Abstract
High Entropy Alloys (HEAs) are one of the new families of alloys that gained the attention of researchers as a new approach to materials design. The special compositional design of these alloys results in unique microstructures and hence distinguished properties. Corrosion resistance is one of the most important properties of any material used for structural components. Therefore, it is of interest to investigate the electrochemical properties of such recent groups of HEAs alloys to understand their corrosion behavior. In the current study, the electrochemical properties of cast Fe35Mn31Ni18Al13Tix HEAs prepared by induction melting were investigated in 3.5% NaCl and 5% H2SO4 solutions. It was observed that both Ti addition (0.0–3 wt%) and heat treatment (900 °C at 10 and 20 h) altered the alloys microstructure and, hence, affected their corrosion behavior. The base alloy without Ti showed higher resistance to corrosion in both NaCl and H2SO4 solutions compared to Ti-containing alloys. Heat treatment for 10 h increased significantly the corrosion resistance of the as-cast alloys regardless of the Ti content. On the other hand, heat treatment for 20 h affected negatively the corrosion resistance of the alloy without Ti. Therefore, the electrochemical properties of HEAs can be well controlled through alloying additives and heat treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last 20 years, the term of high entropy alloys (HEAs) has increasingly appeared in the material science platform. The first evolution of these alloys has been performed by Jien-Wei yeh and Bran Cantor teams.1,2,3,4 The common definition of HEAs is that alloy systems contain five or more principal metallic elements in equiatomic or near equiatomic ratio thus forming one or more solid solution phases. The main cause behind this phenomenon is the high entropy of mixing solid solution phases that enhances their stability compared with intermetallic compounds. Many factors can be classified into two types: thermodynamic and electronic parameters. However, continued research has shown that numerous so-called HEAs, such as the Mo0.5AlNbTa0.5TiZr system, did not have high configurational entropies and present the formation of secondary phases (rather than just solid solution phases). Therefore, some researchers prefer to name these types as compositionally complex alloys (CCAs).5,6 By this time, researchers started a new direction in studying the properties of HEAs to get a complete characterization profile. This concern came from the unique and attractive properties of HEAs that have been observed in the preliminary studies, which give a promising chance for industrial applications. HEAs exhibit many functional properties as optical, thermal and electrochemical, etc. The merit of HEAs properties comes from the overall contribution of the constituting phases by the effect of phase shape, phase distribution, phase boundaries, and related properties of such phase.7,8,9 One of the most important material properties in engineering applications is corrosion resistance. It is commonly known that corrosion negatively affects materials’ proper function, which leads to rapid failure. Furthermore, the cost of corrosion is very high, it destroys more than 3% of the world’s GDP. In HEAs, especially those containing passivating elements as Cr, Ni, Mo, superior corrosion resistance is expected relative to the conventional alloys. Chen et al.9,10 investigated the corrosion behavior of Cu0.5NiAlCoCrFeSi alloy in NaCl and H2So4 solutions and compared it with the austenitic stainless steel (AISI 304 st. st). The main observation was that the HEA exhibited a lower corrosion rate against 304 St.St. Following this study, other works have been performed to investigate the corrosion resistance depending on two main influencing parameters: alloying elements and heat treatment. Lee et al.11 studied the effect of Al on corrosion of AlxCrFe1.5MnNi0.5 in H2So4 and NaCl solutions, the surprising conclusion was that Al-containing HEA alloys have lower corrosion resistance relative to the Al free ones. However, the increase of Al content enhances the passivity of alloy in NaCl solution. Later, Qiu et al.12,13 have performed a wider study to measure Ecorr (corrosion potential) and Epit values for about 20 HEAs in 0.6M NaCl and also reported a preliminary galvanic series for HEAs. The main findings were that the investigated HEAs were found to be significantly nobler than carbon steels and Al alloys. The published Ecorr values of the HEAs were in the range of ferritic and austenitic stainless steels, with some HEAs having nobler values than austenitic stainless steel. The Ecorr, Epit, and icorr values of the HEAs were primarily dependent upon the respective alloying elements that were used to synthesis the HEA.
Wang et al.14 prepared (Fe36Ni18Mn33Al13)100−x Tix HEAs using high purity metals and observed that the mechanical properties of these alloys can be significantly enhanced via controlling their microstructure. Both of titanium addition and heat treatment control the lamellar spacing of the microstructure and hence affect the hardness and strength. In another recent study,15 Fe35Mn31Ni18Al13Tix HEAs were prepared using induction melting and it was noted that heat treatment at 900 °C for 10 and 20 h exhibited significant effects on the cast microstructure and alloy hardness. In spite of the reported controllable mechanical properties of these alloys,14,15 there is no study up-to-date that investigated their electrochemical properties, which is important in various applications.
In the current study, the corrosion behavior of cast Fe35Mn31Ni18Al13Tix HEAs prepared in15 was evaluated using NaCl and H2SO4 solutions as corrosive media. The results were then related to the obtained microstructures.
Experimental Work
Materials
The as-received HEAs were prepared by induction melting as described in.15 Three groups of alloys were obtained with different percentages of titanium. Ti and Mn were added as ferrous alloys cost-wise, while Ni and Al were added as pure metals. Corrosion test samples were poured in shell sand cups to obtain cubes of 2 cm thickness. Three groups of FeMnNiAlTix HEAs were obtained with different Ti percentages and named as 1C, 2C, and 3C, as shown in Table 1.
After casting, square samples were sectioned from the middle of cubes and heat-treated at 900 °C for 10 and 20 h. As shown in the following Table 2, the tagging of as-cast and heat-treated specimens follows the indicated criteria. From the shown chemical composition, Si came unintentionally due to the addition of Ti and Mn as ferroalloys. However, the presence of Si with this percentage (1.5–2%) as alloying elements, is very beneficial for corrosion resistance.
Samples Preparation
All the as-received HEAs samples (cast and heat-treated) were metallographically prepared. The specimens were first ground then polished and etched using 3 wt% Nital and rinsed with water and ethanol and finally washed with anhydrous ethyl alcohol. Optical microstructures were taken to understand the differences between all the investigated conditions. Similar sets of samples were prepared without etching for corrosion tests. According to Table 2, 9 specimens have been metallographically prepared for testing.
Electrochemical Tests
Two electrochemical tests were performed: accelerated corrosion test and immersion test.
Accelerated Corrosion Test
In this test, the specimens were evaluated via a potentiostat instrument (Metrohm AUTOLAB PGSTAT302N). All the measurements were performed, and the output data have been extracted by NOVA software embedded with the AUTOLAB module. The measurements were the electrochemical impedance and potentiodynamic polarization. The electrochemical cell was composed of the anode (working electrode), cathode (platinum electrode), and reference electrode (Ag/AgCl). The exposed area of the working electrode was 0.38 cm2 and a 3.5% NaCl solution was used as the liquid media. In the beginning, the system performs a steady-state open circuit potential (OCP) that ranges between − 0.4 and + 0.4 V and a scan rate of 0.001 V/s was applied. All the tests were performed at room temperature, and the frequency range was between 100 kHz and 0.1 Hz using 0.01 V amplitude. The potential against current plots was obtained along with the impedance plots.
Immersion Corrosion Testing
The specimens for immersion experiments were prepared with the same procedure as explained previously in “Samples Preparation” section. As mentioned previously, there were 9 specimens, however, in this experiment, specimens were distributed into 2 groups. The first group containing 9 specimens, was immersed in 3.5 wt% NaCl, while the second group was immersed in 5 wt% H2SO4 solution. Firstly, all specimens were polished and cleaned in ultrasonic followed by washing in distilled water and drying. Then, weighed before the immersion test and hung freely in the solutions. After 30 days, all the specimens were taken out of the solutions and washed in distilled water then dried. The weight change due to corrosion was then calculated.
Characterization of the Corroded Samples
All the corroded specimens were characterized using optical and Field Emission Microscope (FEM- Quanta FEG 250 model) equipped with EDAX.
Results
Characterization of the As-Received Samples
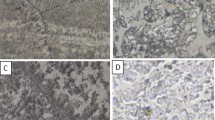
As shown in Figure 1, the optical micrograph for alloy 1C represents a microstructure that consists of two phases: the matrix phase (composed of Ni and Al) and the secondary phase (mainly Fe and Mn) with a dark lamellar structure inside. As mentioned in,15 the distance between lamellas decreased with Ti addition and almost all the elements became together in the solid solutions. Moreover, coarse grains with some Ti precipitates on the grain boundaries formed with the addition of 3.0% Ti. Also, some free Ti dispersoids were frequently detected.
Figures 2 and 3 present the heat-treated samples (at 900 °C) for 10 and 20 h, respectively. It is noted that the lamellar spacing and the percentage of phases to each other significantly changed with heat treatment as described in Refs.14,15 According to the microstructure observation, the electrochemical properties of the alloys are expected to show different behavior depending on Ti content and heat treatment.
Electrochemical Properties
Accelerated Corrosion Test
The polarization behavior of the processed HEAs showed significant changes in their corrosion behavior as a result of Ti addition and heat treatment.
Effect of Ti on Electrochemical Behavior
Figure 4 shows the Tafel plot of the as-cast HEAs with different Ti content. It can be observed that the addition of Ti moved the potential slightly toward more positive values. However, increasing Ti content in the alloy unexpectedly decreased the impedance of the alloy in spite of the well-known resistance of Ti to corrosion. This is apparent from the lower values of impedance for 2C and 3C specimens shown in Figure 5.
Effect of Heat Treatment on the Electrochemical Behavior
Heat treatment of the HEAs without Ti (1C) at 900 °C showed improved corrosion resistance after 10 h, while extending the treatment time to 20 h resulted in negative effects on impedance as clear from Figures 6 and 7. Though they are not presented herein, both of Bod modulus and Bod Phase curves further supported these results, where specimen 1H10 exhibited a higher Z (Ω) value and wider frequency range compared to 1C and 1H20.
On the other side, heat treatment enhanced the corrosion resistance of the samples by 1 wt% Ti (2C) at 10 h and 20 h, as remarked from Figures 8 and 9.
The specimen with the maximum Ti content (3C) exhibited almost the same behavior as (1C) where 3H10 showed better impedance compared to 3C, while the impedance of 3H20 was the lowest, see Tafel plots and Impedance curves of Figures 10 and 11.
Using the above Tafel curves (Figures 4, 6, 8, and 10), Table 3 summarizes the corrosion rates as shown below. This further supports the obtained results.
In order to further understand the corrosion behavior during the polarization test, selected samples Figures 12 and 13 (1C and 2C: representing different Ti contents) and Figure 14 (1H10: heat-treated specimen) were investigated using FEM and the distribution of the elements was analyzed using EDX point analysis from inside the corrosion is passing by the interface to the uncorroded region.
Contrasting Figures 12 and 13 it can be observed that the corroded area in sample 1C is depleted in Fe and Mn and enriched with Ni, while sample 2C showed higher Fe and Mn and lower Ni.
By comparing Figure 14 of 1H10 to Figure 12 of 1C, it can be observed that almost the same way of elemental distribution, where the corroded area has lower values of Fe and Mn while Ni reached 31.9 wt%, which is much higher than the substrate (17 wt%).
Immersion Test
Immersion in NaCl
Concerning the immersion test in NaCl, Figure 15 shows the optical micrographs of the as corroded (NaCl) specimens. It is noted that corrosion products increased in 2C and 3C relative to 1C. Also, from the EDX point analysis, Figure 16, of some frequently detected particles in the 3C sample, it is clear that Ti agglomerated at some points and caused the localized attack. Figure 17 shows the plot of weight loss curve of the investigated HEAs after immersion in NaCl and H2SO4 for 30 days. Specimens 1C exhibited lower values of weight loss than 2C and 3C, which is in agreement with the measurements of the polarization test.
Immersion in H2SO4
The corroded surface morphologies obtained after immersion in H2SO4 are presented in Figure 18a–i. It is apparent from both the weight change curve of Figure 17 and the macrographs of Figure 18 that, the addition of Ti increased relatively the weight of corrosion products in 2C and 3C specimens compared to 1C. Heat treatment for 10 h improved the alloy resistance to corrosion which is clear from Figure 17, where the weight of corrosion products of sample 1C decreased from ~ 0.36 to 0.21 mg. This improvement was also significant in the case of specimen 3C containing Ti, in which the weight gain decreased from 0.42 to 0.33 mg. On the other hand, treatment for 20 h increased the weight of corrosion products on 1C to ~ 0.49 mg for 1H20. In the case of 2C and 3C with the highest Ti content, heat treatment at 20 exhibited severe corrosion attack on the tested samples, see 2H20 and 3H20 in Figure 18i, where deeply localized pits and surface engulfment can be detected.
Discussion
In the current work on Fe35Mn31Ni18Al13Tix HEAs alloys, the obtained Ecorr for the different specimens in NaCl solution ranged between − 329 and – 422 mV which is a value that does not belong to either Fe, Mn, Ni or Al. These results are in agreement with the comment of Qiu et al.12 in their review that HEAs are composed of unique and heterogeneous phases that own special electrochemical characteristics which differ from all the constituting elements. Therefore, more concern should be given to the constituting phases of HEAs rather than their elemental components.
The results of the corrosion test as related to the influence of Ti on the microstructure are in accordance with the work of Ren et al.15 where they owed increasing the general corrosion resistance in NaCl solution with Ti addition in Al0.3CrFe1.5MnNi0.5Tix HEAs to the formation of some intermetallic compounds that act as corrosion sites. Moreover, Qiu et al in their review,13 mentioned that Ti addition mitigates the general corrosion of HEAs while the nobler phases containing Ti may encourage localized corrosion. It is noted in Figure 5 that Ti addition decreased the impedance significantly in 2C and 3C alloys compared to 1C. The reasoning can be clearly found in Figure 16 which shows an example of a localized corrosion attack at a Ti-containing particle.
In regards to the influence of heat treatment, it was observed that 10 h heat treatment positively affected the corrosion resistance, however, extending the heat treatment time to 20 h showed the opposite effect, which was most significant in the alloy with Ti addition. This can be explained through the work of Shi et al.8 who described that heat-treating HEAs at high temperatures homogenizes their microstructures and eliminates the elemental segregations and hence improving the corrosion resistance. However, in some cases, if the treatment temperature was not appropriately selected, some undesired phases and intermetallic may form and affect negatively the corrosion resistance.16 This is expected to be the case in the current work when the treatment time was prolonged to 20 h, which caused more pits and engulfment on the surfaces of the heat-treated specimens 2H20 and 3H20 when immersed in the aggressive H2SO4 solution, see Figure 18h and i.
Conclusions
In the current work, the electrochemical properties of as received-cast Fe35Mn31Ni18Al13Tix HEAs were investigated in 3.5% NaCl and 5% H2SO4 solutions.
-
It was observed that both Ti addition and heat treatment altered the alloy's microstructure and, hence, affected their corrosion behavior.
-
The base alloy without Ti showed higher resistance to localized corrosion in both NaCl and H2SO4 solutions compared to Ti-containing alloys.
-
Heat treatment for 10 h increased significantly the corrosion resistance of the as-cast alloys regardless of the Ti content.
-
Heat treatment for 20 h affected negatively the corrosion resistance of the alloy without Ti.
-
Concluding, the electrochemical properties of HEAs can be well controlled through alloying additives and heat treatment.
References
P.-K. Huang, J.-W. Yeh, T.-T. Shun, S.-K. Chen, Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 6, 74–78 (2004)
J.-W. Yeh, S.-K. Chen, J.-W. Gan, S.-J. Lin, T.-S. Chin, T.-T. Shun, C.-H. Tsau, S.-Y. Chang, Formation of simple crystal structures in Cu–Co–Ni–Cr–Al–Fe–Ti–V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 35A, 2533–2536 (2004)
J.-W. Yeh, S.-K. Chen, S.-J. Lin, J.-Y. Gan, T.-S. Chin, T.-T. Shun, C.-H. Tsau, S.-Y. Chang, Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 6, 299–303 (2004)
B. Cantor, I.T.H. Chang, P. Knight, A.J.B. Vincent, Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 375–377, 213–218 (2004)
E.P. George, D. Raabe, R.O. Ritchie, High-entropy alloys. Nat. Rev. Mater. 4, 515 (2019)
Z.P. Lu, H. Wang, M.W. Chen, I. Baker, J.W. Yeh, C.T. Liu, T.G. Nieh, An assessment on the future development of high-entropy alloys: summary from a recent workshop. Intermetallics 66, 67–76 (2015)
X. Wang, W. Guo, Y. Fu, High-entropy alloys: emerging materials for advanced functional applications. J. Mater. Chem. A 9, 663–670 (2021)
Q. Li, X. Bao, S. Zhao, The influence of AlFeNiCrCoTi high-entropy alloy on microstructure, mechanical properties and tribological behaviors of aluminum matrix composites. Inter. Metalcast. 15, 281–291 (2021). https://doi.org/10.1007/s40962-020-00462-x
H. Sahin, H. Zengin, Microstructure, mechanical and wear properties of low-density cast medium and high entropy aluminium alloys. Inter. Metalcast. (2022). https://doi.org/10.1007/s40962-021-00744-y
Y.Y. Chen, U.T. Hong, H.C. Shih, J.W. Yeh, T. Duval, Electrochemical kinetics of the high entropy alloys in aqueous environments—a comparison with type 304 stainless steel. Corros. Sci. 47, 2679–2699 (2005)
C.P. Lee, C.C. Chang, Y.Y. Chen, J.W. Yeh, H.C. Shih, Effect of the aluminum content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behavior in aqueous environments. Corros. Sci. 50, 2053–2060 (2008)
Y. Qiu, S. Thomas, M.A. Gibson, H.L. Fraser, N. Birbilis, Corrosion of high entropy alloys. Mater. Degrad. 1, 15 (2017)
Y. Qiu, M.A. Gibson, H.L. Fraser, N. Birbilis, Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 31, 1235–1243 (2015)
Z. Wang, M. Wu, Z. Cai, S. Chen, I. Baker, Effect of Ti content on the microstructure and mechanical behavior of (Fe36Ni18Mn33Al13)100−xTix high entropy alloys. Intermetallics 75, 79–87 (2016)
S. El-Hadad, M. Ibrahim, M. Mourad, Effect of heat treatment and titanium addition on the microstructure and mechanical properties of cast Fe31Mn28Ni15Al24.5Tix high-entropy alloys. Adv. Mater. Sci. Eng. 2019, 2157592 (2019)
B. Ren, R.F. Zhao, Z.X. Liu, S.K. Guan, H.S. Zhang, Microstructure and properties of Al0.3CrFe1.5MnNi0.5Tix and Al0.3CrFe1.5MnNi0.5Six high-entropy alloys. Rare Metal 33, 149–154 (2014)
Acknowledgements
The corresponding author would like to acknowledge the fund from the Egyptian Science and Technology Development Fund (grant No. 33401). Dr. Mohamed Mourad from the Metal Casting Dept. Central Metallurgical R&D Institute is also acknowledged for its support during the casting process of the as-received HEAs. The first author also acknowledges the support from the Egyptian Natural Gas Company (GASCO) throughout his M.Sc. study period.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, M., El-Hadad, S., Khalifa, W. et al. Effect of Ti Addition and Heat Treatment on Electrochemical Properties of Cast Fe35Mn31Ni18Al13Tix HEAs. Inter Metalcast 17, 888–899 (2023). https://doi.org/10.1007/s40962-022-00821-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-022-00821-w