Abstract
The main objective of this paper was to assess three leaded brass samples (pending application with Copper Development Association) using optical microscopy and mass spectrometry to compare the distribution of lead. Based on the mass spectrometry data, a great deal of variation was not found within each of the samples based on five different sample locations. Optical microscopy, scanning electron microscopy and energy-dispersive X-ray spectroscopy confirmed that the lead was homogenously distributed in brass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brass is extensively used in numerous market applications such as screws, valves, bearings, fittings and specialty fasteners due to its beneficial corrosion resistance, thermal and electrical conductivity, formability and good mechanical properties.1 Some alloying elements enhance the special characteristics of brass. Lead is one of the most important elements, which can be added to any brass to increase machinability with respect to low melting point of lead and very low solubility of lead in brass. However, other elements such as bismuth (Bi), tin (Sn) and arsenic (As) are used to improve some characterisation of brass.2 , 3 Leaded brass rods can be produced by continuous extrusion forming technology and continuous casting. The main disadvantage of producing a brass alloy rod by continuous extrusion forming technology is the quality of the brass alloy rod.
The use of continuous casting gives a range of advantages in comparison with continuous extrusion forming such as low energy consumption, high productivity, length size of final product and cost.4 , 5 Leaded brass bars with the same composition but with different combinations have been characterised in this work. Chemical composition and microstructure have been studied in order to clarify the distribution of lead (Pb).
Brass Phase Diagram
Figure 1 shows the phase diagram of brass. CuZn alloy system contains intermediate phases. Brass alloys having various Zn content are categorised into different types of brass for example:
-
1.
Alpha brasses [Zinc (%) <35], which contain only one phase, with face-centred cubic (FCC) crystal structure.
-
2.
Alpha–beta brasses [Zinc (%) 35–45] which contains both α and β′ phase; β′ phase is body-centred cubic (BCC) and α phase is FCC.
-
3.
Beta brasses [Zinc (%) 45–50], which contain only one phase, with BCC crystal structure.
Leaded brass is an alpha–beta brass with an addition of lead with excellent machinability.
Experimental Procedure
Leaded Brass Samples
The representative leaded brass samples analysed in this work and their corresponding combination and information is listed in Tables 1, 2 and 3. Leaded brass samples with 0.1–0.2 % Pb contents according to Table 4 were used in this investigation. Nearest Alloy Designation is C28XXX (pending application with Copper Development Association—CDA). Distribution of Pb was investigated by metallography and mass spectrometry analysis.
In this work the charge was first weighed by the operator and then was melted in a graphite crucible using graphite heating element furnace technology (Rautomead horizontal continuous casting machine) as shown in Figures 2a, b and 3.
Metallography
Samples for metallographic examination were prepared by conventional techniques. Metallographic sections were cut with a clean sharp hacksaw and then ground using alumina grinding paper, first using coarse abrasive paper (grit no. 220) and subsequently wet and dry fine paper (grit no. 1200) by 5–10 lbs force and water as a lubricant. Base/head speed of grinding was 100/100 rpm. The samples were then polished using diamond paste beginning with 1 micron and then subsequently using ¼ micron. Base/head speed of polishing was 100/100 rpm and the force was 5–10 lbs. After polishing, the samples were cleaned by acetone in an ultrasonic cleaner and dried with nitrogen gas. According to the ASTM E407-07 (Standard Practice for Micro-Etching Metals and Alloys), the polished samples were etched in a solution of 70 % concentrated nitric acid (HNO3) and water. Samples structure was investigated using a Keyence VHX 3D digital microscope.
Mass Spectrometry Analysis
Mass spectrometry (MS), wet chemistry including gravimetric and titrimetric techniques, spark optical emission spectroscopy (Spark-OES), inductively coupled plasma-optical emission spectroscopy (ICP-OES), X-ray fluorescence (XRF) and X-ray diffraction (XRD) are common analytical chemistry techniques to identify the amount and type of chemicals present in a sample. The mass spectrometer has a few advantages over the other analytical methods such as small sample size, accuracy, fast analysis and less demanding safety issues as compared to the X-ray techniques.6 – 9 In this research mass spectrometry was used (model: AMETEK) as an analytical technique to identify the amount of chemicals present in samples. The samples were prepared using a milling machine. Figure 4 shows photographic images of samples before, during and after mass spectrometry analysis.
SEM/EDX
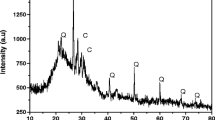
A scanning electron microscope was employed to produce high resolution images. Energy-dispersive X-ray spectroscopy analysis is a well-known X-ray technique used to identify the elemental composition of materials. In this report a JEOL JSM7400F field emission scanning electron microscope was used to identify the ‘dark spots’ assumed to be Pb particles, which are the feature of interest.
Results and Discussion
Results and Discussion from Optical Microscope
The grain structure of the leaded brasses is similar to the unleaded brasses.10 The microstructures of the leaded brasses contain lead particles mainly in the grain boundaries or inter-dendritic regions. Lead is practically insoluble in solid copper and appears as a dark particle in the structure.11 In order to identify lead, the samples were examined under a digital optical microscope—magnification 1000×. Figure 5 shows the typical equiaxial grain morphology structure of brass samples and highly insoluble Pb as a dark particle. The Pb content was too low to draw any important conclusion about the homogeneity of Pb in the samples. However, it loosely appeared to be fairly well distributed.
Results and Discussion from Mass Spectrometry Analysis
The work was continued using the AMETEK spectrometer to examine different areas on each specimen. Areas 1 through 4 are around the circumference of the sample, and area 5 is in the middle. Results show that there is no significant difference in the distribution of any one of the elements based on the mass spectrometry result. Table 5 and Figure 6 present the findings.
Analysis of variance (ANOVA) was carried out on the samples to observe any difference between the groups on some variable using ANOVA. ANOVA is a statistical procedure to analyse the difference between two or more means and is used to analyse general alternately specific difference between means.12 As we see in the above chart, we have three groups of samples, labelled 1, 2, 3. There is a target range for samples: the aim is that the sample should be located within this range, and then the statistical method of ANOVA is applied over these samples. From this analysis, the results fall perfectly in the target range, i.e. the ANOVA analysis of variance failed to reject the null hypothesis with a confidence level of α = 0.05. This means that there is no proven significant difference in any of the samples tested on the spectrometer based on their respective areas of testing.
Results and Discussion from SEM Image Observation and EDX Analysis
Figures 7 and 8 show the structure of brass samples. The peaks corresponding to the elements, which were generated by the EDX analysis, show the overall texture of the sample to be Cu and Zn. In addition, the bright particles have been determined using selected point area EDX analysis and Pb was identified. The SEM/EDX analysis on the samples demonstrated that the distribution of elements inside each individual sample is homogeneous, which confirm the result from the optical microscope and mass spectrometry analysis.
Conclusions and Future Work
The obtained results regarding the investigation of the distribution of lead in three different combinations of brass feedstock by mass spectrometer, digital optical microscope and SEM/EDX can be summarised in the following points:
-
1.
Following on from the mass spectrometry results, the distribution of elements inside each individual sample is homogeneous.
-
2.
Homogenised distribution of lead in three different combinations of brass feedstock by mass spectrometer has been confirmed by ANOVA analysis.
-
3.
Regarding to the optical microscopy and SEM and EDX analysis done on the samples, the distribution of lead inside each individual sample is homogeneous.
-
4.
Based on mass spectrometry we could not find a great deal of variation within each samples based on sampling five different locations.
-
5.
As a future work, this work can link the observation of the surface area oxide from granules versus larger chunks.
-
6.
As for future work, this research can be extended to investigate lead using X-ray fluorescence (XRF) machine, which is categorised as non-destructive analysis.
References
Y.F. Sun, N. Xu, H. Fujii, The microstructure and mechanical properties of friction stir welded Cu–30Zn brass alloys. J. Mater. Sci. Eng. 589, 228–234 (2014)
Ch. Nobel, F. Klocke, Machinability enhancement of lead-free brass alloys, in 6th CIRP International Conference on High Performance Cutting, vol. 14 (2014), pp. 95–100
A. Momeni, Effect of chemical composition and processing variables on the hot flow behavior of leaded brass alloys. J. Mater. Sci. Eng. 626, 1–8 (2015)
B. Li, Flow characteristics of brass rod during continuous extrusion, in 11th International Conference on Technology of Plasticity, ICTP 2014, 19–24 October 2014, Nagoya Congress Center, Nagoya, Japan, Procedia Engineering, vol. 81 (2014), pp. 647–651
I.R.P.F. Cuypers, Continuous Casting in the Copper Industry (Eindhoven University of Technology Netherlands, Department of Industrial Engineering and Management Sciences, 1987)
E. de Hoffmann, Mass Spectrometry Principles and Applications, 3rd edn. (Université Catholique de Louvain, Belgium & Ludwig Institute for Cancer Research, Brussels, Belgium, 2007)
R. García, A.P. Báez, Atomic Absorption Spectrometry (AAS) (Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, Ciudad Universitaria, Mexico City, 2012)
Beckhoff, Handbook of Practical X-ray Fluorescence Analysis (Springer, 2006)
S. Otles, V.H. Ozyurt, Classical Wet Chemistry Methods, Handbook of Food Chemistry (Springer-Verlag, Berlin, Heidelberg, 2014), pp. 1–14
Copper Development Association Inc from http://www.copper.org
P. García, Comparative study of the parameters influencing the machinability of leaded brasses. J. Eng. Fail. Anal. 17, 771–776 (2010)
J. Miller, P. Haden, Statistical Analysis with the General Linear Model (Creative Commons Attribution, USA, 2006)
Acknowledgments
This research was supported by LCL Pty Ltd, one of Australia’s largest producers of high quality copper and bronze alloys who provided insight and expertise that greatly assisted the research. This research project would not have been possible without the support of Rautomead Ltd engineers. The authors would like to thank Mr. Colin Bell, Scott Tocher and Mr. Gavin Marnie. Their guidance helped us throughout this research. The authors would like to thank Sir Michael Nairn, Chairman of Rautomead, for his valuable comments and suggestions to improve the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bagherian, ER., Fan, Y., Cooper, M. et al. Investigation of the Distribution of Lead in Three Different Combinations of Brass Feedstock. Inter Metalcast 10, 322–328 (2016). https://doi.org/10.1007/s40962-016-0055-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-016-0055-1