Abstract
During the in-situ exploitation of oil shale, the evolution of pore structure will affect the heat transfer medium and the transport capacity of products. In this study, using the oil shale from Jimsar region of Xinjiang, in combination with the low-temperature nitrogen adsorption experiment (LNTA) and high-pressure mercury injection experiment (MIP), the effect of temperature on evolution law of its pores during pyrolysis were analyzed according to the LNTA and MIP joint characterization results. The results show as follows: the change curve of oil shale porosity with pyrolysis final temperature in Xinjiang shows an “S” shape. The pore volume increases slightly before 300 °C, and it shows an increasing trend in the subsequent temperature range, with the mesopore volume change being the most noticeable. However, the increase slows down after 600 °C. The evolution of full-scale pores in the pyrolysis process of oil shale is reflected by the joint characterization of LNTA and MIP, which indicates that the temperature has a controlling effect on the evolution of pore, and provides basic theoretical support for how to improve the oil recovery rate of exploitation of oil shale.
Article highlights
-
1.
The influence of temperature on the pore structure of oil shale was studied by multi-means and multi-angle at different scales, and the related mechanism was explained.
-
2.
The pore structures measured at different scales are jointly characterized, so that the characterization results are more comprehensive.
-
3.
The results of the experiment were digitally analyzed from multiple angles, which made the conclusions more accurate and refined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As the world’s population continues to grow, the energy supply becomes an urgent issue to be addressed. With the continuous growth of energy demand, the conventional oil and gas resource reserves are gradually reduced, and the focus of scholars on energy development has gradually developed from conventional reservoir research (Mao et al. 2005; Si Ma et al. 2012) to coal reservoirs (Tang et al. 2005; Yang et al. 2009; Cai et al. 2013; Yao et al. 2008) and shale reservoirs (Hinai et al. 2014; Zhang et al. 2019; Saidian et al. 2014). As a typical unconventional oil and gas resource, oil shale is an alternative energy source with wide development prospects, and the effective development and utilization of it have received worldwide attention (Saif et al. 2019).
The mining technology of oil shale mainly includes surface dry distillation and in-situ mining, the former has not been widely used due to cost, high mining intensity, energy consumption, pollution and other factors. In China, oil shale, as a disposable unconventional energy with huge reserves, will become the best alternative to fossil energy after its scientific and efficient development (Wang et al. 2016; Wu et al. 2016). Due to the low grade and deep burial of oil shale resources in China (Liu et al. 2009), the surface dry distillation technology is limited by the aforementioned issues. Therefore, the theoretical research and technical research of the in-situ mining has become a new development trend (Kang et al. 2011; Liu et al. 2018). In the process of in-situ mining, the internal pore structure of oil shale changes significant due to the physicochemical interactions of its internal substances. These changes have an impact on the flow behavior and heat transfer efficiency of the heat-carrying medium, as well as the diffusion and flow behavior of oil and gas products. Therefore, studying the pore evolution during the pyrolysis of oil shales is necessary.
Since the temperature has a significant impact on the pore structure of oil shale during in-situ mining, many scholars have studied the evolution of it at different temperatures. Based on the fluid intrusion method, Schrodt and Ocampo (1984) passed the N2 and CO2 isothermal adsorption experiments, and discovered that under the nitrogen atmosphere, the surface area of the pores is reduced due to the blockage of the reaction intermediates in the low-temperature stage, and it increases sharply when the temperature is high. But when oil shale is combusted in air, the surface area of pores decreases and the volume of mesopores increases. Zhu et al. (2018) used nitrogen adsorption–desorption and SEM techniques to study the pore structure evolution characteristics of oil shale under the microwave heating mode. The results shows that increasing the microwave heating rate will raise the product generation rate, resulting in the internal pores of oil shale changing from small pores to mesopores. At the same time, the specific surface integral dimension of the pore structure of the oil shale is positively correlated with the heating parameters, while the fractal dimension of the pore volume is opposite. Yang et al. (2016) used the mercury injection method to investigate the pore size evolution of oil shale following heat treatment at various temperatures. The results showed that as the temperature raised, the pore volume, average pore size, and porosity of oil shale would continue to increase. The porosity of oil shale at 600 °C was 8 times that at room temperature, and it was pointed out that the pyrolysis process of oil shale was the process of continuous expansion and coalescence of its internal pore structure. Based on imaging technology, Tiwari et al. (2013) used CT scanning and three-dimensional reconstruction technology to analyze the pore structure evolution of American oil shale under different final pyrolysis temperatures, obtained the porosity of oil shale semi coke, and estimated the permeability by Bolzmann equation based on 3D pore network structure. Rabbani et al. (2017) used CT technique to study the pore characteristics of oil shale in the temperature range of 390–400 °C and found that the average pore-throat ratio of the oil shale was 1.5, although the permeability of oil shale in different directions was anisotropic, the rate of change of permeability was isotropic. Saif et al. (2016) used CT technique to visualize the evolution of pores and micro-rupture networks in the pyrolysis process of oil shale, and the results showed that during 390–400 °C, with the formation of μm-level heterogeneous pores, the porosity increased dramatically. And with the further increase of temperature, the pores steadily expanded, forming a connectivity microcrack network mainly developed along the rich kernifer layer, so the development of pores was directly related to the initial spatial distribution of organic matter. Liu et al. (2019) used the NMR method to study the connectivity characteristics between different levels of pores in oil shale after different temperature treatments, and concluded that the threshold temperature of internal pore connectivity was 300 °C. When the temperature was below 300 °C, it wouldn’t promote the connectivity between pores. However, when it higher than 300 °C, the pore connectivity was significantly improved. It’s pointed out that the porosity of the movable fluid was the main part of promoting pore connectivity. Based on the large temperature range and different heating rates, Bai et al. (2017) studied the internal pore structure of oil shale in the range of 100–800 °C. It was considered that the threshold temperature for the sudden change of internal pores of oil shale was 350–450 °C. In this process, the escape of volatile components in oil shale would lead to the irregularity of the pore surface, and there was a linear relationship between the fractal dimension of pore structure and pore diameter in this temperature range. Han et al. (2006) studied the pore structure characteristics of oil shale before and after pyrolysis at different heating rates, and found that at high heating rates, the internal pore volume would decrease at the early stage of kerogen decomposition, and it would increase rapidly at the decomposition stage, and then decreased again near the completion stage of decomposition. Wang et al .(2020, 2018) studied the evolution characteristics of the internal pore fracture structure of oil shale after pyrolysis by high-temperature steam in the range of 300–600 °C. The results showed that the capillary pores of oil shale after pyrolysis of high temperature steam were the main channels of fluid transport within the oil shale, and the rupture method was mainly the rupture along the laminar surface, and the convection heating efficiency carried out by using high temperature steam as the heat carrier was higher than the efficiency of conductive heating. The above research shows that although the existing research methods are rich and have their advantages, the results are fragmented due to the different test methods and the limitation of accuracy. And since the evolution of oil shale pore structure under the condition of in-situ pyrolysis is extremely complex, which makes the micro-structure characterization methods of oil shale pore structure tend to be diversified and refined.
In summary, the above results have deepened the understanding of oil shale pore and fracture, but most researchers have focused on a certain pore size range, and the full-scale characterization of oil shale pore structure is still not sufficiently studied. Therefore, the purpose of this study is to research the effect of temperature on evolution law of pores during pyrolysis of Jimsar oil shale by combing the low-temperature nitrogen adsorption experiment (LNTA) and high-pressure mercury injection (MIP) experiments, jointly characterize the pore structure measured at different scales, and explain its mechanism from the aspects of mineral composition and pyrolysis characteristics to provide theoretical support and technical guidance for in-situ underground mining of oil shale.
2 Experimental samples and methods
2.1 Sample preparation
The oil shale samples used in the experiment were collected from Jimsar, Xinjiang. After collection, they were sealed with wax sealing and transported to the laboratory to prevent weathering and deterioration. According to Chinese standards (GB/T 19143-2017 and GB212-77), elemental analysis and industrial analysis of Xinjiang oil shale were carried out. The test results are shown in Table 1. The mineral composition of the samples was determined by using a Rigaku D/Max-2500 X-ray diffractometer (manufactured by Shimadzu, Kyoto, Japan) (Table 2). According to Table 2, the main mineral components of the samples are quartz, plagioclase, and dolomite, with the minerals sanidine, calcite, and clay following and lower amounts of pyrite and gypsum.
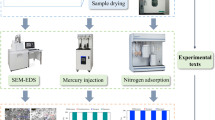
Xinjiang oil shale powder with a particle size of less than 200 mesh was processed in the laboratory for the LTNA experiment, and the specimen with size of 1 × 1 × 2 cm was processed for the MIP experiment. The samples were pyrolyzed in the SGL-1700 precision vacuum atmosphere tube furnace (Fig. 1) and heated to the target temperature (100 °C, 200 °C, 300 °C, 400 °C, 500 °C, 600 °C, 650 °C) in batches at a rate of 2 °C/min. The samples following heat treatment are shown in Fig. 2. And each treated sample was subjected to the above experiments.
2.2 Equipment and experiments
The type of pore structure in this paper was classified according to Hodot's classification scheme, which was divided into micropores (pore size < 10 nm), transition pores (or small pores, 10 nm < pore size < 100 nm), mesopores (100 nm < pore size < 1000 nm) and macropores (pore size > 1000 nm), relevant pore structure parameters were obtained through the LTNA and MIP experiments.
2.2.1 The LTNA experiment
The LTNA experiment was measured in the laboratory, using Bei Shi De 3H-2000PS2 specific surface and pore size analyzer (Fig. 3). The main parameters of the equipment were as follows: the specific surface is more than 0.01 m2/g, the pore size is 0.35–400 nm; the ultimate vacuum reaches 10−2 Pa.
The experiment was carried out in accordance with China Petroleum and Natural Gas Industry Standard SY/T 6169-2021. Dry the oil shale after pyrolysis at different target temperature followed by 2–3 g of each sample. Before the test, the samples were treated with vacuum degassing at 105 °C. Then, the sample tube was placed in the range of liquid nitrogen, and N2 was successively injected into the sample according to the preset partial pressure point to achieve adsorption equilibrium. The adsorption capacity of each partial pressure point was calculated by using the gas state equation, and nitrogen was successively extracted for treatment during desorption. Through the relationship between relative pressure and adsorption capacity and BJH theoretical model, the pore size distribution parameters were obtained and the curve was drawn.
2.2.2 The MIP experiment
The MIP experiment was measured in the laboratory, using AutoPore IV9500 automatic porthole distribution mercury porosimeter (Fig. 4). The mercury porosimeter had a pressure range of 0.43–60,000 psi and a measuring pore size range of 3–360 μm. The volume accuracy of the dilatometer for entering and exiting mercury was less than 0.1 μL, the accuracy level of the analytical balance was 0.001 g, and the data was controlled and collected by a computer. The mercury used in the experiment was of analytical grade with a contact angle of 130°.
During the experiment, the sample was first dried and weighed, then put it into a dilatometer to vacuum, finally injected mercury liquid. The mercury injection pressure was sequentially increased to make the mercury enter the pores of the oil shale, and the amount of mercury injected was recorded; mercury was removed step by step at maximum pressure. Through software automatic record and calculation, draw the pore size distribution curve, obtain porosity, pore volume and other relevant parameters. The maximum test pressure is 413 MPa.
3 Results
3.1 The results of the LTNA experiment
The results of the LTNA experiment will be analyzed from two aspects: the influence of temperature on pore size distribution (PSD) and the influence of temperature on the average pore size of BJH.
3.1.1 The effect of temperature on PSD
Based on the LTNA experiment, the PSD curves of oil shale at different target temperature are shown in Fig. 5.
As shown in Fig. 5, the PSD curve of oil shale under different target temperature changed regularly with temperature. The PSD of oil shale in the temperature range of 23–200 °C is complicated, with multi-peak distribution and concentrated in the micropore range of 2–4 nm, but the peak value is not large. It indicates that there are few pores and most of them are unconnected micropores at this temperature. There is a single peak distribution in the 300–400 °C range, with the peak at 3.8 nm. Bimodal distribution appears in the range of 500–650 °C, and the peak value of transition pore shifts to the right with the increasing temperature, which indicates that the average pore diameter increases with the increase of temperature, and the pore connectivity is improved.
3.1.2 The effect of temperature on the average pore size of BJH
Based on the LTNA experiment, the change in average pore size of BJH in Xinjiang oil shale at different target temperatures is shown in Fig. 6.
As shown in Fig. 6, with the raising pyrolysis temperature, the average pore size of oil shale shows a process of rising-falling-rising-leveling. It reaches the highest value at about 200 °C. Then it shows a linear declining trend between 200 and 350 °C before starting to increase, and stabilizing at 600 °C. In conclusion, the average pore size drastically decreased in 200–350 °C. Combined with Fig. 5, it becomes evident that the oil shale's transition pores were significantly reduced in this stage. This was primarily because of the continuous precipitation of adsorbed water inside the oil shale and the less constraints between its minerals (Liu et al. 2022), which caused some transition pores to expand and form mesopores, which were outside the scale of the LTNA experiment. Therefore, it is crucial to use joint LTNA-MIP to characterize the pore evolution of oil shale.
3.2 The results of MIP experiment
For the results of the MIP experiment, this paper will analyze the influence of temperature on the PSD and the influence of temperature on median pore size and average pore size.
3.2.1 The effect of temperature on the pore size distribution
Based on the MIP experiment, the PSD curves of Xinjiang oil shale under different target temperatures is shown in Fig. 7.
Figure 7 shows that the PSD of Xinjiang oil shale is distributed in two sections of micropores and macropores in the form of a half peak before the temperature is lower than 400 °C, that is, micropores, transition pores and macropores are relatively developed, while mesopores are poorly developed. During 23–300 °C, with increasing temperature, the two-pore size distribution areas of the high-pressure area and low-pressure area expand to the center, and the PSD tends to be uniform. At 400 °C, it becomes a single peak, with a pore size of 554.1 nm. The peak continues to shift to the right in the range of 400–650 °C, reaching 834.8 nm at 650 °C. It demonstrates that the pore size is becoming larger under the influence of temperature at this point. It should be noted that at 500 °C, a small peak appears at 17.1 nm, which shifts to the right with the change of temperature, and becomes 95.4 nm at 650 °C and connects with the main peak, but the peak height does not increase significantly, which shows that the amount of new micropores in Xinjiang oil shale is not obvious after 400 °C. According to the actual situation, this paper considers that the new micropores at high temperature are mainly related to the dehydration of clay minerals (Zhu et al. 2022).
3.2.2 The Effect of temperature on median pore size and average pore size
Median pore size and average pore size are important indexes for evaluating pore characteristics of porous materials. Based on the MIP experiment, the pore size variation of Xinjiang oil shale under different target temperatures is shown in Fig. 8.
As shown in Fig. 8, the median pore size and average pore size vary slightly between 23 and 300 °C. After 300 °C, the median pore size and the average pore size increase greatly, and the former increases much more than the latter, and the average pore size decreases slightly between 400 and 500 °C. The reason is that the median pore is more developed in this temperature range. After 500 °C, the trend of the average pore size deviates from the median pore size. Figure 7 shows that the transition pore concentration region with a peak of 17.1 nm is generated after 500 °C, with a smaller but more numerous pore volume contribution, which lead to an increase in the median pore size and a decrease in the average pore size. At 600 °C, the median pore size decreases substantially mainly due to the increase in the number of micropores caused by inorganic matter water loss (Liu et al. 2022), which is different from the results of LTNA experiment because a large number of micropores smaller than 2 nm produced before 600 °C are not reflected.
4 Joint characterization of pore structure by LTNA and MIP
4.1 Joint characterization principle
Based on the analysis above, the difference between LTNA and MIP experiment was the different range of measured pore sizes. The MIP experiment provided a wide testing range and might characterize pore structure at multiple scales. However, it cannot accurately characterize pores below 10 nm due to pore deformation or even destruction caused by high mercury pressure. The LTNA experiment was more reliable for the characterization of pore structure in the 2–50 nm range, especially for the comparative analysis of the surface area and pore structure morphology. Therefore, they both can complement each other. And in this paper, it is the combination of the advantages of them that is used to jointly characterize the evolution of oil shale pores during pyrolysis using LTNA and MIP joint characterization. Theoretically, the combination of the two should satisfy the same pore size (mercury liquid or liquid nitrogen) corresponding to the same pore size, that is, (dV/dD)LTNA = (dV/dD)MIP (Liu et al. 2015; Schmitt et al. 2013), so that the pore size distribution curves overlapped or intersected within a certain range. Therefore, the LTNA and MIP experiments data at the same temperature in Figs. 5 and 7, respectively, was placed under a unified standard, as shown in Fig. 9 (taking 23 °C and 650 °C data as examples). However, the two curves didn’t entirely overlap, which could be the result of two factors: (1) According to different experimental principles, the MIP experiment results reflected pore throat distribution, whereas BJH theoretical calculation results based on LTNA data contained pore and throat information (Clarkson et al. 2013), so the analysis of certain pore shapes can be biased between them. (2) In the MIP experiment, under the action of mercury pressure, the solid skeleton of porous media compressed (Kuila and Prasad 2013), leading to large measured data. At the same time, as shown in Fig. 9, although the PSD curves of the two test results don’t overlap within their common effective test range, for the same test object, the changing trends of the two for the same test object were very similar. So combine the two to achieve the joint characterization of the PSD at the full scale.
4.2 Realization of joint characterization
In the LTNA experiment, the effective pore size ranges from 2 to 50 nm, while in the MIP experiment, the effective pore size ranges from 10 to 100 μm. These two experiments have a common effective pore size test range (10–50 nm), and for this reason, it is necessary to calculate the pore size at which the measured data of the two experiments are connected. Take the curve of Xinjiang oil shale at 23 °C as an example to illustrate the connection process using a graphical representation:
-
1.
Based on the measured data of the two experiments, the cumulative pore volume is obtained by accumulating from the small pore diameter. Considering that the fitting curve has better stability, the accumulated pore volume needs to be fitted to the curve first in actual operation (Fig. 10). And to control the fitting accuracy, the correlation coefficient R2 of all fitting curves is required to be greater than 0.96. It should be noted that the data based on the MIP method are accumulated from the large pore, and it needs to be recalculated to be accumulated from the small pore.
-
2.
Derive the two cumulative pore volume curves to obtain the intersection point on the two differential curves (Fig. 11). Take the abscissa of the intersection point as the pore size of a connecting point, measuring the pore volume change data by using the LTNA method before the connecting point, and measuring the pore volume change data by using the MIP method after it. Then the distribution curve of pore volume variation in the full-scale range is obtained. The cumulative pore volume is obtained by accumulating the pore volume variation, and the corrected porosity data can be obtained by multiplying the cumulative pore volume by the measured apparent density. Using the same method, the pore volume increment and porosity curves of oil shale at other temperature points can be obtained.
It is worth noting that this method only considers the relationship between the incremental volume and pore diameter obtained by each technique, ignoring the intrinsic properties of the LTNA and MIP techniques, so the results obtained are only useful for the pore diameter-pore volume (and its incremental) distributions and the related data derived therefrom, which is a limitation. But this method makes the pore characterization results more comprehensive and accurate.
4.3 The results of joint characterization
Figure 12 shows the full-scale pore size distribution curves of Xinjiang oil shale characterized by LTNA and MIP joint characterization. As shown in Fig. 12, the pore volume increment distribution curve of oil shale is more prominent at 23–300 °C with the left peak distribution and the peak position fluctuates at 18–120 nm. There are irregularly distributed pores at 10–100 μm on the right, while the changing trend of the porosity distribution curve is not obvious, which means that the porosity is slowly increasing at this stage. At 400 °C, the pore volume increment and porosity increase sharply. In 400–600 °C, the pore volume increment distribution curve shows a multi-peak distribution in the relatively concentrated area (434.2–921.6 nm), and the peak value increases with the increasing temperature. In this range, the pore capacity distribution is dominated by transitional pore and mesopore, and the peak value decreases at 650 °C. However, there is a significant peak at 1329.2 nm in the macropore range. Although the porosity keeps increasing in the range of 400–650 °C, the trend of its distribution curve does not change, and the pore volume distribution is dominated by mesopore and macropore.
Under different target temperature, the pore structure change mechanism of oil shale is different. In the range of 23–300 °C, the effects of temperature on oil shale are mainly the dehydration of free water, the expansion and deformation of organic matter, and the fine-tuning of pore structure caused by the different thermal expansion coefficients of minerals (Liu et al. 2022, 2023). The pore volume of oil shale increases significantly due to the dehydration reaction of gypsum at 100 °C (Strydom et al. 1995). At 400 °C, the organic matter in the oil shale reaches its pyrolysis temperature and a large amount of organic matter is separated out. At this time, the influence of temperature on pore structure is mainly related to the pyrolysis reaction and the degree of pyrolysis. The pyrolysis of organic matter in oil shale can be divided into two obvious stages, and the occupation and blockage of intermediate products will affect the measurement of pore structure at the corresponding temperature (Lai et al. 2016; Liu et al. 2023). After 500 °C, the pyrolysis reaction of organic matter in oil shale is basically completed, and the changes in its pore structure are mainly caused by the thermal effects of temperature on inorganic minerals, such as the removal of crystal water from clay minerals, the decomposition of carbonate minerals, the thermal fracture caused by the expansion of quartz phase change, and the connection and collapse of pore structure at high temperature (Liu et al. 2014; Zhang et al. 2018); At this stage, the changes of pore structure of Xinjiang oil shale are closely related to its mineral composition.
Table 3 shows the porosity of oil shale at different target temperatures and its pore volume changes at different pore scales, from which the specific parameters of porosity and stage pore volume at corresponding temperatures can be obtained from the table.
Based on the fitting of full-scale porosity data, it is found that the change of Xinjiang oil shale porosity with temperature conforms to the logistic function, and the fitting equation is as follows:
where φ is the porosity, %, and T is the temperature, °C.
It can be found from the equation that the porosity varies with temperature roughly in an "S" curve, that is, the porosity increases with the increase of temperature, and the middle section of the curve changes greatly, and the two ends of the curve change gently.
4.3.1 Variation law of stage pore volume with temperature
Figure 13 shows the variation curves of different scales of pore volume with temperature at different target temperature.
As shown in Fig. 13, the stage pore volume of oil shale increases slightly at 300 °C; at 400 °C, the micropore increases slightly to the maximum value in the whole temperature range, and then decreases slowly. The transition pore decreases to the minimum value in the temperature range at 400 °C, then increases slowly to 600 °C, and then decreases at 650 °C. The pore volume of mesopores increases significantly at 400 °C, increases steadily between 400 and 600° C, and decreases at 650 °C. The pore volume of macropores changes weakly with temperature, and shows an increase–decrease-increase trend in the range of 400–650 °C, and it increases significantly at 650 °C.
4.3.2 Variation law of porosity with temperature
Porosity (φ) is a parameter characterizing the size of pore space in rock, which refers to the percentage of the ratio of pore volume (Vp) in oil shale to the total volume of oil shale (V). The curve shown in Fig. 14 is drawn according to the porosity change of Xinjiang oil shale at different temperatures.
It can be seen from the Fig. 14 that the change curve of oil shale porosity with the temperature has an “S” shape, that is, the low-temperature section and high-temperature section change slowly, while the middle section (400 °C) changes dramatically, which is just in line with the fitting equation obtained above. Comparing the porosity change curves obtained by the MIP method and the full-scale method (Fig. 14), it shows that the full-scale method accesses more accurate LTNA test data in the micro and small pore sections, which corrects the problem of high pore volume increment test caused by the compression of the sample skeleton under high pressure by the MIP method. The porosity obtained is generally lower than the mercury injection test results at the corresponding temperature, but the variation trends of the two with temperature were highly consistent.
During the whole heating interval, the porosity increased from 2.3642% at 23 °C to 39.1493% at 650 °C, with the porosity increasing by 16.6 times. It shows that the change of the final pyrolysis temperature controls the change of the porosity, but the high temperature (650 °C) has little contribution to the porosity, which is conducive to the expansion of the pore size distribution to mesopores and macropores.
Based on the above analysis, it was found that the temperature causes the redistribution of oil shale pore structure. In 23–350 °C, the increasing temperature leads to the removal of free water inside the pore and the micro- expansion of inorganic minerals, resulting in the generation of micropores and the expansion of microfissures. From 350 to 550 °C, a large amount of kerogen is pyrolyzed to form pore, which the thermal stress leads to the fracture of inorganic mineral skeleton to form fissures, and the pore morphology and structure are greatly changed under the multiple factors. In 550–650 °C, most of the organic matter is pyrolyzed completely, and the decomposition of the carbonate minerals and the deformation of mineral skeleton form pores and fissures. But due to the insufficient strength of the skeleton, some pores are collapsed and blocked, so that the pore parameters tend to be stable after 600 °C. Therefore, during the in-situ mining, the temperature needs to be maintained between 350 and 550 °C as possible, so that not only the kerogen can be completely pyrolyzed, but also the damage to the mineral skeleton can be reduced to a certain extent, which facilitates the transportation of heat transfer medium and shale oil and gas.
5 Conclusions
In this paper, the pore characteristics of Xinjiang oil shale at different pyrolysis final temperatures were studied by the LTNA and MIP experiments. Based on the two experimental results, the LTNA and MIP joint characterization of the pore was obtained. The main conclusions are as follows:
-
Based on the LTNA experiment: the PSD of Xinjiang oil shale during 23–200 °C is multi-peak and concentrated in the micropore range of 2–4 nm. From 300 to 400 °C, it is single-peak, with a peak at 3.8 nm. In 500–650 °C, it shows a bimodal distribution, and the peak of transition pore shifts to the right with the increasing temperature.
-
Based on the MIP experiment: before 400 °C, the PSD is relatively developed with micropores, transition pores and macropores, and the mesopores are poorly developed. at 400 °C, it becomes unimodal, and the corresponding pore size is 554.1 nm. In 400–650 °C, the peak of it continuously shifts to the right, reaching 834.8 nm at 650 °C.
-
The joint characterization results show that the change curve of oil shale porosity with pyrolysis final temperature in Xinjiang shows an “S” shape. The porosity of the oil shale increases continuously in 23–650 °C, with a slight increase before 300 °C, a continuous increase in porosity from 300 to 600 °C, and a decrease in the increase at 650 °C. Included all pores, the transition pores decreased to a minimum at 400 °C and slowly increased at 600 °C; the change of the mesopores was particularly obvious, with a large increase at 400 °C and a steady increase at 400–600 °C, both of which decreased at 650 °C.
-
The joint characterization can not only take into account the micropores which can not accurately measured in MIP experiment, but also take into account the mesopores and macropores which can’t be accurately characterized in the LTNA experiment. The joint characterization of LNTA and MIP is, the advantages of them can be complementary, to make the characterization results are more comprehensive and accurate.
References
Bai F, Sun Y, Liu Y et al (2017) Evaluation of the porous structure of Huadian oil shale during pyrolysis using multiple approaches. Fuel 187:1–8. https://doi.org/10.1016/j.fuel.2016.09.012
Cai Y, Liu D, Pan Z et al (2013) Petrophysical characterization of Chinese coal cores with heat treatment by nuclear magnetic resonance. Fuel 108(11):292–302. https://doi.org/10.1016/j.fuel.2013.02.031
Clarkson C, Solano N, Bustin R et al (2013) Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 103:606–616. https://doi.org/10.1016/j.fuel.2012.06.119
Han X, Jiang X, Yu L et al (2006) Change of pore structure of oil shale particles during combustion. Part 1. Evolution mechanism. Energy Fuels 20(6):2408–2412. https://doi.org/10.1021/ef0603277
Hinai A, Rezaee R, Esteban L et al (2014) Comparisons of pore size distribution: a case from the Western Australian gas shale formations. J Unconv Oil Gas Resour 8:1–13. https://doi.org/10.1016/j.juogr.2014.06.002
Kang Z, Yang D, Zhao Y et al (2011) Thermal cracking and corresponding permeability of Fushun oil shale. Oil Shale 28(2):273–283. https://doi.org/10.3176/oil.2011.2.02
Kuila U, Prasad M (2013) Specific surface area and pore-size distribution in clays and shales. Geophys Prospect 61(2):341–362. https://doi.org/10.1111/1365-2478.12028
Lai D, Shi Y, Geng S et al (2016) Secondary reactions in oil shale pyrolysis by solid heat carrier in a moving bed with internals. Fuel 173:138–145. https://doi.org/10.1016/j.fuel.2016.01.052
Liu D, Wang H, Zheng D et al (2009) World progress of oil shale in-situ exploitation methods. Nat Gas Ind 29(5):128–132. https://doi.org/10.3787/j.issn.1000-0976.2009.05.032
Liu Q, Han X, Li Q et al (2014) TG–DSC analysis of pyrolysis process of two Chinese oil shales. J Therm Anal Calorim 116(1):511–517. https://doi.org/10.1007/s10973-013-3524-2
Liu T, Lin B, Zou Q et al (2015) Pore characteristics of coal with the treatment of hydraulic slotting and gas pre-drainage in Yangliu coal mine. Nat Gas Geosci 26(10):1999–2008. https://doi.org/10.11764/j.issn.1672-1926.2015.10.1999
Liu Z, Yang D, Hu Y et al (2018) Influence of in situ pyrolysis on the evolution of pore structure of oil shale. Energies 11(4):755. https://doi.org/10.3390/en11040755
Liu Z, Yang D, Shao J et al (2019) Evolution of pore connectivity in the Fushun oil shale by low-field nuclear magnetic resonance spectroscory. Chin J Magn Reson 36(3):309–318. https://doi.org/10.11938/cjmr20182687. (in Chinese)
Liu Z, Ma H, Guo J et al (2022) Pyrolysis characteristics and effect on pore structure of Jimsar oil shale based on TG-FTIR-MS analysis. Geofluids. https://doi.org/10.1155/2022/7857239
Liu Z, Ma H, Wang Z et al (2023) Experimental study on the thermophysical properties of Jimsar oil shale. Oil Shale 40(3):194–211. https://doi.org/10.3176/oil.2023.3.02
Mao Z, He Y, Ren X et al (2005) An improved method of using NMR T2 distribution to evaluate pore size distribution. Chin J Geophys 48(2):373–378. https://doi.org/10.1002/cjg2.668
Rabbani A, Baychev T, Ayatollahi S et al (2017) Evolution of pore-scale morphology of oil shale during pyrolysis: a quantitative analysis. Transp Porous Media 119(1):143–162. https://doi.org/10.1007/s11242-017-0877-1
Saidian M, Godinez L, Rivera S et al (2014) Porosity and pore size distribution in mudrocks: a comparative study for Haynesville, Niobrara, Monterey, and Eastern European Silurian formations. In: Unconventional resources technology conference, Denver, Colorado, 25–27 August 2014. Society of Exploration Geophysicists, American Association of Petroleum Geologists, Society of Petroleum Engineers, pp 1226–1243. https://doi.org/10.15530/urtec-2014-1922745
Saif T, Lin Q, Singh K et al (2016) Dynamic imaging of oil shale pyrolysis using synchrotron X-ray microtomography. Geophys Res Lett 43(13):6799–6807. https://doi.org/10.1002/2016gl069279
Saif T, Lin Q, Gao Y et al (2019) 4D in situ synchrotron X-ray tomographic microscopy and laster based heating study of oil shale pyrolysis. Appl Energy 235:1468–1475. https://doi.org/10.1016/j.apenergy.2018.11.044
Schmitt M, Fernandes C, Da Cunha Neto J et al (2013) Characterization of pore systems in seal rocks using nitrogen gas adsorption combined with mercury injection capillary pressure techniques. Mar Pet Geol 39(1):138–149. https://doi.org/10.1016/j.marpetgeo.2012.09.001
Schrodt J, Ocampo A (1984) Variations in the pore structure of oil shales during retorting and combustion. Fuel 63(11):1523–1527. https://doi.org/10.1016/0016-2361(84)90219-9
Si Ma L, Zhao H, Dai S (2012) Analysis of adaptability of application of NMR logging in igneous rock reservoirs. Prog Geophys 27(1):145–152 (in Chinese)
Strydom CA, Hudson-Lamb DL, Potgieter JH et al (1995) The thermal dehydration of synthetic gypsum. Thermochim Acta 269:631–638
Tang J, Pan Y, Zhang Z (2005) NMRI research on storage and transport of coalbed methane. J Liaoning Tech Univ 24(5):674–676 (in Chinese)
Tiwari P, Deo M, Lin C et al (2013) Characterization of oil shale pore structure before and after pyrolysis by using X-ray micro CT. Fuel 107:547–554. https://doi.org/10.1016/j.fuel.2013.01.006
Wang H, Ma F, Tong X et al (2016) Assessment of global unconventional oil and gas resources. Pet Explor Dev 43(6):925–940. https://doi.org/10.1016/S1876-3804(16)30111-2
Wang L, Yang D, Zhao J et al (2018) Changes in oil shale characteristics during simulated in-situ pyrolysis in superheated steam. Oil Shale 35(3):230–241. https://doi.org/10.3176/oil.2018.3.03
Wang L, Zhao Y, Yang D (2020) Investigation on meso-characteristics of in-situ pyrolysis of oil shale by injecting steam. Chin J Rock Mech Eng 39(8):1634–1647. https://doi.org/10.13722/j.cnki.jrme.2019.0914. (in Chinese)
Wu X, Wang S, Zheng M et al (2016) Standard system establishment for conventional and unconventional hydrocarbon resources assessment techniques and its significance. Nat Gas Geosci 27(9):1640–1650. https://doi.org/10.11764/j.issn.1672-1926.2016.09.1640
Yang Z, Xian B, Jiang H et al (2009) The experimental study on coalbed gas reservoir using nuclear magnetic resonance technique. Chin Coalbed Methane 6(4):20–23 (in Chinese)
Yang L, Yang D, Zhao J et al (2016) Changes of oil shale pore structure and permeability at different temperatures. Oil Shale 33(2):101–110. https://doi.org/10.3176/oil.2016.2.01
Yao Y, Liu D, Tang D et al (2008) Fractal characterization of adsorption-pores of coals from North China: an investigation on CH4 adsorption capacity of coals. Int J Coal Geol 73(1):27–42. https://doi.org/10.1016/j.coal.2007.07.003
Zhang H, Liu J, Kang Z et al (2018) Experimental research of the pyrolytic properties and mineral components of Bogda oil shale, China. Oil Shale 35(3):214–229. https://doi.org/10.3176/oil.2018.3.02
Zhang J, Li X, Zou X et al (2019) Characterization of multi-type pore structure and fractal characteristics of the Dalong formation marine shale in northern Sichuan Basin. Energy Sources 42:2764–2777. https://doi.org/10.1080/15567036.2019.1618988
Zhu J, Yang Z, Li X et al (2018) Evaluation of different microwave heating parameters on the pore structure of oil shale samples. Energy Sci Eng 6(6):797–809. https://doi.org/10.1002/ese3.253
Zhu Z, Yang S, Wang R et al (2022) Effects of high temperature on the linear thermal expansion coefficient of Nanan granite. Acta Geod Geophys 57(2):231–243. https://doi.org/10.1007/s40328-022-00375-7
Funding
This work has been supported by Heilongjiang Provincial Natural Science Foundation of China (No. LH2021E108), the Basic Scientific Research Operating Expenses of Heilongjiang Provincial Universities and Colleges of China (No. 2020-KYYWF-0691), and the National Natural Science Foundation of China (No. 52174075).
Author information
Authors and Affiliations
Contributions
ZL conceived and designed the experiments, ZW, HM and YG conducted the experiments, ZL and GL analyzed the data, ZL wrote the paper, all authors discussed the previous version of the manuscript, read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Ma, H., Wang, Z. et al. Study on the pore evolution of Xinjiang oil shale under pyrolysis based on joint characterization of LNTA and MIP. Geomech. Geophys. Geo-energ. Geo-resour. 9, 154 (2023). https://doi.org/10.1007/s40948-023-00686-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40948-023-00686-1