Abstract
High pressure and high temperature (HPHT) reservoirs have challenging environments for a successful completion program. Generally, low to mid-density range brine-based completion fluids (CF) are commonly used in petroleum reservoirs. Nowadays, the oil and gas drilling industry is moving toward clear high-density completion fluids at HPHT reservoir conditions. Completion fluid is used to complete an oil and gas well. It is positioned in the well to ease final operations before the start of production. These operations involve tasks like installing screens, production liners, packers, downhole valves, or performing perforations in the producing zones. We have experimentally investigated the completion fluid for stability, solid free, low viscosity, and low precipitation to ensure that it has all the desired properties. We have formulated a high-density specific gravity (1.61) completion fluid using Magnesium bromide (MgBr2) in an aqueous medium. The results show that the alkaline pH value of 7.18 and solid free fluid system provide a suitable completion fluid to keep corrosion rates acceptably low. The high density of the completion fluid is an essential parameter for pressure maintenance during well control events. Our experimental results are obtained for different ranges of temperature and pressure (i.e. temperature 25–300 °C, pressure up to 30,000 psi) using a new generation ultra HPHT rheometer. This study investigates the effect of ultra-high temperature and pressure on their rheological properties. Rheological results show that the completion fluid has a low value of apparent viscosity (1.89–6.66 mPa s), which is essential for designing completion fluid at HPHT conditions. These works are helpful in maximizing the completion fluid program for HPHT well for providing an early and timely production.
Article highlights
-
Our work is important for the development of novel high-density clear completion fluids for applications in HPHT wells across the world.
-
This study investigates the effect of ultra-high temperature and pressure on their rheological properties. The effect of temperature on viscosity is more predominant compared to pressure.
-
Solid-free and high-density completion fluid is crucial for well completion to minimize reservoir formation damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The worldwide demand for hydrocarbon is energetic for the petroleum industry to explore HPHT reservoirs (Lee et al. 2012). There is always a big challenge and adverse environmental conditions for drilling and completing a high pressure and high temperature well. Globally, the sharing of some HPHT, ultra HPHT, and extreme HPHT wells located in the Gulf of Mexico, North Sea, Middle East, and Southeast Asia are shown in Fig. 1 (Shadravan and Amani 2012). HPHT well are classified according to a reservoir temperature and reservoir pressure system. As shown in Fig. 2, the HPHT well system has a starting reservoir temperature range of 300 °F to 400 °F and an initial reservoir pressure range from 10,000 to 20,000 psi (Loth 1998; Tony 2016). For example, a tight permeable gas reservoir in the Krishna- Godavari (KG) basin on the east coast of India is considered an HPHT reservoir. The field has an average recorded pressure and temperature of 12,000 psi and 360 °F, respectively (Denney 2013).
HPHT reservoir has a very high pore pressure gradient and must require a high density completion fluid during the installation of completion tolls and remedial workover. HPHT reservoirs are highly prone to gas kick occurrence during the use of low-density completion fluid. Generally, a low to mid-range density completion fluid is currently applicable in the normal reservoir, which is at lower risk of such unwanted pressure events.
Completion fluid plays an important role as the interface between the reservoir and surface production in the last stage of drilling operations. According to the American Petroleum Institute (API) definition of completion fluid, it is defined as a solid-free, clear liquid, high density, low viscosity fluid for completing an oil/gas well (Ezzat 1990). Completion fluid is used in the well to prepare the wellbore and enable final operations before the initiation of production. Completion fluids are used during the installation of sand screens, production liners, packers, artificial lift mandrels and valves, and subsurface safety valves during perforations into the pay zone (Caenn et al. 2011). The main objective of a completion fluid is to control the formation pressure of a well during such installation without damaging the pay zone formation. High-density completion fluid (HDCF) systems play a vital role in the completion of HPHT well. They have essential properties such as high specific gravity, solid-free, thermal and rheological stability, damage-free to reservoir formation, low cost, worldwide availability, and environmentally friendly (Dubberley and Magill 2020). Solids-free brine-based completion fluids (SFBCF) differ from conventional completion fluids in several key aspects, such as solids-free, no fluid loss control additives, and low Viscosity (Dubberley and Magill 2020). Figure 3 shows the solid-free and high-density fluid application in oilfields (TETRA Technologies. Inc 2022).
Completion fluids should be maintained in a solids-free nature during the entire fluid storage and handling process. This requires clean tanks, clean lines, and a good filtering system. Covered tanks will reduce the loss of density by moisture pick-up from surroundings. A solids-free or extremely clean completion fluid is highly ideal during the well perforation and gravel packing (Olivier 1981; McLeod 1982; Klotz et al. 1974). The high density of completion fluid helps in well control of formation pressure and maintains the overbalance to prevent the entry of formation fluid into the wellbore during completion (Hossain and Al-Majed 2015). Any improper selection of chemical additives used in completion fluid formulations can easily lead to thermal degradation and loss of rheological properties. The viscosity of hydrosoluble polymer solutions commonly used in CF formulations strongly decreases as temperature increases above 150 °F (Ibeh et al. 2008).
Generally, Temperature and pressure are important factors when selecting any completion fluid under HPHT conditions. Both temperature and pressure values directly increased with the depth of the reservoir (Lee et al. 2012). To investigate the completion fluids property, we first need to understand the well completion design and existing installed well completion components. Generally, a well-completion process involves installing production tubing, packers, and associated downhole tools, perforating, and well-stimulating completion tools (Hossain and Al-Majed 2015). Figure 4 shows the relevant factors which are affecting the overall well-completion activities (Hossain and Al-Majed 2015). Any completed well must consist of the outcomes of this activity. All parameters of these completion activities directly affect or influence the success and failure of any well completion.
Generally, low to mid-density range brine-based completion fluids are commonly used in oil and gas reservoirs; the options for high-density completion fluids are rare. Brine based completion fluids such as KCl, NaCl, NaBr, NH4Cl, CaCl2, CaBr2, ZnBr2, and cesium formate. There is some limitation to existing completion fluid, such as Zinc bromide (ZnBr2), which is a highly marine pollutant and toxic. KCl, NaCl, NaBr, NH4Cl, and CaCl2 based completion fluids are not suitable for HPHT reservoirs due to their density limitation (< 1.2 s.g.). However, cesium formate (CsCOOH) has a high density and is currently used as an HPHT completion fluid. The issue with cesium formate is that the cost is not economical, and there is a very limited worldwide supply (Al-Bagoury and Steele 2016). Secondly, cesium is a rare earth metal, and the oil and gas industry does not want to rely totally on this for their highly demanding HPHT completion project. In view of the following limitations, completion programs have a rising demand for exploring alternative engineering solutions. Our work is an effort in this direction to provide an alternative solution to the industry. Our motivation for developing MgBr2 based completion fluid is inspired because of some of the highly relevant fundamental properties associated with alkaline magnesium, and MgBr2 based completion fluid formulation has shown high specific gravity with the solid free system, high solubility in water, low toxicity, and alkaline nature (Singh et al. 2022a, b). At HPHT reservoirs, wellbore stability is one of the significant success elements of the drilling operations, which is dependent upon the chemistry of the fluid formulation (Ali et al. 2022).
In HPHT operations with narrow operating windows, pore pressures and fracture pressures are very close to each other, a situation commonly encountered in deep HPHT wells. The selection of thermally stable, high-density completion fluid is highly desired to prevent the formation and lost circulation, as these issues may lead to well control and wellbore stability problems. We have investigated the effects of high temperature and pressure on the formulated high density completion fluid rheology. We have also investigated the stability of formulated HDCF to know about their interaction effects at high temperatures and pressure conditions. A low value of viscosity is advantageous for completion fluid and ensures good wellbore stability. This minimizes the Equivalent Circulation Density (ECD) to prevent the formation and lost circulation, thereby averting potential well control and wellbore stability issues. High viscosity would result in elevated pump pressure and increased frictional pressure drop during circulation, leading to heightened pressure at the wellbore bottom. We have obtained an optimum high density, alkaline pH, fluid mixing rate, and mass composition. Our work is important for the development of novel clear completion fluids for applications in HPHT well.
2 Materials and methods
2.1 Completion fluid formulation
In order to formulate completion fluid, we need desirable properties data for the selection criteria of completion fluid for HPHT Well. This study includes HPHT stability and rheological studies for various aspects of completion fluid formulation. Figure 5 shows the highly relevant properties of the completion fluid at HPHT conditions. Based on these criteria, we have selected MgBr2 salt as the base completion fluid. MgBr2 is an ionic compound and consists of giant ionic lattices at room temperature. The strong attraction between the positive and negative ions needs a lot of heat energy to break, so it has high melting and boiling points (Lide 1998). However, when it melts, it undergoes electrolysis and gets dissolved in water with neutral to alkaline properties (pH of approximately 7) due to the alkaline nature of magnesium. Figure 6 shows the highly relevant and meaningful properties of the Magnesium Bromide (Lide 1998). Magnesium bromide is highly water-soluble. Most heavy brines are quite hygroscopic (Spies et al. 1983). The high-density brines are strong salt solutions. Wear safety goggles to prevent eye contact, have eye-wash facilities available, and avoid extensive skin contact when handling these hygroscopic fluids, which is a standard safety practice in the petroleum industry.
We use the MgBr2 (Purity of 98%) manufactured by Nice Chemicals Private Limited, India. MgBr2 is an ionic compound consisting of alkaline earth metal magnesium. The high density, high solubility in water, and high melting and boiling points make this compound favourable and suitable for formulating completion fluid at the HPHT reservoir. We have used 100 ml of distilled water provided by Merck Life Science Private Limited, India. Our completion fluid formulation is a single-salt water-based formulation without any solid additives. We have prepared the sample CF1 and CF2 according to API standard (API Recommended Practice 13B-1 2003) practice for the formulation of completion fluid (API Recommended Practice 13B-1 2003). The sample was mixed using a high shear mixture by Qingdao Haitongda Special Instrument Co. Ltd, China. To get clear HDCF, we use a high shear rate (3000, 4000, and 6000 rpm) along with a high mixing time (8, 10, 15, and 20 min). After formulation, we obtained the specific gravity range of the completion fluid sample from 1.51 to 1.61. We have used a digital weighing balance (Atma Technologies, India) for accurate weight measurements. The pH of the sample was measured using a digital pH Meter by Hangzhou Lohand Biological Co., Ltd, China. We have to follow the pH measurement, which consists of the first calibration of the pH meter using water, and then the pH measurement of the prepared sample is performed for 20 min. After the pH test of the sample, unplug the pH meter, clean the probe with distilled water, and then place the cap on the probe. Table 1 shows the specific gravity and pH value for completion fluid samples CF1 and CF2 using Magnesium bromide.
2.2 Rheology of completion fluid: HPHT experimental setup
We have investigated the rheological properties of samples CF1 and CF2 using Grace Instrument M7500 ultra HPHT Rheometer by Grace Instruments Company, USA. As shown in Fig. 7, the experimental setup at HPHT conditions consists of an M7500 Rheometer, air compressor, water circular chiller bath, and data logging system. Pressure cell assembly installation and the bob shaft assembly installation are required prior to loading the sample in the pressure tower. We have considered the following test design configurations for testing the completion fluid sample. The temperature range is considered from ambient to 600 °F. The pressure range considered extends from atmospheric pressure to 30,000 psi, with a conversion factor of 1 psi (field unit) equal to 6.89 kilopascals (SI unit). The test sample size is 132 ml. We have used a water circular bath chiller with a volume capacity of 20 L by Medlab Scientific Equipment, India. The purpose of using a bath chiller is to cool down the temperature of Rheometer M7500 after the end of each cycle. We have considered the glycol and water mixture ratio (60G/40W). At the end of each test sequence, the automatic command passes to the M7500 Rheometer system to engage the cooling circuit to cool down the temperature of the pressure cell. Generally, the cooling cycle will continue until the sample temperature in the pressure cell reaches 109 °F. We have used an air compressor for providing 120 psi pressure to the M7500 Rheometer system by Grainger Inc. USA. The system creates loud noise during test cell pressurization and depressurization. This noise is the normal operating noise of the pump and pressure release valve. There must be safety precautions in experiments dealing with HPHT conditions. The bath chiller unit circulation pump should remain ON during the running test. Supply Pressurization oil Container should always be filled with oil before and after purging and before starting the test. During purging, the pressure release valve should remain open and close the valve after the completion of purging. The pressure release valve should be closed before starting the test. After completion of the test, wait to cool the sample up to 40 °C and then open the pressure release valve very slowly to release the cell pressure.
2.3 Rheology model and measurements
A reliable rheology model plays a vital role in all types of well-completion fluids. Completion fluid rheology depends on the effect of high temperature, pressure, and shear stress during the completion process. A comprehensive study of the reservoir characteristics at the downhole conditions is essential for the completion of fluid design (Caenn et al. 2011). The common practice is to measure a completion fluid’s flow characteristics under HPHT downhole conditions. The mobility of the CF systems is affected by an increase in pressure and a decrease in temperature, which leads to an increase in overall apparent viscosities and visco-elastic relaxation times (Ibeh et al. 2008). Generally, the effect of pressure is expected to be greater with oil-based systems having high oil phase compressibility as compared to a water-based system having less compressibility. Newtonian fluid has a linear proportionality of shear stress and shear rate. The slope of the graph and the constant of proportionality define the Newtonian viscosity. We consider a Newtonian fluid rheological model for water-based completion fluids as follows:
where \(\tau\) is the shear stress, \(\gamma\) is the shear rate, S−1, and \({\mu }_{a}\) is the apparent viscosity. The rheological property was calculated based on the API standard, and we have used the following general formula for converting the rpm readings into the Newtonian rheological parameter (API Recommended Practice 13B-1 2003):
where \(\mu a\), apparent viscosity; \(\theta N\), dial reading (deg.) at rotary speed N rpm.
3 Results and discussions
3.1 Effect of pH and salt concentration on completion fluid
We have used a single salt system formulation without any additives. As shown in Table 1, completion fluid density, salt mass concentration, and pH value were measured at ambient temperature and pressure. The pH value of the sample is in the alkaline region (pH 7.18), which is highly desired for the completion fluid to keep very low corrosion rates. The tendency for corrosion will be greater for a low value of pH (Bush 1974; Place et al. 1980). The pH of the completion fluid (CF) slightly increases to a more alkaline side as brine density increases. Increasing the weight percent of base salt in CF leads to enhancing alkaline pH. This is believed to be a consequence of two complementary mechanisms: an increase in ion activity as brine density is increased and a complementary increase in Mg(OH)2 solubility as brine density increases. It should be noted that this pH of water-based completion fluids should be maintained at alkaline sides when completing critical sour wells. Maintaining an elevated pH is a well-established and cost-effective method for reducing corrosion rates in water-based completion fluids (Bush 1974). The density of brines gradually increases with increasing the solute mass fraction. We found a high solubility of approximately 80.8 wt% at room temperature, and its maximum density approaches 1.61 g/cm3.
3.2 Comparison of existing solutions of density limitation using CF1 and CF2
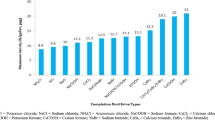
In our work, we have reported the specific gravity of CF1 and CF2 as 1.51 and 1.61, respectively. We have obtained the high specific gravity completion fluid without any additives. High density formulation will provide well control at HPHT conditions and reduce the overall cost by eliminating further any solid weighting material. Nowadays, existing completion fluids are potentially formation damaging. Figure 8 shows the available range of completion fluid (Caenn et al. 2011). Comparing the existing completion fluid density with our formulated sample, we have observed that most existing fluids are either low to mid-range density, and suitability at HPHT conditions is not well explored. Although few types of brine have high density, they are either highly corrosive or toxic and expensive; e.g. ZnBr2 is highly toxic and causes corrosion in completion tools. Zinc is also a marine pollutant and can cause issues in the processing stage if residual zinc is in the oil sent to the refinery. Nowadays, cesium formate (CsCOOH) is the only available choice for HPHT completion fluid. Cesium being a rare earth metal, the cost of CF is very expensive with less supply (Al-Bagoury and Steele 2016). Due to these limitations, there is a rising demand for exploring an alternative HDCF that meets all suitability requirements for completion fluids at HPHT reservoirs. This work is an effort in this direction. Our formulated completion fluids showed a high value of specific gravity, having solid free and clear fluids with no settling of particles observed in the sample, resulting in good completion fluid.
3.3 Effect of temperature and pressure on completion fluid density
The Higher Specific gravity of base CF plays an important role during high bottom-hole temperature conditions. The density of a CF weakens at higher temperatures due to the liquid's warm volumetric extension (TETRA Technologies. Inc. 2022). However, high bottom-hole temperature causes a reduction in CF density and results in well control-related problems (Spies et al. 1983). An adequate density is required to control the formation pressure and maintain overbalance into the wellbore during completion. The fluid density is typically chosen to surpass the formation pressure, in addition to an additional safety factor. Well control is a particularly troublesome concern. Regularly utilized overbalance levels are 200 psi for oil wells and 300 psi for gas wells (Hossain and Al-Majed 2015). The density of the completion fluid in the field unit is determined based on the true vertical depth (TVD) of a well and the overbalance pressure.
where \(\rho\) is the equivalent density of CF, lb/gallon, \({\rho }_{o}\) is original CF density, \({P}_{ov}\) is overbalance pressure, psi), and \(TVD\) is the true vertical depth (feet) of the well (Hossain and Al-Majed 2015).
Completion fluid density can be cut by the hydration process and because of air entrainment when surface facilities such as shale shakers, degassers, and pit agitators are used. CF density loss can also occur by mixing with formation fluids because of swabbing effect pressure and forming emulsions (Spies et al. 1983). Completion fluid density can also be affected due to crystallization phenomena. Salts will crystallize out of the solution if the temperature falls below a certain critical value, and this behavior depends on the composition of the brine (Adams 1981; Hubbard 1984; Milhone 1983). CaCl2 and CaBr2 brine mixture of 14.8 Ib/gal (1.77 s.g.) will crystallize at a lower temperature below 63 °F (17.2 °C) (Caenn et al. 2011). Crystallization of CF in surface facilities can be a major problem during working in winter as it can be solidified during winter. However, crystallization was not a major problem during working in the summer. The use of electrically heated tanks during the winter allowed the minimization (Spies et al. 1983). The true crystallization temperature (TCT) is defined as the temperature at which the brine becomes saturated and salt crystals begin to form (Davidson et al. 2017). The TCT is typically measured at atmospheric pressure and gives a measure of the lowest temperature at a given brine that can be used. Using brine below its TCT can lead to serious consequences as the salt falls out of the solution, and the fluid density is severely reduced. Generally, for deep-water applications, a TCT significantly less than 30 °F (around − 1 °C.) is required, but TCT in a range of about 20 °F to about 60 °F (about − 6.7 °C to about 16 °C) is useful for shallower water applications where the seabed temperature is not as low. Magnesium Bromide has a density of 13.2 ppg and has a true crystallization temperature (TCT) of 32 °F (Arthur 2017). Heavy completion fluid brines can crystallize if exposed to lower temperatures or higher pressures. Applying pressure to divalent brine at a density above the eutectic point will lead to an increase in density, which in turn can lead to crystallization. Fluid crystallization can cause blockage to tubular in a wellbore on the surface. Crystallization inhibitors such as methanol and ethylene glycol can be used to lower the TCT but can also result in a reduction of the density of the brine. This will make it unsuitable for the original purpose of HDCF, which means that more solid divalent salt has to be added to bring the density of the brine back to the operational density.
3.4 Effect of ultra-high pressure and temperature on the rheological properties of completion fluids
The rheology of the fluid depends on many factors, such as temperature, pressure, shear stress, and fluid composition (Ibeh et al. 2008; Spies et al. 1983). Any viscosified brines base fluid behaved as near Newtonian fluids with the increase of temperature due to the presence of high salt concentration in the solution. Generally, a high salt concentration involves huge amounts of active ions, and it helps in destroying water structure or polymer bonding (Khatibi et al. 2016). Salinity also affects the rheology of water-based mud under high pressures and high temperatures (Amani and Hassiba 2012). Generally, a completion fluid experiences two opposing effects temperature and pressure under HPHT conditions. An increase in wellbore pressure can result in increases in the completion fluid’s viscosity due to its compressibility effect. An increase in temperature reduces the fluid viscosity due to an increase in the random motion of the macromolecules dissolved in the completion fluid matrix. At a particular temperature and pressure profile, these two opposing effects may cancel out, resulting in a uniform fluid viscosity /density along with the depth of the well that is equal to that at the surface (Ibeh et al. 2008). The effect of temperature on rheology is more predominant than the pressure’s effect. However, if the temperature is lower than the failure point flow, increasing the pressure results in higher viscosity and yield point (Amani 2012). Accurate rheological data and exact prediction of completion fluids behavior at HPHT conditions are the most significant issues in completing deep, complex reservoirs.
The effect of pressure and temperature on the rheology of formulated high-density completion fluid was fully investigated for applications in HPHT reservoirs. Our experimental results are obtained for high ranges of temperature, pressure, and densities (i.e. temperature range up to 301 °C, specific gravity 1.51 to 1.61, and pressure up to 30,000 psi). We have investigated the formulated completion fluid (CF1), having a specific gravity of 1.51 and the completion fluid (CF2), having a specific gravity of 1.61 at high shear rates. Tables 2 and 3 show the test results using the M7500 Ultra HPHT Grace Rheometer (Rotor Number: R1, Bob Number: B1, Bob Radius (cm): 1.724) of completion fluid CF1 and CF2 having a specific gravity of 1.51 and 1.61 respectively.
Apparent viscosity is defined as the resistance to the flow of a fluid and is calculated using Eqs. (2). It is a key parameter of completion fluid, and the value must be kept within the optimum limits for an efficient, well completion process. AV should be as low as reasonably possible to minimize Equivalent Circulating Density (ECD) (Shadravan and Amani 2012). High AV is caused by a viscous base fluid having excess colloidal solids. AV should have a low value due to clear completion fluid so it can minimize formation damage and minimize high surge and swab pressure losses. The apparent viscosity of Magnesium bromide based completion fluid having 1.45 specific gravity has been investigated at HPHT reservoir conditions and found suitable for completion fluid (Singh et al. 2022a, b). The apparent viscosity of CF also has an inversely proportional relationship with temperature. In the case of fluid having various additives, CF viscosity decreased due to the consequence of the thermal degradation of the solid and polymer components of the fluid samples. This behavior will expand the molecular distances of the fluid and lower the resistance of the fluid flow (Amani 2012). A relationship between apparent viscosities, pressure, and temperature, with the test time of formulated CF1 having a specific gravity of 1.51 and CF2 having a specific gravity of 1.61, is shown in Fig. 9. Figure 10 shows the apparent viscosity, pressure, and temperature of the completion fluid in 3D formats. As shown in Fig. 9a, we have investigated the sample CF1 having a specific gravity of 1.51. We have applied a high shear rate of 300 rpm and 600 rpm. The temperature range was taken from 46.1 to 301.7 °C, and the pressure range from 10,000 to 29,246 psi. Our results show the maximum value of apparent viscosity of 5.76 mPa s at temperature 46.1 °C, pressure 10,009 psi, shear rate 300 rpm, and the minimum value of apparent viscosity is 1.89 mPa s at HPHT condition of temperature 301.7 °C, pressure 29,246 psi and shear rate 600 rpm. We have divided the entire (Fig. 9a) into two regions based on pressure and temperature range. The first one is the early-stage region, having a temperature varying from 46.2 to 294.4 °C, a time duration of up to 116.5 min, the shear rate was 300 rpm, and pressure was maintained at 10,000 psi.
As shown in Fig. 11a, we have observed that the apparent viscosity profile continuously decreases with increasing temperature in this region. Similarly, the shear stress profile has also shown a decreasing trend, which is shown in Fig. 12a. At the last stage of CF1, we have observed that an increasing pressure of 10,000 to 30,000 psi causes a slight reduction in apparent viscosity and shear stress at a constant high temperature of around 300 °C and a constant high shear rate of 600 rpm (Fig. 13). Generally, at constant high temperature and rpm, if pressure increases, then also it does not causes a very high increase in apparent viscosity and shear stress. Apparent viscosity normally does not depend much on pressure. Gases are more compressible than liquids. An increase in pressure doesn't bring the liquid's molecules significantly closer together. We have observed the lowest value of apparent viscosity and shear stress in this region due to the combined effect of HPHT, and the predominant effect of high temperature causes a reduction in apparent viscosity and shear stress.
Similarly, as shown in Fig. 9b, we have investigated the sample CF2 having a specific gravity of 1.61. The temperature range was taken from 27.2 to 197.2 °C, and pressure varies from 9 to 30,000 psi. Sample CF2, having a specific gravity of 1.61, shows a maximum value of apparent viscosity of 5.87 mPa s at ambient conditions such as the temperature of 27.2 °C, pressure of 9 psi, a shear rate of 600 rpm, and minimum apparent viscosity is 4.97 mPa s at temperature (197.2 °C), pressure (26,275 psi), and shear rate 300 rpm. We have divided the entire CF2 process into two regions based on pressure and temperature input. The early-stage region has temperature varying from 27.2 to 96.1 °C, time duration of up to 49.2 min, and pressure of 9 psi to 30,062 psi. As shown in Fig. 11b, we have observed that the apparent viscosity profile continuously decreases, and the rate of decreasing the apparent viscosity is higher than in the other regions, and it further decreases as pressure is raised to 30,000 psi at constant temp 96.1 °C. Initially, viscosity was low due to the high shear rate of 600 rpm. Similarly, the shear stress profile has also shown a higher decreasing trend, which is shown in Fig. 12b. In the late stage region, having temperature varies from 96.1 to 194 °C, the time duration was 49.2–114 min, the pressure was maintained at 10,000 psi, and the shear rate was 300 rpm.
We have noticed a rising shift in the apparent viscosity profile at 100 °C, and the apparent viscosity profile was decreasing at a slower rate and maintained at 10,000 psi. At the end of the late stage (temperature is constant around 197 °C, time duration 114–118 min, pressure varies from 10,000 to 30,000 psi, shear rate 300 rpm), apparent viscosity and shear stress have the least value in this region. From this observation, we can say that at high temperature (197 °C), the effect of temperature on the apparent viscosity is more predominant compared to pressure and, hence, results in a drop in apparent viscosity and shear stress.
4 Conclusions
Although low to mid-density range completion fluids are used in petroleum reservoirs. For HPHT reservoirs, there is a need for high-density clear completion fluid. In this paper, we have formulated water-based CF using magnesium bromide, which is a novel work. This study investigates the rheological properties of prepared completion fluid from ambient to high pressure up to 29,246 psi and temperatures 301 °C. This is important because, at present, oil and gas exploration and production are moving towards high-pressure, high, temperature challenging regions. So, these brine-based high-density clear CF systems can arise as sustainable completion fluid choices for deep high-temperature wells.
We report a high specific gravity, solid free completion fluid of s.g.1.61, and it also exhibits an alkaline pH value (pH 7.18), which is essential for the completion fluid at the HPHT reservoir. The apparent viscosity (AV) has been found to have a low value (< 7 mPa s), which is good for any completion fluid and wellbore stability at HPHT conditions. We have observed the lowest value of apparent viscosity and shear stress in this region due to the predominant effect of high temperature resulting in a reduction in apparent viscosity and shear stress property. The effect of temperature on apparent viscosity is more predominant compared to pressure. Our works are likely to accelerate the design and development program of high density clear completion fluid for the HPHT field. There is always a scope for designing stable, solid-free, and high-density completion fluid to ensure early and timely production. Breakthrough is still possible.
Abbreviations
- HP/HT:
-
High-pressure/high-temperature
- HDCF:
-
High density completion fluid
- BP:
-
Bingham plastic model
- CF:
-
Completion fluid
- TCT:
-
True crystallization temperature
- API:
-
American petroleum institute
- SFBCF:
-
Solids free brine-based completion fluids
- KG:
-
Krishna-Godavari
- TVD:
-
True vertical depth
- TCT:
-
True crystallization temperature
- τ:
-
Shear stress
- γ:
-
Shear rate, unit (s−1)
- AV:
-
Apparent viscosity
- ϕ:
-
Dial reading, degree
- ppg:
-
Lb/gallon
- s.g.:
-
Specific gravity (dimensionless)
- \({P}_{ov}\) :
-
Overbalance pressure, (1 psi = 6.89 kilopascal)
References
Adams N (1981) Workover well control conclusion—how to use fluids to best advantage. Oil Gas J 52:254–275
Al-Bagoury M, Steele C (2016) Liquid weight material for drilling & completion fluids. In: SPE/IADC middle east drilling technology conference and exhibition, Abu Dhabi, UAE. https://doi.org/10.2118/178157-MS
Ali JA, Hamadamin AB, Ahmed SM, Mahmood BS, Sajadi SM, Manshad AK (2022) Synergistic effect of nanoinhibitive drilling fluid on the shale swelling performance at high temperature and high pressure. Energy Fuels 36(4):1996–2006
Amani M (2012) The rheological properties of oil-based mud under high pressure and high temperature conditions. APED Adv Petrol Explor Dev 3(2):21–30. https://doi.org/10.3968/j.aped.1925543820120302.359
Amani M, Hassiba KJ (2012) Salinity effect on the rheological properties of water based mud under high pressures and high temperatures of deep wells. In: SPE Kuwait international petroleum conference and exhibition, Kuwait. https://doi.org/10.2118/163315-MS
API Recommended Practice 13B-1(2003) Recommended practice for field testing water-based drilling fluids. American Petroleum Institute, Washington, DC
Arthur GM (2017) High density, low TCT divalent brines and uses thereof. International Publication Number WO2017165762A1
Bush HE (1974) Treatment of drilling fluid to combat corrosion. In: SPE-5123-MS. https://doi.org/10.2118/5123-MS
Caenn R, Darley HC, Gray GR (2011) Composition and properties of drilling and completion fluids, 6th edn. Gulf Professional Publishing, Oxford
Davidson MR, Rosa S, Andrew D, Moctesuma RT, Mahir DA, Stephen M, Jennifer R (2017) High-density completion fluid. United States Patent Application Number US 201701 45284A1, Houston, TX, USA
Denney D (2013) Offshore HP/HT gas well: drilling and well testing. J Pet Technol 65(04):111–115. https://doi.org/10.2118/0413-0111-JPT
Dubberley S, Magill S (2020) A technical review of solids free brine-based drilling fluids. In: American Association of Drilling Engineers Technical Conference and Exhibition on Fluids, Houston, Texas
Ezzat AM (1990) Completion fluids design criteria and current technology weaknesses. In: SPE Formation damage control symposium. https://doi.org/10.2118/19434-MS
Hossain ME, Al-Majed AA (2015) Fundamentals of sustainable drilling engineering. Wiley, New York
Hubbard JT (1984) How temperature and pressure affect clear brines. Petrol Eng 5:58–64
Ibeh C, Schubert J, Teodoriu C (2008) Investigation on the effect of ultra-high pressure and temperature on the rheological properties of oil-based drilling fluids. In: AADE conference and exhibition, Houston, Texas
Khatibi M, Potokin N, Time RW (2016) Experimental investigation of effect of salts on rheological properties of non-Newtonian fluids. Annu Trans Nordic Rheol Soc 24:117–126
Klotz JA, Krueger RF, Pye DS (1974) Maximum well productivity in damaged formations requires deep, clean perforations. In: Formation damage symposium, SPE Paper No. 4792, New Orleans, February 7–8. https://doi.org/10.2118/4792-MS
Lee J, Shadravan A, Young S (2012) Rheological properties of invert emulsion drilling fluid under extreme HPHT conditions. In: IADC/SPE drilling conference and exhibition, San Diego, California, USA. https://doi.org/10.2118/151413-MS
Lide DR (1998) Handbook of chemistry and physics, 87 edn. CRC Press, Boca Raton, pp. 4–67. ISBN 0-8493-0594-2
Loth WD (1998) Subsea engineering for health and safety executive. HPHT wells: perspective on drilling and completion from the field. HSE Books, ISBN 0-7176-1594-4
McLeod Jr, HO (1982) The effect of perforating conditions on well performance. In: Formation damage symposium, SPE Paper No. 10649, Lafayette, LA
Milhone RS (1983) Completion fluids for maximizing productivity-state of the art. J Petrol Technol 35(1):47–55. https://doi.org/10.2118/10030-PA
Olivier DA (1981) Improved completion practices yield high productivity wells. Pet Eng
Place J, Paul JR, Sigalas (1980) A high density clear fluids for completions and workovers. In: European offshore technology conference and exhibition. London, United Kingdom. https://doi.org/10.2118/261-1980-MS
Shadravan A, Amani M (2012) HPHT 101—what every engineer or geoscientist should know about high pressure high temperature wells. In: SPE Kuwait international petroleum conference and exhibition, Kuwait City, Kuwait. https://doi.org/10.2118/163376-MS
Singh R, Sharma R, Rao GR (2022) A deep sea completion fluid technology—novel high density brine—based completion fluid for applications in high pressure and high temperature petroleum reservoirs. In: OCEANS 2022 Chennai, pp 1–5. https://doi.org/10.1109/OCEANSChennai45887.2022.9775222
Singh R, Sharma R, Rao GR (2022b) Aging effects on the rheological properties of novel magnesium bromide hexahydrate-based completion fluids for oil and gas reservoirs. Arab J Sci Eng. https://doi.org/10.1007/s13369-022-06798-2
Spies RJ, Himmatramka AK, Smith JR, Thomas DC (1983) Field experience utilizing high-density brines as completion fluids. J Petrol Technol 35(05):881–888. https://doi.org/10.2118/9425-PA
TETRA Technologies.Inc (2022) Engineered solution guide for clear brine fluids and filtration [online]. 2nd edn, Chapter-2, 5. https://tetratec.com/resources/engineered-solutions-guide. Accessed 20 April 2022
Tony S (2016) High pressure high temperature. Oilfield Review, Schlumberger. https://www.slb.com/resource-library/oilfield-review/defining-series/defining-hpht
Acknowledgements
The authors acknowledged the Oil and Natural Gas Corporation Ltd. (ONGC), India, and the Indian Institute of Technology (IIT), Chennai, India, for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, R., Sharma, R. & Rao, G.R. Investigation of the effects of ultra-high pressure and temperature on the rheological properties of a novel high-density clear completion fluids using magnesium bromide for applications in HPHT reservoirs. Geomech. Geophys. Geo-energ. Geo-resour. 10, 9 (2024). https://doi.org/10.1007/s40948-023-00724-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40948-023-00724-y