Abstract
In this research work, the effects of different amounts of acid hardener (30%, 40%, 60%, 80% weighted to the resin) on the hardening characteristics and hot-distortion properties of no-bake furan and no-bake phenolic bonded sand cores were studied. Bending tests were conducted on test bars with storage times of 1, 2, 3, 5, 7, 24h. Hot-distortion tests were carried out on specimens with storage times of 4h and 24h. The bending tests revealed that in the case of the furan binder system, the acid hardener is best utilized in terms of higher bending strength, in an amount of 40–60%, while in the case of the phenolic binder system, the amount of 60–80% acid hardener resulted in higher bending strength of the sand specimens. Too low (30%) acid hardener (catalyst) level produced low bending strength. Too high (80%) amount of acid hardener decreased the strength of the no-bake furan sand samples, and as can be seen from the SEM analysis, it damaged the binder bridges between the sand grains. The hot-distortion tests showed that there is a correlation between the catalyst content and the max. Deformation of the samples both in the furan and in the phenolic no-bake sand cores, which can be described with a maximum curve. Increasing the acid hardener changes the thermoplastic behavior of the phenolic resin, thus the binder bridges become more rigid and brittle. The acid hardener above 40% decreased the thermal stability of the furan and phenolic bonded test pieces. The research work also revealed significant differences between the specimens made with furan and phenolic binder and the effect of the storage time in terms of the bending strength and hot-distortion properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over more than 60 years, no-bake methods have been widely used in the foundry industry for producing expendable molds and cores during the manufacturing of medium and large-sized castings of nearly all alloys.1
During the no-bake process, two or more binder components cure at ambient temperature after they are blended with foundry sand. The curing process begins immediately after all components of the sand mixture are combined. The produced sand mixture is usable for a limited time, which allows filling of the core or mold boxes. After an additional time, when the curing reaction is advanced, the hardened core or mold can be removed from the box. The time difference between the two steps can range from a few minutes to several hours depending on the used binder system, the type and amount of curing agent, the sand temperature, and other environmental conditions.2,3,4,5
In core making, the term “no-bake process” includes several methods that use self-setting, liquid catalyst-cured organic or inorganic binders such as furan resins, phenolic resins, phenolic-urethane resin, ester-cured phenolics, and ester-cured silicates. Among them, the furan and phenolic no-bake methods were widely accepted in foundry practice during the last decades.6
The furan no-bake method was introduced in 1958. The binder system consists of two liquid components: a resin, and a catalyst. Furan resins are based on furfuryl-alcohol, which is produced from agricultural by-products such as corn cobs, rice hulls, and sugar cane bagasse. Usually, the furfuryl alcohol is modified with urea, formaldehyde, or phenolic resin to improve resin quality. The cross-linking of the furan resin is promoted by an acidic hardener that can be phosphoric acid, or organic sulfonic acid (such as benzenesulfonic acid, or BSA; toluenesulfonic acid, or TSA; xylene-sulfonic acid, and other combination of these). During the production of the sand mixture, first, the hardener is added to the sand, then the resin. At ambient temperature, the resin reacts with the acid hardener through an exothermic polycondensation reaction. The primary by-product of the reaction is water. The produced furan no-bake sand cores usually have excellent dimensional accuracy and strength properties, moderate erosion resistance against the melt, and good shake-out properties.1,6,7,8
The production of furfuryl alcohol depends upon the availability of raw materials. To substitute the expensive furan binder, another organic resin was developed in the 1960s and widely accepted in Europe and the United States as well.9 In the phenolic no-bake process the binder system includes two liquid components, the phenolic resole resin and the acid hardener. The polycondensation reaction occurs at ambient temperature with water as a by-product. The steps of the sand core production are the same as in the case of the furan no-bake method. To achieve the required quality of the sand cores, strong acid catalysts are applied, such as PTSA (p-Toluenesulfonic acid) and BSA. Compared to the furan resin, the phenolic resole contains a larger amount of water (8–25 %).6 Because of that, the degree of cross-linking is lower, thus the produced sand cores show lower strength properties. Despite that, the phenolic no-bake process gradually spread among the foundries, because the binder system ensures good handling, ease of compaction, high hot strength, erosion resistance, good plasticity/elastic nature to the produced sand cores. The disadvantage of using phenolic binders is its limited storage stability.6,7,9
In both no-bake methods, the curing mechanism is affected by several variables such as the type and amount of acid hardener, the sand temperature, the amount of moisture in the sand, the ambient temperature, the relative humidity, and the impurities of the sand.6 In the second half of the XX. century, numerous research works9,10,11,12,13 were conducted to investigate the effects of these factors on the properties of furan and phenolic no-bake sand cores. This research works mostly focused on the influence of the type of acid catalysts and the detrimental factors on the strength and gas evolution of sand cores. Since then, furan and phenolic no-bake binders have undergone significant improvements.14 In the XXI. century, foundries had to comply with increasingly strict environmental regulations. These regulations aim to reduce the amount of harmful, carcinogenic gases and odors that are generated from the sand molds and cores during the mold/core making and pouring processes.15,16 In 2010, one of the regulations classified furan resins containing more than 25% free furfuryl alcohol as toxic, while furan resins, which contain a lower level of free furfuryl alcohol, are regarded as harmful.17 Research work proved that furan resins containing lower than 20% of furfuryl-alcohol provide strongly decreased strength and hot distortion properties to the sand cores.17 On the other hand, experiments18,19 showed that the main source of emission gases during the application of sand cores produced with furan and phenolic no-bake processes is mostly derived from the acid catalysts. Because of that, binder manufacturers are forced to develop such binder systems that are characterized by low emissions during the mixing and pouring process while they are providing optimal performance to the sand mixtures.20
During mold and core making processes, to ensure good quality to the sand mixture with these newly developed, it is very important to determine the optimum binder-catalyst ratio.
In the case of furan and phenolic no-bake sands, for choosing the optimal type and amount of acid catalyst, strength tests are usually conducted to record the hardening characteristics of the investigated sand mixtures.3,21
On the other hand, strength tests carried out at ambient temperature cannot provide sufficient information about the behavior of sand cores and molds when they are interacting with high-temperature liquid metal during the pouring process. In the literature, relatively few publications22,23 can be found about the investigation of the properties of no-bake sands at elevated temperatures. Wan et al.24,25 developed a new high-accuracy instrument and an optimal sample geometry for measuring the high-temperature compressive strength of phenol-formaldehyde resin-coated sand, no-bake furan bonded sand, and TEA based resin-bonded sand. To the opinion of Wan et al.24,25, during the experiments, the most advantageous test temperature, holding time, and an optimal hollow cylinder sample geometry was determined that truthfully reflects the high-temperature performance of resin-bonded sand.
The hot-distortion test provides useful information about how sand cores and molds react to thermal loading during the pouring and cooling of the liquid alloy.26 Instruments were developed for measuring the hot-distortion parameters such as the Hot-Distortion Tester,26 DMA apparatus,27 and its modified instruments28 or the Thermal Distortion Tester.29 Several high-temperature deformation-related experiments30,31,32,33,34,35,36,37,38,39,40,41,42,43 were conducted on different organic and inorganic bonded sand cores. Dobosz et al.17 investigated the hot-distortion properties of no-bake sands. In the experiments, the effect of binder type and the furfuryl-alcohol content of the furan resin on the hot-distortion properties of no-bake resin bonded sands were investigated. These experiments were conducted with the DMA apparatus, one of the widely used instruments for measuring hot-distortion parameters. It was found that the nature of thermal deformation differences depends on the used binder. Decreasing the furfuryl-alcohol content in the no-bake furan resin leads to an increased tendency to thermal deformation, thus early cracking and fracture of the sand cores during thermal loading.17
However, the type and amount of acid catalyst used during the curing of the resin strongly influence the hot-distortion characteristics of no-bake resin bonded sands. Furthermore, the properties of the no-bake sands change over time because of the above-described curing mechanism. The effect of these parameters on the hot-distortion properties was not researched in publicized experiments.
To fill this gap of knowledge, this study aims to investigate the influence of the amount of acid catalyst and the holding time/storage time of samples on the strength and hot-distortion properties of no-bake furan and phenolic no-bake sand cores.
Materials and Experimental Procedure
Experimental Scheme
The present research work was focused on the investigation of the effects of the different amounts of acid hardener on the bending strength and hot distortion properties of no-bake furan and phenolic sand cores. In the first part of the experiments, characteristics of the base sand, such as the sand morphology, sand distribution, specific sand surface area, moisture content, and LOI (Loss on ignition) were investigated. The second part of the experiments included the preparation of sand core mixtures, the production of specimens, and the realization of bending- and hot-distortion tests.
During the research, commercially available silica sand was used as refractory base sand. To bond, the sand grains GIOCA NB86F type furan binder and COROFEN PREMIUM W/D type phenolic binder was applied. Härter 760 type acid hardener was used to ensure the cross-linking of the binders.
The various stages of the experiments are summarized in Figure 1, which will be described in detail in the following sections.
Preliminary Tests of the Base Sand
In terms of strength, gas permeability, and dimensional stability of sand cores at ambient and high temperatures, the grain structure of the base sand plays a significant role. The moisture and volatile organic impurity content of the base sand6,11,44,45,46,47,48 have an adverse effect on the properties of the sand cores. The moisture present on the sand grain surface retards the curing reaction, which results in lower flowability and reduced strength. According to the literature,6 moisture levels above 0.25% should be avoided. The LOI value of the base sand provides information about the amount of organic and gas-forming impurities.49 Contaminations can change the pH value of the foundry silica sand50 and, usually, their basic pH neutralizes the effect of the acid hardener,35 and this way reducing the degree of cross-linking. Impurities on the surface of the foundry silica sand grains, such as potash, alkali feldspars, soda, have a detrimental effect on the adhesive strength between the binder bridge and sand grains, as well as the cohesive strength of the binder bridges.46,47,51 Because of that, it is very important to analyze the characteristics of the refractory sand.
The preliminary tests consisted of microscopic examination, sieve analysis, specific surface area test, moisture analysis, and LOI test of the base sand. Figure 2 shows the measuring instruments used in the preliminary tests.
The used measuring devices for the determination of the sand characteristics: (a)—Zeiss Stemi 2000-c stereomicroscope, (b)—Fritsch Analysette 3 Pro sieve shaker, (c)—DISA POF-type specific surface testing apparatus, (d)—Kern DBS-60 moisture analyzer, (e)—muffle furnace with the three ceramic crucibles.
First, the sand grain morphology of the silica sand was investigated using a Zeiss Stemi 2000-C stereomicroscope (Figure 2a). Then, the grain size distribution of the sand was measured with Fritsch Analysette 3 Pro sieve shaker (Figure 2b). The measuring apparatus has 6 pieces of meshes with aperture sizes of 63-, 125-, 250-, 355-, 500-, and 710 μm. The sample weight was 50 g. The amplitude of the sieving was 2 mm, and the sieving time was 15 minutes. The sieve analysis was conducted following the AFS 1105-12-S standard.52 After the sieving procedure, the retained sand on each mesh was weighted.
From the results, the grain size distribution, the average grain size, the grain fineness number (GFN) were calculated according to AFS 1105-12-S and AFS 1106-12-S standards.52 For the evaluation, the average of two consecutive measurements was used. To measure the specific surface of the sand, a DISA POF type-specific surface testing equipment (Figure 2c) was used. The tests were executed following the AFS 1108-12-S standard.52 The measurement procedure is based on the Blaine method. During the test, 50 grams of clean, sludge-free sand is put to the burette of the device, and the volume of the sand is noted. Based on the time required for a fixed volume of air to pass through the sand sample, the actual specific surface [cm2/g] can be evaluated by using the graph given by the manufacturer of the equipment. The specific surface of the sand was derived from the average of three consecutive measurements. From the quotient of the measured and the theoretical specific surface, another parameter, the coefficient of angularity can be calculated. This coefficient describes the angularity of sand grains, which is interpreted as the deviation from the spherical shape. The theoretical specific surface and the coefficient of angularity were calculated according to AFS 1109-12-S.52
The determination of the moisture content of the silica sand sample was performed with the Kern DBS-60 moisture analyzer (Figure 2d), following the AFS 2216-19-S standard.52 The procedure of the conventional LOI test was carried out as described in the AFS 5100-12-S.52 The tests were performed in a muffle furnace using three ceramic crucibles (Figure 2e). The average of three consecutive measurements was used for the characterization. The results of the preliminary tests will be described in detail in Sect. 4.
To determine the alkalinity or acidity of the base sand and to characterize the amount of contaminants that are soluble in dilute acid, pH test and acid demand value (ADV) test were conducted. The former procedure was performed as described by the AFS 5113-18-S standard52 applying HI2211 pH/ORP Meter device. The ADV test was carried out according to AFS 1114-18-S. The average of three consecutive measurements of the tests is presented in Table 3.
Preparation of the Specimens for the Bending and Hot-Distortion Tests
In the second part of the experiments, test specimens for the bending tests and hot-distortion tests were prepared from no-bake furan and no-bake phenolic sand core mixtures containing different amounts of acid hardener. The various components were blended using equipment, see Figure 3, which is a laboratory blade-type mixer. The amount of each component was weighed on a Kern EG 4200 precision scale. The sand mixtures were prepared using 2 kg of silica sand. First, the acid hardener was added to the sand and mixed for 2 min at 80 rpm, followed by the binder, which was blended for an additional 2 min. In Table 1 the compositions of the sand core mixtures used in the experiments are summarized. The binder content was based on the sand weight, while the amount of acid hardener was weighed relative to the binder content.
Test specimens were produced from sand mixtures with hand ramming in core boxes. To determine the hardening characteristics of a given composition of sand mixture, two pieces of six-cavity core-boxes were used during the production of bending test bars. For the hot-distortion test pieces, eight cavity core-box was applied. After 20 min from the mixing process had ended, test specimens were removed from the boxes. In Table 2 the conditions of the preparation procedure are summarized. The stages of the sample production are illustrated in Figure 3.
As a result of the sample preparation, rectangular cross-sectioned test bars having dimensions of 22.4 × 22.4 × 180 mm and hot-distortion test pieces with dimensions of 114.3 × 25.4 × 6.35 mm were produced. The weight of each specimen was determined with Kern Precision balance EW-N with an accuracy of 0.01 g. The average weight of the rectangular cross-section test specimens was 153 ± 4 g, while the average weight of the hot-distortion test pieces was 26 ± 2 g.
Experimental Methods
To study the hardening characteristics of the furan -, and phenolic no-bake sand cores containing different amounts of acid hardener, 3-point bending tests were conducted with a MULTISERW LRu-2e universal strength testing machine (see in Figure 4.). The tests were performed on test specimens after storage times of 1h, 2h, 3h, 5h, 7h, and 24h. Storage times were selected based on the foundry practice, and they were calculated from the end of the mixing process. The average value of the bending strength (\({R}_{gI}^{b}\)) was calculated from the results of 2 measurements. The analysis of storage times with 1h, 2h, 3h, 5h, and 7h give information about the handling strength of sand cores while they are manipulated and transported immediately after their removal from the core box. The results of the bending tests after 24h give information about the maximum bending strength and the storage ability of the no-bake sand cores.
To represent the harmful effect of the acid hardener, Field Emission Scanning Electron Microscopic (FE-SEM) analysis was executed on the fracture surface of the bending specimens containing different amounts of acid catalyst. Backscattered electron (BSE) images of the overall shape and the fracture surface of the resin bridges were made with a Hitachi S-4800 scanning electron microscope.
Hot-distortion tests were executed to investigate the deformation behavior of no-bake sand cores when they are exposed to heat. The tests were performed on samples after storage times of 4h and 24h using a Simpson Gerosa® Hot-Distortion Tester. With these conditions, it became possible to analyze the effects of the storage time on the deformation behavior of chemically bonded no-bake sand cores. In each case, 4 samples were investigated. During the hot-distortion tests, one end of the rectangular cross-sectioned test piece is fixed, while on the other end of it, a displacement sensor is placed. The accuracy of the sensing mechanism is up to 0.01 mm. The sand sample was heated from the bottom using a gas burner controlled by a gas flowmeter. For the experiments, the used ratio of natural gas and air was 50:30, and the temperature of the heating media (gas flame) was around 900 °C. When the test specimen is exposed to heat loading, the sample distorts from the source of the heating, and the deformation of the end of the specimens can be measured over time. The recorded hot-distortion curve provides useful information about the deformation behavior, collapsibility, and the binder burn-out process of chemically bonded sand cores. Figure 5. illustrates the experimental settings on the Hot – Distortion Tester.
Results and Discussion
The Characteristics of the Refractory Base Sand
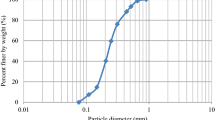
The first part of the evaluation of the conducted investigations is the characterization of the base sand. Figure 6 shows the stereomicroscopic image of the investigated silica sand grains at a magnification of 10x (Figure 6a) and 20x (Figure 6b). The results of the sieve analysis are shown in Figure 7. The bar graph shows the retained sand in the function of the different sizes of the meshes. Details about the properties of the base sand are presented in Table 3.
Comparing the stereomicroscopic images with the published chart from the literature,53 it can be established that the base sand has grains that are sub-angular with medium sphericity. As can be seen from Figure 7, the investigated sand grains mostly fall into three size regions: 0.15–0.25 mm, 0.25–0.355 mm, and 0.355–0.5 mm. The calculated average grain size and grain fineness number indicate that the base sand mostly contains coarse sand grains. According to the bar graph (see Figure 7.), the silica sand is concentrated in 3 sieves.
The sand is characterized by a, respectively, low specific surface with a value of 104 cm2/g, which can be attributed to the coarse grain size and the sub-rounded sand morphology. Based on the angularity factor, the sand can be classified into sub-angular group.
The moisture content of the base sand is neglectable, which means that it has not affected negatively the curing reaction. The conducted LOI test indicates that the base sand has a very low amount of gas-forming impurities (less than 0.4%).
The conducted pH tests showed that the applied silica sand is chemically neutral; thus, it has not influenced the curing process of the organic resins negatively.
From the results of the ADV tests, it can be stated that the used silica sand is pure and low in impurities, which can react with acid catalyst.
Summarizing the results of the preliminary tests, it can be established that the investigated silica sand shows relatively good properties, which makes it suitable as a refractory matrix for producing no-bake sand cores.
The Effect of the Acid Hardener on the Hardening Characteristics of the Furan and the Phenolic No-Bake Sand Cores
It is generally said that the quality of the acid hardener, its pH value, and the amount of acid hardener strongly influence the curing mechanism of the resin, thus the strength properties of the no-bake sand cores.1,2,4,6,8,11
Results of the bending tests are summarized in Figure 8a in the case of furan no-bake sand cores and Figure 8b in the case of phenolic no-bake sand cores. The strength values are represented as a function of the storage time.
Tables 4 and 5 summarizes the bending strength values, which are the arithmetic means of the two bending test results.
From Figure 8a, it can be observed that as a function of the storage time, the bending strength of the furan no-bake sand specimens is increasing continuously. However, the tendency of the strength growth does not follow a linear relationship. In the first hours after the production of the core mixtures, intensive strength growth takes place. After a storage time of 7 hours, the intensity of the strength growth decreases, but the bending strength is increasing continuously thereafter during the 24-hour storage time.
In contrast, the hardening characteristics of the phenolic no-bake sand core mixtures (see Figure 8b) follow a curve with a maximum point. From the results, it can be concluded that the storability of the no-bake furan bonded sand cores is better than that of the no-bake phenolic bonded sand cores. It can be generally said that in the case of 30–40% amount of acid hardener, sand specimens containing furan binder showed better bending strength characteristics than the no-bake phenolic bonded sand samples. The only exception is the bending strength of the phenolic bonded sand specimens containing 30% level at 2 hours of storage time. Cores produced from the investigated no-bake furan bonded sand mixtures are useable even up to 24h.
In different sources,1,3,5,6,7,8 greatly different optimal catalyst levels can be found for the no-bake furan and phenolic sand cores. Several factors affect the optimal amount of acid hardeners, such as ambient temperature, humidity level, quality of the binder, sand temperature, and moisture content of the sand.4,6,7,8,11
Further analyzing the data in Figure 8 it can be stated that the conducted bending tests revealed the problem of too little or too much acid hardener addition. Adequate bending strength could not be developed in phenolic bonded sand specimens containing 30% of catalyst. The test pieces deformed when they were removed from the box. Bending tests on furan bonded sand specimens containing the same amount of acid hardener show less than 40 N/cm2 of bending strength. This can be explained by the low degree of cross-linking. As can be seen in Figure 9, the slow cure rate results in weak furan bonded sand specimens showing plastic behavior, and as a result, distortion or even fracture may occur when they are removed from the box and during the handling stage.
Based on the bending strength values in Tables 4 and 5, it can be concluded that by increasing the catalyst level up to 60%, higher initial and final bending strength can be achieved for the furan bonded sand cores than in the case of the phenolic resin bonded sand samples. However, this tendency changed after the addition of more than 60% acid hardener. At 60% and 80% catalyst levels, results indicate higher bending strength of the phenolic bonded sand specimens compared to the furan bonded sand samples. Catalyst levels above 60% in the no-bake furan sand specimens resulted in decreased strength values. However, for the phenolic no-bake system, the initial strength is higher for 80% acid hardener as well.
In the case of the no-bake furan sand specimens, an over-curing phenomenon may occur, in which case the excessive amount of acid hardener (level of 60–80%) destructs the formed resin bridges between the sand grains.
During the FE-SEM analysis, BSE images of the overall shape and the fracture surface of the resin bridges were made that are summarized in Figure 10 in the case of the phenolic no-bake sand cores, and Figure 11 in the case of the furan no-bake sand cores.
At a magnification of 200x, funnel-shaped binder bridges can be observed in the phenolic bonded no-bake sand cores containing 30% of acid hardener. These resin bridges are characterized by cohesive fracture. At a magnification of 700x, it can be seen that the cross-section of the investigated binder bridge was a solid, dense cross-linked polymer structure. Its fracture surface is characterized in part by a slight striation.
In the case of the phenolic sand cores containing 40% of acid hardener, adhesive and cohesive fracture surfaces of the resin bridges can be observed at a magnification of 200x. On the fracture surface of the binder bridges, two distinct layers with different structures can be found. The outer part of the resin bridge contains a solid, dense polymer structure, while thin plate-shaped solid cross-linked resin material weaves the inner part of the binder bridge (magnification of 700x).
Further increasing the acid hardener content up to 60%, the shape of the binder bridges changes significantly. The bridges broke and cracked at several places. At higher magnification (700x), a dense, plate-like structure with large pores can be observed in the inner layer of the binder bridges. The edges of the resin bridges are sharper, cracked, and in some parts, characteristics of a brittle fracture can also be found.
In the case of sand cores containing 80% of acid hardener, significant degradation of the bridges can be observed. The binder shattered in several places, fragmented on the surface of the sand grains. At higher magnification, cracks and brittle fracture surfaces can also be observed inside the binder bridges.
SEM analysis of the no-bake furan sand cores (see Figure 11.) showed significantly different binder bridge morphology than in the case of the no-bake phenolic sand cores.
In the case of the 30% acid hardener containing sand cores, relatively few binder bridges can be observed on the surface of the sand grains. At a magnification of 700x, it can be seen that the edges of the binder bridges are sharp, and the characteristics of the brittle fracture can also be observed, which can be derived from the nature of the furan resin. In the inner part of the binder bridges, a fine solid structure can be found.
Increasing the acid hardener level up to 40%, thicker binder bridges were developed on the surface of the sand grains. In the BSE image, it can be seen that the binder bridges are porous. Furthermore, cracks and fractures can be observed in the inner part of the resin bridges. At higher magnification, it can be seen that the edges of the broken bonding bridges are sharp, corrugated, and fragmented, with cavities inside. Increasing the acid hardener content up to 60% drastically changes the morphology of the resin binder bridges. Thick bonding bridges are located in the volume portion between the sand grains. Throughout the cross-section of the bonding, the bridges are porous. Fragmented and detached binder particles can also be observed on several bonding bridges. The solid internal resin structure is made up of a fine lamellar structure with hollow spaces between them. In the case of the furan no-bake sand cores containing 80% acid hardener, remains of the fragmented and broken bonding bridges can be observed on the surface of the sand grains. At higher magnification, it seems like the binder bridges are built up from fine porous pallets. The center of the bonding bridge shown in the image is detached from the surface of the sand grain.
From the results of the conducted SEM analysis, it can be concluded that the acid hardener content influences the structure of the emerging bonding bridges by the curing rate of the resin. A level of 60 and 80% acid hardener significantly modifies the structure and morphology of the crosslinked resin binder bridges. According to the researches of Frank Iden et al.,54 the amount of solvent present in the resin and the acid hardener also significantly affect the structure of the formed bonding bridges. It can be assumed that in the present experiment, the amount of solvent added by increasing the amount of acid hardener also influences the porous structure of the crosslinked bonding bridges.
In the no-bake methods, the catalyst is evenly distributed in the formed bonding bridges, so the crosslinking starts simultaneously. The solvent remains enclosed in the evenly distributed cross-linked binder. This can also result in porous bonding bridges for both binder systems at 60–80% catalyst content. However, the detrimental effect of this phenomenon on the bending strength can be observed only in the case of the furan bonded sand samples. For a better understanding of the role of the acid hardener in the curing process, it is very important to further investigate the type and amount of acid hardener on the morphology of the cross-linked bonding bridges of the no-bake sand systems.
Hot-Distortion Properties of No-Bake Furan and Phenolic Bonded Sand Cores
In the next part of the research work, hot-distortion tests were conducted to study the thermal behavior of the furan and phenolic no-bake sand cores. During the evaluation process, the recorded hot-distortion curves were analyzed, and hot-distortion parameters were determined, such as the max. deformation and degradation time. The former means the maximum of the positive deformation of the test piece, while the latter implies the length of time from the initial specimen heating to its breakdown.26
Figure 12 reveals the thermal behavior of the furan no-bake sand specimens that have been stored for 4h (Figure 12a) and 24h (Figure 12b). The hot-distortion curve of the phenolic no-bake sand specimens is shown similarly in Figures 13a and b. The presented results in the figures reflect the result closest to the mean deformation and the mean degradation time of five consecutive measurements.
As can be seen from the results, the quality of the used resin strongly influences the thermal behavior of the chemically bonded sand cores. Significant differences can be observed between the thermal deformation resistance of no-bake sand cores produced with furan and phenolic resin.
Analyzing the hot-distortion curves in Figure 12a, it can be established that the results of the hot-distortion tests look similar to the ones presented by Dobosz et al.17,35,40 and Ignaszak.33 During the tests, when the furan no-bake test pieces were exposed to heat, the samples started to distort from the source of heating. This phenomenon is derived from the thermal expansion of the sand grains. Further thermal loading of the specimens resulted in steadily increased deformation. When the maximum deformation was reached, the degradation of the furan resin bridges between the sand grains took place. The test pieces broke down by brittle fracture. This kind of mechanical failure can be clearly seen from the steep slope of the hot-distortion curves. The brittle fracture of the test pieces can be explained by the nature of the cross-linked furan resin. According to the literature,8 this brittleness of the cured furan resin makes it easier to remove it from the sand grains during the mechanical reclamation process.
The hot-distortion tests of the phenolic no-bake sand cores (see Figure 13a) resulted in different outcomes. Observations made during the hot-distortion measurements have allowed us to state that the positive deformation of test pieces can be divided into two stages. At the beginning of the heat loading, first, an intensive deformation can be observed, which is followed by a slower increment of deformation prolonged in time. Test pieces bend upward. Under further thermal exposure, due to the contraction of the cured resin bridges, specimens deform downward. This stage is characterized by thermoplastic thermal behavior. When the phenolic bonded hot-distortion specimens can not withstand further thermal exposure, the fracture will occur.
Because of the thermoplastic property of the phenolic resin, the removal of the resin film from the surface of the sand grains could become difficult by mechanical attrition during the reclamation process. Furthermore, the thermoplastic behavior of the phenolic resin may also affect the collapsibility of the sand core.
Since the degradation time evaluated from the hot-distortion curve represents the hot strength of the chemically bonded molding material,25 it can be concluded that the phenolic no-bake sand cores show higher hot strength than the sand cores made with furan resin. Because of that, the phenolic binder is an excellent choice for no-bake sand molds and cores for producing steel castings. The higher thermal stability of the phenolic resin can be explained by the research work of Svidró et al.,55 who investigated the furan and the phenolic binder degradation characteristics of the 3D printed sand cores using inverse Fourier thermal analysis. According to their study, the decomposition behavior of the phenolic binder is characterized by a more complex, multi-staged process compared to the furan resin binder.
Further analyzing the results, it can be established that the acid hardener strongly influences the hot-distortion properties of the furan and phenolic no-bake sand cores through the curing of the resin.
The investigation revealed that in the case of the furan no-bake resin, sand cores containing a 30% amount of acid hardener with a storage time of 4 h have very low thermal deformation resistance. The test results in Figure 12a show that in the specimens, this low acid hardener level did not ensure such a degree of cross-linking of the resin to withstand the thermal exposure. At the beginning of the hot-distortion test, the furan no-bake sand samples deformed immediately. However, the complete degradation of the test pieces did not occur. A small positive deformation can be observed, which may be caused by the thermal expansion of the sand grains and the thermal-induced post-curing of the resins. On the other hand, in the case of the phenolic no-bake samples, an intensive deformation growth results in 1.54 mm max. deformation.
An interesting correlation can be observed between the catalyst level and the max. deformation of the test pieces. Figure 14a presents the max. deformation as a function of the acid hardener level in the case of the furan no-bake hot-distortion test pieces with storage times of 4 and 24h, whereas Figure 14b shows the results of the phenolic no-bake test pieces with storage times of 4 and 24h. The correlation between these two parameters can be described with a maximum curve. In the case of furan no-bake sand cores, the highest deformation value (2.53 mm) was measured on test pieces with 40% of acid hardener content, while the recorded highest deformation value of the phenolic no-bake sand cores is 2.05 mm at a catalyst level of 40%. Based on the variation of the maximum deformation values, the furan resin is more susceptible to the effects of the acid hardener content on the hot-distortion properties.
It can be established that the amount of acid hardener strongly influences the thermal deformation resistance of the furan and phenolic no-bake sand cores.
The authors explain the correlation between the acid hardener and the max. deformation with the following hypothesis.
It can be seen from the hot-distortion tests of the furan no-bake sand cores (see in Figure 12a), conducted after 4h storage time, that 30% of acid hardener level is insufficient to withstand the thermal loading, test pieces deformed immediately. Since this kind of composition of sand cores shows plastic behavior (see Figure 12a) and low bending strength (see Figure 8a), the sand grains during the thermal expansion are able to compress the binder bridges. As a result, the sand grains can expand in volume easily in the inner part of the sand specimens. This is the reason for the low thermal deformation properties. Increasing the acid hardener level up to 40% results in advanced cross-linking of the resin. Due to that, the bending strength of the sand cores is higher, and the sand grains adjacent to each other are not able to compress the stronger (more rigid) binder bridges. As a result, higher thermal deformation can be experienced. This tendency prevails up to the catalyst content of 80%. As can be seen from the scanning electron microscopic analysis, this amount of acid hardener destructs the developed binder bridges. The excess catalyst level decreases the thermal stability of the resin as well. Because of that, there is not enough time for the sand grains for a similar degree of thermal expansion as in the other cases, which explains the lower thermal deformation of furan no-bake test pieces.
Based on the results, the thermal stability of the phenolic resin is higher than that of the furan resin, the sand grains have enough time for the thermal expansion when the sand cores are exposed to heat loading. As a result, except for the furan bonded sand specimens with a storage time of 24h, a higher positive deformation could be experienced in the case of the phenolic bonded sand specimens with an acid hardener level of 30 and 40% (see Figure 13b).
A similar phenomenon, as discussed above, can be observed in the case of phenolic bonded test pieces. It can be said that up to a given acid hardener level (40%), increasing the amount of catalyst in the sand cores leads to higher positive deformation. Above 40% amount of acid hardener, the thermoplastic behavior of the resin changes, which results in more rigid, fragile binder bridges. This can be seen from the degradation stage of the hot-distortion curves characterized by a less steep slope.
Taking into consideration the economic aspects and the fulfillment of the required dimensional tolerances, understanding the above-discussed results are very important when the amount of acid hardener has to be set during the production of the sand cores.
The acid hardener influences the degradation/thermal stability of the no-bake furan and phenolic bonded sand cores. Figures 15a and b present the degradation time of the hot-distortion test pieces with storage times of 4 and 24h. Based on the results, it can be said that except for the sand specimens containing 30% acid hardener with a storage time of 4h, the higher the amount of acid hardener, the lower the thermal strength of the sand cores is. This can be explained by the over-curing of the resin. This phenomenon may cause casting defects such as breakdown failure when interacting with high-temperature liquid metal. On the other hand, too high thermal strength may lead to poor collapsibility during core removal.
Since the cross-linking of the no-bake binders is a time-dependent process, the determination of the optimal storage time is critical.
In this research work, the effect of the storage time on the hot-distortion properties of the no-bake sand cores was also investigated. Hot-distortion tests were conducted on furan and phenolic no-bake test pieces with storage times of 4h and 24h [see Figures 12(b) and 13(b)]. A significant change in the hot-distortion properties can be observed. Test pieces stored for 24h showed higher max. deformations and longer degradation times in both investigated binder systems. This means that longer storage times result in lower thermal deformation resistance and better thermal strength against the thermal stresses induced by the high-temperature liquid metal during casting. While the former statement is very important in terms of the fulfillment of the dimensional tolerances, the latter influences the shake-out properties of the sand cores.
Conclusion
The present research work was carried out to investigate the effects of the acid hardener content on the bending strength and hot-distortion properties of furan and phenolic no-bake sand cores. It can be concluded that the acid hardener content strongly influences the strength and thermal behavior of the no-bake sand cores through the curing reaction of the resin. By inadequate setting of the amount of catalyst, it is possible to produce sand cores with good mechanical properties, but when it is exposed to thermal load during the interaction with the high-temperature liquid alloy, high deformation and/or short breakdown time might occur. This may cause casting defects during casting production.
Based on the results, the following conclusions can be drawn:
-
Results of the bending tests show that in the case of the furan binder system, the acid hardener is best utilized in terms of higher bending strength, in an amount of 40–60%, while in the case of the phenolic binder system, the amount of 60–80% acid hardener resulted in higher bending strength of the sand specimens.
-
Too low amounts of acid hardener (30%) result in low bending strength of the specimens. At this acid hardener level, phenolic no-bake test pieces with a storage time of 1h could not be removed from the core boxes without deformation. This can be explained by the low curing rate of the resin. In the case of the furan no-bake binder system, sand specimens can be easily removed from the core boxes. They are characterized by low bending strength.
-
In the case of the furan binder system, too high amounts of acid hardener (80%) are harmful because the excess catalyst destructs the binder bridges between the sand grains, which decreases the bending strength of the samples. However, phenolic bonded sand samples containing 60% and 80% of acid hardener show increasing bending strength in the function of the storage time.
-
The acid hardener strongly influences the thermal behavior of the furan and the phenolic no-bake sand cores. A correlation can be found between the acid hardener content and the max. deformation of samples, which is described with a maximum curve.
-
The furan resin binder is more susceptible to the effects of acid hardener content changing on the thermal behavior than the phenolic resin.
-
In the case of the furan no-bake sand samples, 30% amount of acid hardener was insufficient to withstand the thermal exposure during the hot-distortion test. The samples deformed immediately after the early stage of the thermal loading.
-
Increasing the acid hardener content above 40% results in decreased thermal stability/degradation time of the furan resin bonded sand cores.
-
In the case of the phenolic no-bake sand cores, the highest deformation resistance and degradation time can be achieved by the addition of 30% of acid hardener.
-
For the two investigated binder systems, furan resin provides higher bending strength and better storage ability than the phenolic resin.
-
According to the results of the hot-distortion tests, the furan no-bake sand specimens are characterized by a steadily increasing deformation growth, while in the case of the phenolic bonded sand specimens, an intensive deformation growth can be observed. The latter may negatively influence the dimensional accuracy when the sand cores are exposed to heat during the pouring process.
-
At the end of the hot-distortion tests, the furan no-bake sand samples had been broken with brittle fracture. The phenolic no-bake sand specimens deformed plastically under the thermal loading.
-
Since the curing of the no-bake resin systems is a time-dependent process, the storage time influences not only the strength properties but the thermal behavior of the sand cores as well.
The conducted research work may raise additional questions:
-
Is there any correlation between the positive deformation of the no-bake hot-distortion specimens and the deformation of the real sand cores?
-
The short degradation time of the hot-distortion samples indicates low thermal strength. Can this parameter predict the collapsibility of the sand cores?
-
The acid hardener influences the thermoplastic behavior of the phenolic resin. How does this affect the removal of residual resin from the surface of the sand grains during the mechanical reclamation process? It would be interesting to know that there is any kind of relationship between the degradation time of the furan and phenolic no-bake sand cores derived from the hot-distortion tests and the LOI test conducted after the mechanical reclamation process.
References
M. Holtzer, A. Kmita, Mold and core sands in metalcasting: chemistry, Chapter 6 cold-setting processes (No-Bake), pp. 145-162 (Springer Nature Switzerland AG 2020) https://doi.org/10.1007/978-3-030-53210-9
J.J. Archibald, Benchmarking the NoBake binder systems. AFS Trans. 101, 967–978 (1993)
J.R. Brown, Foseco Ferrous Foundryman’s Handbook, Chapter 13. Resin bonded sand, pp. 167-180, (Butterworth Heinemann, Linacre House, Jordan Hill, Oxford, 2000), ISBN 0 7506 4284 X
Prof. Dr.-Ing. habil. Eckart Flemming, Doz. Dr.-Ing. habil. Werner Tilch, Formstoffe und Formverfahren, Kapitel 6. Verfahren mit kalthärtenden Formstoffen, pp. 288-298 (Deutscher Verlag für Grundstoffindustrie, Leipzig Stuttgart 1993)
P. Beeley, Foundry technology, Chapter 4. The moulding material: properties, preparation and testing, pp. 208-210, Second Edition (Butterworth-Heinemann, Oxford, 2001)
S. Viswanathan et al., In No-Bake Sand Molding, ASM Handbook Volume 15: Casting pp. 567-580, (ASM International, Materials Park, Ohio, 2008), https://doi.org/10.1361/asmhba0005354
M. Holtzer et al., Microstructure and properties of ductile iron and compacted graphite iron castings, Chapter 2 Molds and Cores Systems in Foundry pp. 31-37 (SpringerBriefs in Materials, 2015), https://doi.org/10.1007/978-3-319-14583-9_2
L. Pilato, Phenolic resins: a century of progress, Chapter 18 Foundry pp. 470-474 https://doi.org/10.1007/978-3-642-04714-5
Y.D. Kim, Phenolic nobake binders for core and mold production. AFS Trans. 84, 287–294 (1976)
R.I. Naro, R.D. Tenaglia, Influence of Nobake binder processing variables on metal penetration gray iron castings. AFS Trans. 86, 343–356 (1979)
B.R. Nelson, An evaluation of toluenesulfonic acid (TSA) and phospohoric acid as catalysts for Furan no-bake foundry binders. AFS Trans. 81, 153–157 (1973)
J. Robins, L.I. Toriello, R.J. Schafer, The effects of sand and foundry parameters on the preformance of NoBake binders. AFS Trans. 99, 623–630 (1991)
N.Y. Huang, G.X. Su, Investigation of bonding efficiency of chemically bonded sand. AFS Trans. 96, 109–114 (1988)
E. Ireland, K. Chang, J. Kroker, New horizon in nobake binder technology. AFS Trans. 110, 623–630 (2002)
Directive (EU) 2017/164 - indicative occupational exposure limit values
Directive (EU) 2017/2398 on the protection of workers from the risks related to exposure to carcinogens or mutagens at work
St. M. Dobosz et al., The influence of the content of furfuryl alcohol monomer on the process of moulding sand’s thermal destruction, Archives of Metallurgy and Materials, Volume 59, Issue 3, pp. 1093-1096, https://doi.org/10.2478/amm-2014-0188 (2014)
H. Zhang et al., Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as the binder catalyst. J Therm Anal Calorim (2014). https://doi.org/10.1007/s10973-013-3553-x
M. Holtzer et al., Influence of the Hardener on the Emission of Harmful Substances from Moulding Sands with Furan Resin in the Pyrolysis Process, Archives of Foundry, Volume 16. Issue 1, (2016), https://doi.org/10.1515/afe-2016-0012
C. Fourberg, Dr. I. Bacanu, Furan-phenol hybrid resin a choice for high-quality castings, 2nd South African metal casting conference, WFO technical forum 2017
S.G. Acharya, J.A. Vadher, M. Sheladiya, A furan no-bake binder system analysis for improved casting quality. Inter Metalcast 10, 491–499 (2016). https://doi.org/10.1007/s40962-016-0059-x
H. Bargaoui, F. Azzouz, D. Thibault, G. Cailletaud, Thermomechanical behavior of resin bonded foundry sand cores during casting. J. Mater. Process. Technol. (2017). https://doi.org/10.1016/j.jmatprotec.2017.03.002
E.A. Chernyshova et al., Thermomechanical properties of molds based on different binders and casting quality. Steel Transl. 46(8), 544–547 (2016). https://doi.org/10.3103/S0967091216080064
P. Wan, L. Li et al., Development of a new instrument for measurement of high temperature mechanical properties of resin-bonded sand. China Foundry (2017). https://doi.org/10.1007/s41230-017-6023-4
P. Wan, L. Li et al., Research on testing method of resin sand high temperature compressive strength. China Foundry (2016). https://doi.org/10.1007/s41230-016-6013-y
S. McIntyre, S. M. Strobl, Adapting hot distortion curves to process control, Foundry Management and Technology (1998)
J. Jakubski, S.M. Dobosz, Analysis of thermal deformation of masses using a DMA apparatus. Arch. Foundry 3(9), 246–251 (2003)
Z. Ignaszak, Discussion on the methodology and apparatus for hot distortion studies. Arch. Foundry Eng. 18(2), 141–145 (2018)
A.J. Oman et al., Next generation thermal distortion tester, AFS Transactions, pp. 323-329 (American Foundry Society, 2013)
G.C. Fountaine, K.B. Horton, Hot distortion of cold-box systems. AFS Trans. 98, 9–16 (1990)
S.N. Ramrattan, S. Vellanki, O. Jideaku, C. Huang, Thermal distortion in process control of chemically bonded sands. AFS Trans. 152, 161–165 (1997)
R. Iyer, S.N. Ramrattan, J. Lannutti, W. Li, Thermo-mechanical properties of chemically-bonded sands. AFS Trans. 109, 965–973 (2001)
Z. Ignaszak, Chosen aspects of thermo-mechanical phenomena in resin bonded sands by use of hot distortion tests. Arch. Foundry Eng. 8, 137–142 (2008)
J. Jakubski, The thermal deformation of core and moulding sands according to the hot distortion parameter investigations. Arch. Metall. Mater. 52(3), 421–427 (2007)
S.M. Dobosz et al., Influence of quartz sand quality on bending strength and thermal deformation of moulding sands with synthetic binders. Arch. Foundry Eng. 15(2), 9–12 (2015). https://doi.org/10.1515/afe-2015-0028
S.M. Dobosz et al., New materials in the production of moulding and core sands. Arch. Foundry Eng. 15(4), 25–28 (2015). https://doi.org/10.1515/afe-2015-0073
K. Major-Gabryś, S.M. Dobosz, D. Drożyński, J. Jakubski, The compositions: biodegradable material - typical resin, as moulding sands’ binders. Arch. Foundry Eng. 15(1), 35–40 (2015)
L. Mikóné Mádi, L. Varga, T. Mikó, Examination of the properties of resin bonded core mixtures, Mater. Sci. Forum Volume 885, pp. 171-177., (Trans Tech Publications, Switzerland, 2017), https://doi.org/10.4028/www.scientific.net/MSF.885.171
Z. Ignaszaka, J.-B. Prunier, Synergy of practical knowledge of molding sands reclamation in heavy casting foundry of iron alloys. Arch. Foundry Eng. 13(3), 30–36 (2013). https://doi.org/10.2478/afe-2013-0054
S.M. Dobosz, A. Grabarczyk, K. Major-Gabryś, Elasticity of moulding sands–a method of reducing core cracking. Arch. Foundry Eng. 17(1), 31–36 (2017). https://doi.org/10.1515/afe-2017-0006
E.C. Silva, I. Masiero, W.L. Guesser, Comparing sands from different reclamation process for use in the core room of cylinder heads and cylinder blocks production. Int. J. Metalcast. 14, 706–716 (2020). https://doi.org/10.1007/s40962-019-00400-6
S. Ramrattan, L. Wells, P. Patel, J. Shoemaker, Qualification of chemically bonded sand systems using a casting trial for quantifying interfacial defects. Int. J. Metalcasting 12(2), 214–223 (2017). https://doi.org/10.1007/s40962-017-0166-3
R. Tuttle, S. Ramrattan, L. Wells, As-cast surface characterization for steel using disk-shaped chemically bonded sand specimens. Inter Metalcast 15, 382–390 (2021). https://doi.org/10.1007/s40962-020-00520-4
G.S. Lukacek et al., Humidity–it’s effect on no-bake binders. AFS Trans. 91, 455–463 (1983)
R.H. Toeniskoetter, Effects of sand surface characteristics on adhesion of resinous binders. AFS Trans. 81, 280–288 (1973)
Molding Methods and Materials, Des Plaines (American Foundrymen’s Society, Illinois, 1962)
V.L. Weddington, C.E. Mobley, Influence of sand surface chemistry on bonding. AFS Trans. 99, 825–870 (1991)
J. Zych, Behavior of chemically bonded molding sands in dry air. Inter Metalcast 3, 17–27 (2009). https://doi.org/10.1007/BF03355445
S.N. Ramrattan, P.G. Ikonomov, Five minute LOI test. AFS Trans. 114, 429–434 (2006)
G. Gyarmati, I. Budavári, G. Fegyverneki, L. Varga, The effect of sand quality on the bending strength and thermal distortion of chemically bonded sand cores. Heliyon 7(7), e07624 (2021). https://doi.org/10.1016/j.heliyon.2021.e07624
S.P. Thomas, Laura Moreno, Mold & Core Test Handbook, 5th edn., (American Foundry Society, Schaumburg, IL, USA, 2019), ISBN 978-0-87433-467-8
J.R. Brown, Foseco Ferrous Foundryman’s Handbook, Chapter 12. Sand and green sand, pp. 147, (Butterworth Heinemann, Linacre House, Jordan Hill, Oxford, 2000), ISBN 0 7506 4284 X
Dipl.-Ing./Dipl.-Wirtsch.-Ing. Frank Iden et al. Strukturen von Cold-Box-Bindersystemen und die Möglichkeit ihrer Veränderung, Giesserei-Rundschau 58, Heft 1/2, pp. 3-8 (2011)
J. Tóth, J.T. Svidró, A. Diószegi et al., Heat absorption capacity and binder degradation characteristics of 3D printed cores investigated by inverse fourier thermal analysis. Inter Metalcast 10, 306–314 (2016). https://doi.org/10.1007/s40962-016-0043-5
Acknowledgment
The authors thank Csaba Mekler and Dr. Sycheva Anna, who is working in Innovation Laboratory Ltd., for their assistance in the making of SEM images and EDS analysis.
Funding
Open access funding provided by University of Miskolc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Budavári, I., Gyarmati, G. & Varga, L. The Influence of Acid Hardener on the Strength and Hot-Distortion Properties of No-Bake Sand Cores. Inter Metalcast 16, 1415–1431 (2022). https://doi.org/10.1007/s40962-021-00700-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-021-00700-w