Abstract
Phosphorus in surface waters accelerate algal growth and eutrophication, considerably influencing water quality. Spatiotemporal changes in phosphorus concentration are crucial for environmental issues. We aimed to study the temporal and spatial changes in water quality in a river and in a drainage water system considering different land uses. To this aim, 15 water samples were collected from the origin of the river to the estuary, in the Bostankar River watershed (N-Iran), during spring and winter. Further samples were collected from agricultural drainage water in rice fields, tea, flower, orange as well as kiwi gardens, and forests during spring and winter. EC, pH, TDS, and three forms of phosphorus (total, particulate, and soluble) were measured in the water samples. The results showed that water quality changes in agricultural drainage water were time-dependent; the average total phosphorus was 0.4 mg l-1 lower in the spring than in the winter. The highest phosphorus concentration (1.29 mg l-1) occurred in the winter in the drainage water of the orange gardens. Temporal and spatial changes of the river showed that water quality reduced from the river upstream (jungles and grasslands) towards the downstream (different agricultural land uses), and the amount of phosphorus increased from 0.25 to 0.5 mg l− 1. The TDS increased from 60 to 220 mg l− 1 in the river in the winter. Finally, the results showed that human activities were the main factor in river water quality reduction due to agricultural activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus is one of the vital components of plant growth (Gigolashvili and Kopriva 2014; Rennenberg and Dannenmann 2015; Castro-Rodríguez et al. 2017). Compounds including adenosine diphosphate, adenosine triphosphate (ADP, ATP), and nucleic acids are important phosphorus compounds in plants (Tisdale et al. 1985; Scheerer et al. 2019). Providing sufficient food for the rapidly growing world population has created more pressure on agricultural lands (Sharpley 2003). Thus, cultivated agricultural soils would lose nutrients (particularly phosphorus). To maintain soil fertility, manure and fertilizers are added to the soil. Phosphorus, as one of the vital components of plants, belongs to the type of fertilizer that is added to soil more than its needs. Indeed, the input phosphorus is more than the discharge phosphorus (absorption by plant) (Sharpley 2003; Fan et al. 2018; Ebrahimi et al. 2022). Phosphorus is usually released with soil runoffs into two forms particulate phosphorus and soluble phosphorus (Shoja et al. 2017; Asadi et al. 2018; Latifi et al. 2018). 80% of the phosphorus loss in the surface runoff from cultivated lands includes particulate phosphorus (Sharpley et al. 1992; Ebrahimi et al. 2022b).
Eutrophication is the main factor in surface water quality reduction (Environmental Protection Agency, United States 1996) and is caused by excess phosphorus, nitrogen, and carbon in the surface water (Conley et al. 2009; Schindler et al. 2016). This results in increased algal growth; algae stay on water surfaces as a barrier and decline fishery, entertainment, and industrial activities (Eastman et al. 2010; Schindler et al. 2016). Other consequences of eutrophication include dissolved oxygen reduction due to the decomposition of algae, increasing suspended solids, and decreasing variety of animal and aquatic plant species (Migliaccio et al. 2007). Increased growth of cyanobacteria is another consequence in these waters, which would sicken humans and livestock if consumed (Sharpley 2003). Algal growth releases toxins and volatile organic compounds, resulting in nerve damage and causing concerns over eutrophication (Burkholder and Glasgow Jr 1997). Nitrogen and carbon are vital for aquatic animal growth, but these components are difficult to control owing to atmospheric and water cycles, and phosphorus has been identified as the most limiting component (Sharpley 2003). Many pieces of evidence have confirmed that phosphorus is the most limiting component of aquatic organisms. Note that when moving from fresh waters towards coastal and ocean waters, the limiting component changes from phosphorus to nitrogen (Ryther and Dunstan 1971; Berthold and Schumann 2020; Diatta et al. 2020). According to laboratory examinations, the phosphate concentration providing moderate algal growth is diverse and differs from 0.003 to 0.8 mg l− 1 (Grover 1989). Nevertheless, it was observed that if the phosphate concentration increased to 15 mg l− 1, the amount of carbon fixation and chlorophyll concentration in Lake Michigan increased drastically. However, no confirmed phosphorus concentration was accepted by any of the experts. In accordance with these recommendations, to reach a concentration that causes eutrophication, water phosphorus can be measured instead of soluble phosphorus (Correll 1998). Some researchers (Smith et al. 1993; Foy and Withers 1996) believe that phosphorus concentration around 0.01 to 0.015 mg l− 1 is a concentration responsible for the extreme growth of harmful algae in waters. Based on studies conducted in New Zealand, concentrations around 0.015–0.03 mg l− 1 have been confirmed (Nguyen and Sukias 2002). Sharpley (2003) suggested that if the phosphorus concentration in lake water is more than 0.02 mg l− 1, it accelerates eutrophication. Note that the amount of phosphorus concentration in the water required to measure the threshold concentration depends on the under-studied region.
Considering the importance of phosphorus and nitrogen in surface waters and their role in eutrophication, it is essential to study the temporal and spatial changes in this component as well as its sources. Catchments affected by mining, urban development, and agricultural activities and high levels of water pollution have been realized because of significant nutrient, metalloid, and trace metal contamination loads (Radeva and Seymenov 2021; Seymenov 2022). Nutrient loss due to sediment runoff is a dynamic phenomenon that depends on the time and location of its sources. The largest amounts of nitrogen, phosphorus, and organic matter were observed in summer and the lowest in the winter (Bijayalaxmi Devi and Yadava 2006). Agricultural land use and landscape pattern changes affect the infiltration to runoff ratio, which underlines the need for integrated soil and water conservation (Madarász et al. 2011; Nagy et al. 2020); 70% of phosphorus pollution was found to be the consequence of agricultural activities (Alexander et al. 2008). Various studies have been conducted on the temporal and spatial changes in land use and landscape patterns (Bui and Mucsi 2022), but no comprehensive study has examined the concentration of dissolved solids and various forms of phosphorus from different land drains (Ebrahimi et al. 2022a).
In the Bostankar river watershed, situated in Mazandaran province in the north of Iran, due to the lack of industrial factories, food companies, and sand factories of large cities that are important causes of increasing phosphorus pollution, there is a possibility of separation of agricultural units. This watershed is a good standard for similar regions at the international level, owing to different vegetation types, humid climates, and proximity to the sea. Therefore, the objectives of this research were: (1) to study concentration changes in various forms of phosphorus discharge from different agricultural units and forests in two seasons, spring and winter, and (2) to perform temporal and spatial change analysis of phosphorus pollution in the Bostankar River in two seasons.
Materials and methods
Study area
The Bostankar River watershed, with an area of 4569.5 ha, is situated in Shirud, one of the suburbs of Tonekabon city in Mazandaran province in the north of Iran. From the west, Tonekabon is connected to Ramsar city; from the east to Chalus city; from the southern side, it is connected to the Alborz Mountains; and from the north, it ends in the Caspian Sea (Fig. 1). This river originates in the Tonekabon Mountains, joins the Shirud River, and enters the sea. This watershed supplies various land uses including cultivation, forests, kiwi and orange gardens, tea gardens, and flower gardens.
Among the available land uses in the region, only those with drainage were investigated. In this watershed, farmers apply almost the same management for each land use type. This watershed includes 166 ha of forest land, 957 ha of garden land, 3405 ha of irrigated gardening, and 42 ha of floodplains. The specific sediment and annual sediment load of this watershed are 66.4 tons ha year− 1 and 3035.1 tons year− 1, respectively, which were formed in the Triassic (Organization of Cardamom Forest, Pastures, and Watershed Management of Iran 2002). The river in this watershed is directly connected to the Tiromrood River in the Shirud region, and after traversing approximately 2.5 km, it ends in the Caspian Sea, which is very prominent.
For a better understanding, the predominant land uses within the area have been categorized into three sections: F, characterized by a predominance of forested areas; G, characterized by diverse gardens; and R, characterized by the prevalent cultivation of rice or paddy lands.
Water sampling and analysis
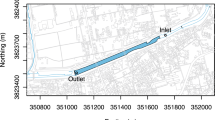
River water samples were collected at 15 points (Fig. 2a), beginning from #1, located upstream of the watershed, and continuing to #15 downstream with three repetitions at only two flood events in the winter and spring. All points were measured using a GNSS device. Water samples (1.5 l) were collected from the centerline of the river, at a depth of 25 cm. The study area can be delineated into two main regions exhibiting different land-use characteristics, as demonstrated by the Sentinel-2 Land Cover map from the time of sample collection (Fig. 2a, Karra et al. 2021). Forest and garden units were situated in the southern part of the studied watershed, whereas the agricultural land use was predominantly paddy fields in the northern region (Fig. 2a). Three water samples were collected from each land-use drainage (i.e., from paddy fields, tea, flower, orange, and kiwi gardens and forests) during the two flood events in both seasons. All samples from the river and drainages were collected on the same day in the winter and spring (Fig. 2b).
Water samples were transferred to the laboratory and kept below 4 °C to prevent changes due to biological and chemical transformations. They were analyzed for less than 24 h after sampling to prevent extensive changes in water phosphorus concentration (Kovar and Pierzynski 2009). In the laboratory, the pH value of the samples was measured by a pH meter (WTW-2010), the electrical conductivity (EC) by an EC meter, and the TDS reading (Total Dissolved Solids) was accomplished by a TDS meter (HI99300-2005) (1999).
Measuring phosphorus forms
Various forms of phosphorus exist in nature, but the phosphate forms, including PO43−, orthophosphate H2PO4−, and HPO42−, are measurable. Thus, the phosphorus present in the samples must be converted to phosphate and orthophosphate for measurement. Accordingly, we first digested the samples with potassium persulfate. As soon as digestion was performed, the amount of phosphorus was gauged using the ascorbic acid method (Carlson and Simpson 1996).
The equation used to calculate the total P is the following (Bridgewater et al. 2017):
where: Total P = the phosphorus concentration is the sample expressed in milligrams per liter.
mgP = the amount of phosphorus determined from the calibration curve and.
mlsample = the volume of the sample.
Soluble phosphorus (SP) measurement is almost identical to total phosphorus, except that samples must be screened using Whatman 44 filter paper before digestion. Note that the samples were screened through three layers of filter paper (Carlson and Simpson 1996). The amount of particulate phosphorus (PP) was calculated as the difference between the total phosphorus and soluble phosphorus content.
Statistical analysis
Water chemical variables were analyzed using a robust ANOVA (applying trimmed means of 20%). We performed a robust ANOVA with trimmed means and Yuen’s test for pairwise comparisons (H0: there was no difference among means of land-use classes or seasons). Common effects of land use and seasons were analyzed with a 2-way factorial ANOVA involving the interactions.
We performed a Principal Component Analysis (PCA) to evaluate the effect of land use in a multivariate method. EC, TDS, pH, TP, PP, and SP were involved with the log-transformed data to improve normality and to ensure the same magnitude for all variables. Besides, we used the standardized PCA using the correlation matrix, and the varimax rotation to get uncorrelated principal components. Kaiser-Meyer-Olkin measure of sampling adequacy (KMO) and the root mean square residual (RMSR) were used to evaluate the success of ordination. The number of principal components (PCs) was determined based on the Kaiser’s rule. The result was visualized in a biplot diagram with the land use classes.
We divided the river samples into 4 subsections (Fig. 2a) from the highest parts to the coastal region (mouth). Due to their high correlations within the PCs, they were used as variables in the further analyses, we performed robust ANOVA with trimmed means and Yuen’s test for pairwise comparisons with PC1 and PC2 (H0: there was no difference among means of land use classes, and H0: there was no differences among river sections).
Statistical analyses were conducted in R 4.3.3 (R Core Team, 2024) with the ggstatsplot (Patil 2021) and FactoMineR (Lê et al. 2008) packages.
Results
TDS, EC, and pH in the drainage water by land use types and seasons
The EC, TDS, and pH were different and had significant differences by land use types, and regarding the effect sizes, all three effect sizes (ξ) were > 0.85. Forest had the lowest values, and the rice fields were the largest. In the case of pH, the lowest value was 6.76, almost in the range of neutral range in the forest land use type, and the highest belonged to the rice fields, 7.69 indicating slightly alkalic pH, the mean values of all land uses were close to the neutral range. EC and TDS also indicated low loads in the water (Fig. 3).
TDS, EC, and pH of the drainage samples were not significantly higher (i.e., p > 0.05) in the winter than in the spring (Fig. 4), but, regarding the TDS, the p = 0.07 was close to significant in statistical terms, and the effect size justified that seasons had a large effect on the concentrations (ξ = 0.71); at sampling point #11, winter EC and TDS values were almost twice those of spring. The pH did not change much during spring, but in the winter, it increased due to water addition having alkaline pH (Fig. 5).
The increase of the EC and TDS had a trend in both seasons from the source to the estuary. In the case of pH, the trend was not obvious, as in winter it decreased at #9 and right after that, reached its maximum at #12. In the winter, after a slight increase, from #11, pH started to decrease (Fig. 6).
Phosphorus forms in the drainage water by land use types and seasons
Although there were no significant differences among the land use types in any case of phosphorus forms (Fig. 6), the effect sizes were around 0.3 indicating moderate effects. The smallest loads were found in drainages of forests, while gardens and rice fields caused 1.5-2 times higher loads in some cases, but the differences were still not significant.
Seasons had a relevant effect on the phosphorus concentrations, in all phosphorus forms. Drainages had significantly larger (4–7 times higher) loads in the winter (Fig. 7). In the winter, the concentrations had wider ranges, pointing to the diverse sources of phosphorus in the watershed.
In the spring, changes in TP and PP rates increased in the river to sampling point #4; showed no trend up to sampling point #10, and then decreased to the mouth of the river (Fig. 8). The SP trend along the river length fluctuated and was close to zero at sample points #12–14 (Fig. 8). All forms of phosphorous increased from upstream to downstream of the river, and the analysis showed significant differences with high effect size (even larger than 2 standard deviations) in all forms of phosphorus between seasons.
Common effects of land use and seasons
PCA was successful according to the KMO (0.67, i.e., mediocre), and RMSR (0.06, i.e., good). Two PCs explained 89% of the total variance. PC1 correlated with the TP, PP, and SP accounting for 46% of the variance, while PC2 correlated with the EC, TDS, and pH accounting for 43%. The biplot diagram (Fig. 9) showed discriminated groups of the land use classes the forest formed a completely different group, and the rice and garden classes had a slight overlap. ANOVA justified that PC2 has a discriminating effect (PC1: F = 1.868, df = 2, p = 0.174; PC2: F = 56.34, df = 2, p < 0.001) where all pairwise comparisons showed significant differences (p < 0.001); accordingly, TDS, EC, and pH had the main effect.
Regarding PC1 (TP, SP, PP), there were no significant differences (Fig. 10), all sections had similar values, only the ranges differed slightly, which was reflected in the effect size (0.41). However, in the case of PC2 (EC, TDS, pH) values had an increasing trend from section #1 to #3 with significant differences, but sections #3 and #4 did not differ, the effect size was 0.86 indicating a large effect.
Total phosphorus had the highest concentrations in the winter and regarding land use, we experienced the largest concentrations in rice land drainages. In the spring, gardens had larger concentrations of TP than the rice fields and forests, but the magnitudes were relevantly smaller than those in the winter (Fig. 11). Based on the adjusted R2, land use and seasons explained 96.3% of the variance. Both factors were significant and had large effects on the TP concentrations (Table 1), and the interactions.
In the case of EC, ranges were significantly different for all land use types in both seasons (Fig. 12). In the winter, rice lands had the largest interquartile range, and the forest had the smallest. In the spring, ranges were small for all land use types. Adjusted R2 was 0.772, indicating a strong relationship, and both the land use type and the season as independent variables had significant and strong effects (Table 2), however, there was no interaction.
Discussion
Surface erosion in the Bostankar watershed area after rainfall increased the concentration of runoff and elemental minerals. In general, due to the lack of vegetation in the winter, the resilience of the soil is small, and raindrops hit the soil surface directly and destroy the soil grains, which increases surface erosion (Ebrahimi et al. 2022b). Forests had more phosphorus released in the winter than in the spring consistent with the previous observations (Hubbard et al. 2004; Buck et al. 2004). This can be related to the splash effect of larger raindrops under taller trees in forests; water gathers on leaves, and larger raindrops falling from 4 to 5 m height have more kinetic energy to destroy soil aggregates, and smaller particles can easily cause erosion (Park and Cameron 2008). As a result, large aggregates disintegrate and turn into particles, which are easily transferred by water currents (Ojani et al. 2022). In the spring, unlike winter, vegetation is suitable and prevents direct raindrops strike considerably, hence, the erosion rate decreases. We justified this process using values measured in the river and drainage systems. In particular, phosphorus bound to soil particles is a direct indicator of erosion; a higher concentration indicates suspended particles in water (Alexander et al. 2008). Cultivated surfaces in flower gardens are usually larger than those in forests, other gardens, and tea gardens; thus, soil particle release in these land use types is high. Accordingly, the TDS level was the highest (398 mg l− 1) in the drainages of this land use type. The minimum TDS concentration was associated with forest drainage in the spring (59 mg l− 1) where both vegetation density and the leaf area index were the highest. Ebrahimi et al. (2022b) found that TDS changes were time-dependent, and their quantities were not the same across different seasons, which is in line with the result of this research, i.e., concentrations are higher in the winter.
Cultivated lands (i.e., human activity), as opposed to forests, are more responsible for phosphorus release (Buck et al. 2004). Since the organic and synthetic fertilizers that have been used for a long time are beyond the plants’ needs, this excess phosphorus in the soil is released with runoff, which, after some interactions, pollutes the river. Buck et al. (2004) identified the impacts of land use changes on water quality, explaining that agricultural activities were the main sources of deposits and water surface pollution. Our results justified those pollutants had higher concentrations in downstream areas, where the presence of agricultural land use types was more dominant than in upstream areas. In both winter and spring, the role of gardens in phosphorus release from rice fields, tea gardens, and flower gardens was more significant. Assessment of land uses indicates that farmers use phosphorous chemical fertilizers and heavy tillage for agricultural land uses especially rice cultivation that both reasons can contribute to fertility erosion. Supporting this observation, a study by Mostaghimi et al. (1992) found the worst water quality scenarios occurred when either sludge or chemical fertilizer was surface-applied under a conventional tillage system.
The river water quality parameters (pH, EC, TDS, and P) increased in both seasons along the river from sampling points RP01 to RP15, and the pollution increased from the headwaters to the mouth. Runoff from cultivated land increased EC and TDS. Alam et al. (2007) showed that the concentration of solid particles was reduced longitudinally in Surma River, Bangladesh. The TDS rate was higher in the winter than in the spring because in the spring, there was more vegetation and less surface erosion, in accordance with the work of Asadi et al. (2018), that is, EC and pH changed in rivers depending on land use, date, and rainfall in five watersheds of Guilan province in N-Iran. The TDS rates of various agricultural drainages were higher in the winter than in the spring. These drainages join the main river and enhance the TDS concentration (Fig. 4). The pH in both seasons was around 7, and the river water qualitative standard (Nabizadeh et al. 2013), pH variations, in both seasons, remained between 6.5 and 8.5, in the winter these rates increased. The primary contributor to the increased phosphorus concentration in the river was the addition of pollution sources, particularly agricultural plots releasing phosphorus. The results indicate that land uses in the downstream region’s area R drainages experienced significant changes in the TP parameter, as evidenced by points #11 to #15 in the river. In area G, the primary land uses are gardens, such as kiwi, orange, and flower, which have different management practices compared to rice. The last area, F, is predominantly forested, with no visible anthropogenic activities. The drainages and river points in these three sections, particularly in winter, indicate that the TP loading from drainage, especially in winter, is highest in area R. According to Tong and Chen (2002) research, agricultural and urban lands produce a much higher level of nitrogen and phosphorus than other land surfaces. The concentration of PP was higher than that of SP; with SP rarely leaching from the soil, and typically originating from soil sediment deposits (Asadi et al. 2018). Phosphorus is often transported in the form of PP and is gradually separated from the soil during the river length, eventually transforming into SP (Shoja et al. 2017). Asadi et al. (2018) demonstrated that TP, PP, and SP changes in a year in cold seasons and land uses without vegetation result in higher phosphorous output, which is consistent with the findings of this research. Latifi et al. (2018) showed that phosphorus changes along the river length are unstable in the Siahrud River, and phosphorus concentration varies based on pollution sources bound to the river by minor branches.
Conclusions
We aimed to reveal the role of land use in the spatiotemporal distribution of water pollutants in the Bostankar River and the drainage along its close vicinity, focusing on phosphorus forms. The findings indicated that this was due to the loading of agricultural drainage to the river. The difference in EC between the upstream and downstream areas was significant, increasing in both seasons two times in the spring and three times in the winter downstream. Phosphorus changes in river water were time-dependent: in the winter the concentration was 20–25 times greater than in the spring. In drainage water, the largest EC and TDS were experienced in the flower gardens, while TP concentration was the highest in the winter and kiwi gardens in the spring. The results revealed that sources of phosphorus loadings of drainages were associated with cultivated lands and forests were not relevant factors, nor were the quality of drainages or polluters of rivers. This study can be useful for hydrologists, soil scientists, agricultural specialists, and farmers to plan and control activities and fertilize agricultural lands.
References
Alam MJB, Islam MR, Muyen Z et al (2007) Water quality parameters along rivers. Int J Environ Sci Technol 4:159–167. https://doi.org/10.1007/BF03325974
Alexander RB, Smith RA, Schwarz GE et al (2008) Differences in Phosphorus and Nitrogen Delivery to the Gulf of Mexico from the Mississippi River Basin. Environ Sci Technol 42:822–830. https://doi.org/10.1021/es0716103
Asadi H, Latifi V, Ebrahimi E (2018) Study of the Phosphorus losses from different watersheds in Guilan Province. Amirkabir J Civ Eng 4:199–202. https://doi.org/10.22060/ceej.2017.12803.5274
Berthold M, Schumann R (2020) Phosphorus Dynamics in a eutrophic lagoon: uptake and utilization of nutrient pulses by Phytoplankton. Front Mar Sci 7
Bijayalaxmi Devi N, Yadava PS (2006) Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-East India. Appl Soil Ecol Sect Agric Ecosyst Environ 31:220–227. https://doi.org/10.1016/j.apsoil.2005.05.005
Bridgewater LL, Baird RB, Eaton AD et al (eds) (2017) Standard methods for the examination of water and wastewater, 23rd edition. American Public Health Association, Washington, DC
Buck O, Niyogi DK, Townsend CR (2004) Scale-dependence of land use effects on water quality of streams in agricultural catchments. Environ Pollut 130:287–299. https://doi.org/10.1016/j.envpol.2003.10.018
Bui DH, Mucsi L (2022) Predicting the future land-use change and evaluating the change in landscape pattern in Binh Duong Province, Vietnam. Hung Geogr Bull 71:349–364. https://doi.org/10.15201/hungeobull.71.4.3
Burkholder JM, Glasgow HB Jr. (1997) Pfiesteria piscicida and other Pfiesreria-like dinoflagellates: Behavior, impacts, and environmental controls. Limnol Oceanogr 42:1052–1075. https://doi.org/10.4319/lo.1997.42.5_part_2.1052
Carlson R, Simpson J (1996) A coordinator’s guide to Volunteer Monitoring. North American Lake Management Society
Castro-Rodríguez V, Cañas RA, De La Torre FN et al (2017) Molecular fundamentals of nitrogen uptake and transport in trees. J Exp Bot 68:2489–2500. https://doi.org/10.1093/jxb/erx037
Conley DJ, Paerl HW, Howarth RW et al (2009) Controlling Eutrophication: Nitrogen and Phosphorus. Science 323:1014–1015. https://doi.org/10.1126/science.1167755
Correll DL (1998) The role of Phosphorus in the eutrophication of receiving Waters: a review. J Environ Qual 27:261–266. https://doi.org/10.2134/jeq1998.00472425002700020004x
Diatta J, Waraczewska Z, Grzebisz W et al (2020) Eutrophication induction Via N/P and P/N ratios under controlled conditions—effects of temperature and water sources. Water Air Soil Pollut 231:149. https://doi.org/10.1007/s11270-020-04480-7
Eastman M, Gollamudi A, Stämpfli N et al (2010) Comparative evaluation of phosphorus losses from subsurface and naturally drained agricultural fields in the Pike River watershed of Quebec, Canada. Agric Water Manag 97:596–604. https://doi.org/10.1016/j.agwat.2009.11.010
Ebrahimi E, Asadi H, Joudi M et al (2022a) Variation entry of sediment, organic matter and different forms of phosphorus and nitrogen in flood and normal events in the Anzali wetland. J Water Clim Change 13:434–450. https://doi.org/10.2166/wcc.2021.456
Ebrahimi E, Asadi H, Rahmani M et al (2022b) Effect of precipitation and sediment concentration on the loss of nitrogen and phosphorus in the Pasikhan River. J Water Supply Res Technol-Aqua 71:211–228. https://doi.org/10.2166/aqua.2022.097
Foy RH, Withers PJA (1996) Contribution of Agricultural Phosphorus to Eutrophication. Proc Fertil Soc 1–32
Gigolashvili T, Kopriva S (2014) Transporters in plant sulfur metabolism. Front Plant Sci 5. https://doi.org/10.3389/fpls.2014.00442
Grover JP (1989) Phosphorus-dependent growth kinetics of 11 species of freshwater algae: algal growth kinetics. Limnol Oceanogr 34:341–348. https://doi.org/10.4319/lo.1989.34.2.0341
Hubbard RK, Newton GL, Hill GM (2004) Water Quality and the Grazing Animal. Publ USDA-ARS UNL Fac 274:255–261
Kovar JL, Pierzynski GM (2009) Methods of Phosphorus Analysis for Soils, sediments, residuals, and Waters. South Coop Ser Bull 408
Latifi V, Asadi H, Ebrahimi E, Mousavi A (2018) Study of temporal variations of Phosphorus Pollution along Siahroud River in Guilan Province. J Water Soil Conserv 25:43–59. https://doi.org/10.22069/jwsc.2018.13864.2858
Lê S, Josse J, Husson F (2008) FactoMineR: an R Package for Multivariate Analysis. J Stat Softw 25. https://doi.org/10.18637/jss.v025.i01
Madarász B, Bádonyi K, Csepinszky B et al (2011) Conservation tillage for rational water management and soil conservation. Hung Geogr Bull 60:117–133
Migliaccio KW, Chaubey I, Haggard BE (2007) Evaluation of landscape and instream modeling to predict watershed nutrient yields. Environ Model Softw 22:987–999. https://doi.org/10.1016/j.envsoft.2006.06.010
Mostaghimi S, Younos TM, Tim US (1992) Effects of Sludge and Chemical Fertilizer Application on Runoff Water Quality1. JAWRA J Am Water Resour Assoc 28:545–552. https://doi.org/10.1111/j.1752-1688.1992.tb03176.x
Nabizadeh R, Valadi Amin M, Alimohammadi M et al (2013) Development of innovative computer software to facilitate the setup and computation of water quality index. J Environ Health Sci Eng 11:1. https://doi.org/10.1186/2052-336X-11-1
Nagy G, Lóczy D, Czigány S et al (2020) Soil moisture retention on slopes under different agricultural land uses in hilly regions of Southern Transdanubia. Hung Geogr Bull 69:263–280. https://doi.org/10.15201/hungeobull.69.3.3
Nguyen L, Sukias J (2002) Phosphorus fractions and retention in drainage ditch sediments receiving surface runoff and subsurface drainage from agricultural catchments in the North Island, New Zealand. Agric Ecosyst Environ 92:49–69. https://doi.org/10.1016/S0167-8809(01)00284-5
Ojani M, Ghajar Sepanluo M, Bahmanyar MA, Danesh M (2022) Investigation of temporal and spatial changes of Nitrogen pollution in drainage of lands with different land-uses in Shirood Watershed Located in Mazandaran Province. J Watershed Manag Res 13:34–42. https://doi.org/10.52547/jwmr.13.26.34
Park A, Cameron JL (2008) The influence of canopy traits on throughfall and stemflow in five tropical trees growing in a Panamanian plantation. Ecol Manag 255:1915–1925. https://doi.org/10.1016/j.foreco.2007.12.025
Patil I (2021) Visualizations with statistical details: the ggstatsplot approach. J Open Source Softw 6:3167. https://doi.org/10.21105/joss.03167
Radeva K, Seymenov K (2021) Surface water pollution with nutrient components, trace metals and metalloidsin agricultural and mining-affected river catchments: a case study for three tributaries of the Maritsa River, Southern Bulgaria. Geogr Pannonica 25:214–225. https://doi.org/10.5937/gp25-30811
Rennenberg H, Dannenmann M (2015) Nitrogen Nutrition of Trees in Temperate forests—the significance of Nitrogen availability in the Pedosphere and Atmosphere. Forests 6:2820–2835. https://doi.org/10.3390/f6082820
Ryther JH, Dunstan WM (1971) Nitrogen, Phosphorus, and Eutrophication in the Coastal Marine Environment. Science 171:1008–1013. https://doi.org/10.1126/science.171.3975.1008
Scheerer U, Trube N, Netzer F et al (2019) ATP as Phosphorus and Nitrogen Source for Nutrient Uptake by Fagus sylvatica and Populus x canescens roots. Front Plant Sci 10:378. https://doi.org/10.3389/fpls.2019.00378
Schindler DW, Carpenter SR, Chapra SC et al (2016) Reducing phosphorus to Curb Lake Eutrophication is a success. Environ Sci Technol 50:8923–8929. https://doi.org/10.1021/acs.est.6b02204
Seymenov K (2022) An example of the adverse impacts of various anthropogenic activities on aquatic bodies: Water quality assessment of the Provadiyska river (Northeastern Bulgaria). Geogr Pannonica 26:142–151. https://doi.org/10.5937/gp26-38196
Sharpley AN (2003) Agricultural phosphorus and eutrophication. USDA
Sharpley AN, Smith SJ, Jones OR et al (1992) The transport of Bioavailable Phosphorus in Agricultural Runoff. J Environ Qual 21:30–35. https://doi.org/10.2134/jeq1992.00472425002100010003x
Shoja H, Rahimi G, Fallah M, Ebrahimi E (2017) Investigation of phosphorus fractions and isotherm equation on the lake sediments in Ekbatan Dam (Iran). Environ Earth Sci 76:235. https://doi.org/10.1007/s12665-017-6548-2
Smith CM, Wilcock RJ, Vant WN et al (1993) Towards sustainable agriculture: freshwater quality in New Zealand and the influence of agriculture. MAF Policy Tech Pap 93
Tisdale SL, Nelson WL, Beaton JD (1985) Soil fertility and fertilizers, 4th edn. Macmillan; Collier Macmillan, New York : London
Tong STY, Chen W (2002) Modeling the relationship between land use and surface water quality. J Environ Manage 66:377–393. https://doi.org/10.1006/jema.2002.0593 (1996) Environmental Indicators of Water Quality in the United States. United States Environmental Protection Agency, Office of Water (1999) Standard Analytical Procedures for Water Analysis. Hydrology Project Technical Assistance
Acknowledgements
Project no. TKP2021-NKTA-32 was implemented with support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme. The research presented in the article was carried out within the framework of the Széchenyi Plan Plus program with the support of the RRF 2.3.1 21 2022 00008 project.
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojani, M., Sepanlou, M.G., Bahmanyar, M. et al. The role of land use on phosphorus release and longitudinal changes of pollution in an agricultural watershed, Bostankar river, Iran. Sustain. Water Resour. Manag. 10, 157 (2024). https://doi.org/10.1007/s40899-024-01141-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01141-z