01.117
M. Sikora-Jasinska, E. Mostaed, A. Mostaed, R. Beanland, D. Mantovani, M. Vedani, Fabrication, mechanical properties and in vitro degradation behavior of newly developed Zn[sbnd]Ag alloys for degradable implant applications. Mater. Sci. Eng. C. 77, 1170–1181 (2017). https://doi.org/10.1016/j.msec.2017.04.023
Z.O. Králová, R. Gorejová, R. Oriňaková, M. Petráková, A. Oriňak, M. Kupková, M. Hrubovčáková, T. Sopčák, M. Baláž, I. Maskaľová, A. Kovalčíková, K. Kovaľ, Biodegradable zinc-iron alloys: Complex study of corrosion behavior, mechanical properties and hemocompatibility. Prog. Nat. Sci. Mater. Int. 31, 279–287 (2021). https://doi.org/10.1016/j.pnsc.2021.01.002
P. Xue, M. Ma, Y. Li, X. Li, J. Yuan, G. Shi, K. Wang, K. Zhang, Microstructure, mechanical properties, and in vitro corrosion behavior of biodegradable Zn-1Fe-xMg alloy. Materials (Basel). 13, 1–15 (2020). https://doi.org/10.3390/ma13214835
H. Gong, K. Wang, R. Strich, J.G. Zhou, In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed Mater. Res. - Part B Appl. Biomater. 103, 1632–1640 (2015). https://doi.org/10.1002/jbm.b.33341
R. Krieg, A. Vimalanandan, M. Rohwerder, Corrosion of Zinc and Zn-Mg Alloys with Varying Microstructures and Magnesium Contents. J. Electrochem. Soc. 161, C156–C161 (2014). https://doi.org/10.1149/2.103403jes
A.A. Mazilkin, O.A. Kogtenkova, B.B. Straumal, B. Ruslan, B. Baretzky, Formation of nanostructure during high-pressure torsion of Al-Zn, Al-Mg and Al-Zn-Mg alloys. Defect Diffus. Forum 237–240, 739–744 (2005). https://doi.org/10.4028/www.scientific.net/DDF.237-240.739
S.C. Jin, J.U. Lee, J. Go, H. Yu, S.H. Park, Effects of Sn addition on the microstructure and mechanical properties of extruded Mg–Bi binary alloy. J. Magnes. Alloy. 10, 850–861 (2022). https://doi.org/10.1016/j.jma.2021.04.015
B. Ratna Sunil, T.S. Sampath Kumar, V. Uday Chakkingal, M.D. Nandakumar, V. Devi Prasad, M. Raghunath, In vitro and in vivo studies of biodegradable fine grained AZ31 magnesium alloy produced by equal channel angular pressing. Mater. Sci. Eng.: C 59, 356–367 (2016). https://doi.org/10.1016/j.msec.2015.10.028
H.R. Bakhsheshi-Rad, E. Hamzah, H.T. Low, M. Kasiri-Asgarani, S. Farahany, E. Akbari, M.H. Cho, Fabrication of biodegradable Zn-Al-Mg alloy: mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C. 73, 215–219 (2017). https://doi.org/10.1016/j.msec.2016.11.138
C. Panaghie, R. Cimpoesu, G. Zegan, A.M. Roman, M.C. Ivanescu, A.A. Aelenei, M. Benchea, N. Cimpoeu, N. Ioanid, In vitro corrosion behavior of Zn3Mg0.7Y biodegradable alloy in simulated body fluid (SBF). AppL. Sci. 12(5), 2727 (2022). https://doi.org/10.3390/app12052727
X. Liu, J. Sun, F. Zhou, Y. Yang, R. Chang, K. Qiu, Z. Pu, L. Li, Y. Zheng, Corrigendum to “Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application.” Mater. Des. 96, 377 (2016). https://doi.org/10.1016/j.matdes.2016.01.135
H.R. Bakhsheshi-Rad, E. Hamzah, H.T. Low, M.H. Cho, M. Kasiri-Asgarani, S. Farahany, A. Mostafa, M. Medraj, Thermal Characteristics, mechanical properties, in vitro degradation and cytotoxicity of novel biodegradable Zn–Al–Mg and Zn–Al–Mg–xBi Alloys. Acta Metallurgica Sinica (English Letters) 30(3), 201–211 (2017). https://doi.org/10.1007/s40195-017-0534-2
Z. Tang, H. Huang, J. Niu, L. Zhang, H. Zhang, J. Pei, J. Tan, G. Yuan, Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 117, 84–94 (2017). https://doi.org/10.1016/j.matdes.2016.12.075
M.E. Moussa, S. El-Hadad, M. Shoeib, Influence of Dendritic Fragmentation through Mg Addition on the Electrochemical Characteristics of Zn–0.5 wt% Al Alloy. Inter. J. Metalcast. 16(2), 1034–1044 (2022). https://doi.org/10.1007/s40962-021-00662-z
A.A. Francis, S.A. Abdel-Gawad, M.A. Shoeib, Toward CNT-reinforced chitosan-based ceramic composite coatings on biodegradable magnesium for surgical implants. J. Coatings Technol. Res. 18, 971–988 (2021). https://doi.org/10.1007/s11998-021-00468-y
S. Farahany, L.H. Tat, E. Hamzah, H.R. Bakhsheshi-Rad, M.H. Cho, Microstructure development, phase reaction characteristics and properties of quaternary Zn-0.5Al-0.5Mg-xBi hot dipped coating alloy under slow and fast cooling rates. Surf. Coat. Technol. 315, 112–122 (2017). https://doi.org/10.1016/j.surfcoat.2017.01.074
J. Venezuela, M.S. Dargusch, The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: a comprehensive review. Acta Biomater. 87, 1–40 (2019). https://doi.org/10.1016/j.actbio.2019.01.035
E. Mostaed, M. Sikora-Jasinska, J.W. Drelich, M. Vedani, Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 71, 1–23 (2018). https://doi.org/10.1016/j.actbio.2018.03.005
C. Yao, Z. Wang, S.L. Tay, T. Zhu, W. Gao, Effects of Mg on microstructure and corrosion properties of Zn-Mg alloy. J. Alloys Compd. 602, 101–107 (2014). https://doi.org/10.1016/j.jallcom.2014.03.025
S.A. Abdel-Gawad, W.M. Osman, A.M. Fekry, Characterization and corrosion behavior of anodized Aluminum alloys for military industries applications in artificial seawater. surf. Interf 14, 314–323 (2019). https://doi.org/10.1016/j.surfin.2018.08.001
S.A. Abdel-Gawad, M.A. Sadik, M.A. Shoeib, Preparation and properties of a novel nano Ni-B-Sn by electroless deposition on 7075–T6 aluminum alloy for aerospace application. J. Alloys Compd. 785, 1284–1292 (2019). https://doi.org/10.1016/j.jallcom.2019.01.245
M.A. Shoeib, M.M. Kamel, S.M. Rashwan, O.M. Hafez, Corrosion behavior of electroless Ni-P/TiO2 nanocomposite coatings. Surf. Interface Anal. 47, 672–680 (2015). https://doi.org/10.1002/sia.5764
S. Liu, G. Esteban-Manzanares, J. Llorca, First-principles analysis of precipitation in Mg-Zn alloys. Phys. Rev. Mater. 4, 93609 (2020). https://doi.org/10.1103/PhysRevMaterials.4.093609
X. Liu, J. Sun, Y. Yang, F. Zhou, Z. Pu, L. Li, Y. Zheng, Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn-Mg-Sr alloys as biodegradable metals. Mater. Lett. 162, 242–245 (2016). https://doi.org/10.1016/j.matlet.2015.07.151
E. Mostaed, M. Sikora-Jasinska, A. Mostaed, S. Loffredo, A.G. Demir, B. Previtali, D. Mantovani, R. Beanland, M. Vedani, Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 60, 581–602 (2016). https://doi.org/10.1016/j.jmbbm.2016.03.018
C. Yao, H. Lv, T. Zhu, W. Zheng, X. Yuan, W. Gao, Effect of Mg content on microstructure and corrosion behavior of hot dipped Zn–Al–Mg coatings. J. Alloys Compd. 670, 239–248 (2016). https://doi.org/10.1016/j.jallcom.2016.02.026
Q. Ge, X. Liu, A. Qiao, Mu. Yongliang, Compressive properties and degradable behavior of biodegradable porous zinc fabricated with the protein foaming method. J. Funct. Biomater. 13(3), 151 (2022). https://doi.org/10.3390/jfb13030151
S. Erdibil, S. Cesur, R. İpek, Mg/Zn composites produced by mechanical alloying and hot pressing and in-vitro biodegradation. Usak Univ. J. Eng. Sci. 2019, 22–38 (2019)
P. Volovitch, T.N. Vu, C. Allély, A. Abdel Aal, K. Ogle, Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn-Al-Mg coatings on steel. Corros. Sci. 53, 2437–2445 (2011). https://doi.org/10.1016/j.corsci.2011.03.016
A. Srinivasan, Y. Huang, C.L. Mendis, C. Blawert, K.U. Kainer, N. Hort, Investigations on microstructures, mechanical and corrosion properties of Mg-Gd-Zn alloys. Mater. Sci. Eng. A. 595, 224–234 (2014). https://doi.org/10.1016/j.msea.2013.12.016
Acknowledgements
The corresponding author would like to acknowledge the partial fund from the Science Technology and Development Fund-Egypt, Grant No. 26565
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hammam, R.E., Abdel-Gawad, S.A., Moussa, M.E. et al. Study of Microstructure and Corrosion Behavior of Cast Zn–Al–Mg Alloys. Inter Metalcast 17, 2794–2807 (2023). https://doi.org/10.1007/s40962-022-00944-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-022-00944-0