Abstract
Purpose
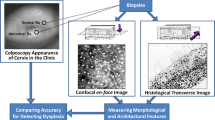
Cervical cancer is a major health issue in developing countries. Early diagnosis and treatment of the disease play a crucial role in the healthcare industry. At present, the diagnostic methods have limitations; newer technologies and techniques are developing, and confocal microscopy is one of them. Classification of cervical precancer stages and treatment is currently based on histopathological classification which has limits due to inter- and intra-variable observation. Confocal microscopy detects cervical precancerous lesions automatically in a non-invasive manner as a point-of-care technology which in turn benefits patient care with limited follow-up procedures. This review encompasses the advantage of confocal microscopy over the current diagnostic techniques.
Results
Optical methods are proving more appropriate to indicate changes associated with tissue including cell morphology, metabolic activity and differentiation, stromal angiogenesis, as well as epithelial–stromal communication. Digital image analysis techniques identify and focus on the abnormal areas with high sensitivity. Suspicious regions can be further confirmed using high-resolution techniques. Quantitative analysis of confocal fluorescence images discriminates high-grade Cervical Intra-epithelial Neoplasia (CIN) lesions versus low-grade CIN lesions and normal tissues, at various depths of imaging.
Conclusion
Portable and cost-effective models of confocal microscopy are under trial phase which would be used for primary screening in a real-time manner especially in low- and middle-income countries in future.


Similar content being viewed by others
Data availability
Not applicable.
References
Sağnıç S. Human papillomavirus and cervical cancer. In: Cervical cancer - a global public health treatise. IntechOpen; 2021. https://doi.org/10.5772/intechopen.98490.
Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020;40(5):602–608. https://doi.org/10.1080/01443615.2019.1634030. Epub 2019 Sep 10. Erratum in: J Obstet Gynaecol. 2020;40(4):590.
Ginsburg O, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–60.
Collier T, Lacy A, Richards-Kortum R, Malpica A, Follen M. Near real-time confocal microscopy of amelanotic tissue: detection of dysplasia in ex vivo cervical tissue. Acad Radiol. 2002;9(5):504–12.
Castellanos MR, Nehru VM, Pirog EC, Optiz L. Fluorescence microscopy of H&E stained cervical biopsies to assist the diagnosis and grading of CIN. Pathol Res Pract. 2018;214(5):605–11. https://doi.org/10.1016/j.prp.2018.03.021. (Epub 2018 Mar 26 PMID: 29627221).
Lifsics A, Groma V, Cistjakovs M, Skuja S, Deksnis R, Murovska M. Identification of High-Risk Human Papillomavirus DNA, p16, and E6/E7 Oncoproteins in Laryngeal and Hypopharyngeal Squamous Cell Carcinomas. Viruses. 2021;13(6):1008. https://doi.org/10.3390/v13061008. (PMID: 34072187; PMCID: PMC8229053).
Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination-Review of Current Perspectives. J Oncol. 2019;2019:3257939. https://doi.org/10.1155/2019/3257939. (PMID: 31687023; PMCID: PMC6811952).
Gaikwad V, Gaikwad S, Yalla S, Salvi P. A prospective comparative study between pap smear, visual inspection with acetic acid, visual inspection with Lugol’s Io-Dine, colposcopy and histopathology for diagnosis of cervical intraepithelial neo-plasia and early carcinoma cervix. J Pharm Negat Results. 2023;1817–26. Retrieved from https://www.pnrjournal.com/index.php/home/article/view/7603
Eeikhzadeh F, Ward RK, Carraro A, et al. Quantification of confocal fluorescence microscopy for the detection of cervical intraepithelial neoplasia. BioMed Eng OnLine. 2015;14:96. https://doi.org/10.1186/s12938-015-0093-6.
Güzel C, van Sten-van’t Hoff J, de Kok IMCM, Govorukhina NI, Boychenko A, Luider TM, Bischoff R. Molecular markers for cervical cancer screening. Exp Rev Proteomics. 2021;18(8):675–91. https://doi.org/10.1080/14789450.2021.1980387
Chang SK, Mirabal YN, Atkinson EN, Cox D, Malpica A, Follen M, Richards-Kortum R. Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt. 2005;10(2): 024031. https://doi.org/10.1117/1.1899686.
Rashid N, Nawaz H, Poon KWC, Bonnier F, Bakhiet S, Martin C, O’Leary JJ, Byrne HJ, Lyng FM. Raman microspectroscopy for the early detection of pre-malignant changes in cervical tissue. Exp Mol Pathol. 2014;97(3):554–64. https://doi.org/10.1016/j.yexmp.2014.10.013.
Castellanos MR, Szerszen A, Gundry S, et al. Diagnostic imaging of cervical intraepithelial neoplasia based on hematoxylin and eosin fluorescence. Diagn Pathol. 2015;10:119. https://doi.org/10.1186/s13000-015-0343-8.
Ragazzi M, et al. Ex vivo (Fluorescence) confocal microscopy in surgical pathology: state of the art Adv. Anat Pathol. 2016;23(3):159–69.
Macé V, Ahluwalia A, Coron E, et al. Confocal laser endomicroscopy: a new gold standard for the assessment of mucosal healing in ulcerative colitis. J Gastroenterol Hepatol. 2015;30(S1):85–92.
Degueldre M, Vandromme J, de Wind A, Feoli F. Real-time in vivo microscopic imaging of the cervix using confocal laser endomicroscopy: preliminary observations and feasibility study. Eur J Cancer Prev. 2015;25(4):335–43.
Schlosser C, Bodenschatz N, Lam S, Lee M, McAlpine JN, Miller DM, Van Niekerk DJ, Follen M, Guillaud M, MacAulay CE, Lane PM. Fluorescence confocal endomicroscopy of the cervix: pilot study on the potential and limitations for clinical implementation. J Biomed Opt. 2016;21(12): 126011. https://doi.org/10.1117/1.JBO.21.12.126011. (PMID:27999860;PMCID:PMC8357321).
Shukla S, Sah AN, Hatiboruah D, Ahirwar S, Nath P, Pradhan A. Design, fabrication and testing of 3D printed smartphone-based device for collection of intrinsic fluorescence from human cervix. Sci Rep. 2022;12(1):11192. https://doi.org/10.1038/s41598-022-15007-x. (PMID:35778460;PMCID:PMC9249735).
Grant BD, Quang T, Possati-Resende JC, Scapulatempo-Neto C, de Macedo MG, Mauad EC, Stoler MH, Castle PE, Guerreiro Fregnani JHT, Schmeler KM, Richards-Kortum R. A mobile-phone based high-resolution microendoscope to image cervical precancer. PLoS ONE. 2019;14(2): e0211045. https://doi.org/10.1371/journal.pone.0211045. (PMID:30726252;PMCID:PMC6364962).
Buys TP, Cantor SB, Guillaud M, et al. Optical technologies and molecular imaging for cervical neoplasia: a program project update. Gend Med. 2012;9(1):S7-24.
Heo JH, Lee JW, Kannappan S, Lee JH. Optical DNA based sensors for cervical cancers. In: Rayappan JBB, Lee JH, editors. Biomarkers and biosensors for cervical cancer diagnosis. Singapore: Springer; 2021. https://doi.org/10.1007/978-981-16-2586-2_6.
Koeneman MM, et al. Natural history of high-grade cervical intraepithelial neoplasia: a review of prognostic biomarkers. Expert Rev Mol Diagn. 2015;15(4):527–46.
El Hallani S, Poh C, Macaulay C, Follen M, Guillaud M, Lane P. Ex vivo confocal imaging with contrast agents for the detection of oral potentially malignant lesions. Oral Oncol. 2013;49(6):582–90.
Wright T, Ronnet B, Kurman R, Ferenczy A. Precancerous lesions of the cervix. In: Blastein’s pathology of the female gental tract. 6th ed. Springer; 2011.
Novikova T. Optical techniques for cervical neoplasia detection. Beilstein J Nanotechnol. 2017;6(8):1844–62. https://doi.org/10.3762/bjnano.8.186. (PMID:29046833;PMCID:PMC5629403).
Bodenschatz N, Lam S, Carraro A, Korbelik J, Miller DM, McAlpine JN, Lee M, Kienle A, MacAulay C. Diffuse optical microscopy for quantification of depth-dependent epithelial backscattering in the cervix. J Biomed Opt. 2016;21(6):66001. https://doi.org/10.1117/1.JBO.21.6.066001.PMID:27251077;PMCID:PMC8357336.
Pandey K, Bhagoliwal A, Jain S. Optical Imaging: Future Tool in Detection of Pre-cancerous and Cancerous Lesions of Cervix and Its Comparison to Colposcopy. J Obstet Gynaecol India. 2015;65(3):176–80. https://doi.org/10.1007/s13224-014-0511-x. (Epub 2014 Jul 15. PMID: 26085739; PMCID: PMC4464571).
Quinn MK, Bubi TC, Pierce MC, Kayembe MK, Ramogola-Masire D, Richards-Kortum R. High-resolution microendoscopy for the detection of cervical neoplasia in low-resource settings. PLoS ONE. 2012;7(9): e44924. https://doi.org/10.1371/journal.pone.0044924.
Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63(9):1999–2004.
Hendee WR. Confocal microscopy: a new technique for in vivo imaging. Acad Radiol. 2002;9(5):503. https://doi.org/10.1016/s1076-6332(03)80325-2.
Ragazzi M, Piana S, Longo C, Castagnetti F, Foroni M, Ferrari G, Gardini G, Pellacani G. Fluorescence confocal microscopy for pathologists. Mod Pathol. 2014;27(3):460–71. https://doi.org/10.1038/modpathol.2013.158.
Reddy SP, Ramani P, Nainani P. Confocal microscopy and exfoliative cytology. J Oral Maxillofac Pathol. 2013;17(2):217–21. https://doi.org/10.4103/0973-029X.119746.
Tang Y, Zhang AXJ, Chen G, Wu Y, Gu W. Prognostic and therapeutic TILs of cervical cancer Current advances and future perspectives. Mol Ther Oncolytics. 2021;22:410–30. https://doi.org/10.1016/j.omto.2021.07.006.
Miebach L, Berner J, Bekeschus, S. Review - In ovo model in cancer research and tumor immunology. Front. Immunol. 2022; VL13 SN - 1664-3224. https://www.frontiersin.org/articles/10.3389/fimmu.2022.1006064
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None to disclose.
Writing assistance
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil-Takbhate, B., Khopkar-Kale, P. & Tripathy, S. Role of Confocal Laser Scanning Microscopy for the Detection of Cervical Cancer. Indian J Gynecol Oncolog 22, 56 (2024). https://doi.org/10.1007/s40944-024-00811-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-024-00811-2